Abstract
Background. Diffusive clearance depends on blood and dialysate flow rates and the overall mass transfer area coefficient (KoA) of the dialyzer. Although KoA should be constant for a given dialyzer, urea KoA has been reported to vary with dialysate flow rate possibly because of improvements in flow distribution. This study examined the dependence of KoA for urea, phosphate and β2-microglobulin on dialysate flow rate in dialyzers containing undulating fibers to promote flow distribution and two different fiber packing densities.
Methods. Twelve stable haemodialysis patients underwent dialysis with four different dialyzers, each used with a blood flow rate of 400 mL/min and dialysate flow rates of 350, 500 and 800 mL/min. Clearances of urea, phosphate and β2-microglobulin were measured and KoA values calculated.
Results. Clearances of urea and phosphate, but not β2-microglobulin, increased significantly with increasing dialysate flow rate. However, increasing dialysate flow rate had no significant effect on KoA or Ko for any of the three solutes examined, although Ko for urea and phosphate increased significantly as the average flow velocity in the dialysate compartment increased.
Conclusions. For dialyzers with features that promote good dialysate flow distribution, increasing dialysate flow rate beyond 600 mL/min at a blood flow rate of 400 mL/min is likely to have only a modest impact on dialyzer performance, limited to the theoretical increase predicted for a constant KoA.
Keywords: dialysate flow rate, dialyzer, haemodialysis, KoA, mass transfer coefficient
Introduction
Diffusive clearance depends on blood flow rate, dialysate flow rate and the product of the overall mass transfer coefficient and membrane surface area (KoA). Ko is a measure of the overall resistance to mass transfer for a given solute and incorporates the resistances associated with diffusion of the solute to the membrane surface on the blood side, diffusion through the membrane and diffusion across the slow-moving boundary layer on the dialysate side of the membrane [1]. KoA is usually assumed constant for a given dialyzer–solute combination. However, Leypoldt et al. showed that increasing dialysate flow rate from 500 to 800 mL/min in a variety of dialyzers increased urea KoA by 14% in vitro [2]. They suggested this increase could result from improved flow distribution through the dialysate compartment or decreased dialysate boundary layer resistance to mass transfer. Subsequently, we showed urea KoA also depends on dialysate flow rate during clinical dialysis [3], and Hauk et al. found a greater than predicted increase in urea Kt/V when dialysate flow rate was increased from 500 to 800 mL/min [4]. Newer dialyzers incorporate features, such as hollow fiber undulations and spacer yarns, that improve flow distribution through the dialysate compartment [5]. However, even in these dialyzers, urea KoA has been reported to increase with increasing dialysate flow rate in vitro [6]. These observations suggest that the boundary layer and maldistribution of the dialysate flow remain important factors limiting the efficiency of urea removal by dialysis.
In addition to dialysate flow rate, dialysate distribution and boundary layer thickness might also be affected by the packing density of hollow fibers in the dialyzer. A low packing density might allow channeling of dialysate through the fiber bundle, leading to areas of semi-stagnant flow, while a high packing density might impede free flow of dialysate through the fiber bundle. To determine the impact of these factors on dialyzer performance during clinical dialysis, we examined the dependence of KoA on dialysate flow rate for two different membrane packing densities using three different test solutes, urea, phosphate and β2-microglobulin.
Materials and methods
Study design
Solute transport was evaluated in a cross-over study with four different dialyzers containing undulating fibers, Revaclear and Revaclear MAX (Gambro Renal Products, Lakewood, CO) and Optiflux F160NR and Optiflux F200NR (Fresenius Medical Care, Lexington, MA) (Table 1). The dialyzers were used in random order, each for three consecutive treatments, and were not reused. Treatments were performed using Phoenix dialysis machines (Gambro).
Table 1.
Dialyzers
| Dialyzer | Membranea | Shell diameter (mm) | Number of fibers | Fiber length (mm) | Fiber inner diameter (μm)b | Membrane area (m2)c | Membrane wall thickness (μm)b | Fiber packing density (%)d | Sterilization |
|---|---|---|---|---|---|---|---|---|---|
| Optiflux F160NR | PS/PVP | 40.4 | 10 700 | 232.5 | 200 | 1.56 | 40 | 51 | Electron beam |
| Optiflux F200NR | PS/PVP | 48 | 13 400 | 232.5 | 200 | 1.96 | 40 | 46 | Electron beam |
| Revaclear | PAES/PVP | 34 | 9600 | 236.3 | 190 | 1.36 | 35 | 56 | Steam |
| Revaclear MAX | PAES/PVP | 38 | 12 000 | 236.3 | 190 | 1.70 | 35 | 56 | Steam |
PS = polysulfone; PVP = polyvinylpyrrolidone; PAES = polyarylethersulfone.
Manufacturer’s data.
Calculated from the number of fibers and fiber dimensions.
Calculated as the ratio of membrane cross-sectional area to the internal cross-sectional area of the dialyzer housing expressed as a percentage. The cross-sectional area of membrane was calculated from the number of fibers and the manufacturer’s data for fiber diameter and wall thickness.
Dialyzer performance was determined during the third treatment with each dialyzer. Clearances of urea, phosphate and β2-microglobulin were determined during the first 45 min of dialysis at the minimum ultrafiltration rate allowed by the dialysis machine (100 mL/h), a blood flow rate of 400 mL/min and dialysate flow rates of 350, 500 and 800 mL/min, used in random order.
The study was registered with ClinicalTrials.gov (identifier NCT00636077).
Subjects
Subjects were enrolled from patients receiving dialysis through the University of Louisville Kidney Disease Program. For inclusion in the study, subjects were required to be older than 18 years, have a stable haemodialysis prescription, a native fistula or Gore-Tex graft capable of providing a blood flow of 400 mL/min and an expected fluid removal of no more than 3 L per treatment. Exclusion criteria included non-compliance with the dialysis prescription, a haematocrit <28% or an active infection. The Human Studies Committee of the University of Louisville reviewed the protocol and all subjects provided informed consent before participating in the study.
Measurement of clearance
Clearances of urea, phosphate and β2-microglobulin were determined using standard methods. Briefly, blood and dialysate flow rates were set at the desired values, and the net ultrafiltration rate was set to minimum, using the dialysis machine displays. After 15 min, blood samples were drawn from the inlet and outlet blood lines of the dialyzer, and a dialysate sample was obtained from the outlet dialysate line. Plasma was obtained by centrifugation. Before plasma separation, a well-mixed aliquot was taken from the inlet blood sample to determine haematocrit.
Analytical methods
Urea concentrations were determined by routine clinical laboratory methods. Phosphate concentrations were measured by the method of Chen et al. [7] adapted for a microplate spectrophotometer (SPECTRAmax Plus, Molecular Devices, Sunnyvale, CA). β2-Microglobulin concentrations were measured by an immunometric assay (Immulite, Diagnostic Products Corporation, Los Angeles, CA).
Data analysis
Instantaneous solute clearances (K) were calculated from arterial (CPi) and venous (CPo) plasma concentrations and the outlet dialysate concentration (CDo) [8]:
Where QBeffi is the effective blood-side flow rate for a given solute at the inlet to the dialyzer and QUF is the instantaneous ultrafiltration rate. For urea, QBeffi was assumed to be the blood water flow rate at the inlet to the dialyzer (QBWi), whereas for phosphate and β2-microglobulin QBeffi was assumed to be the plasma water flow rate at the inlet to the dialyzer (QPWi) [9]. These flow rates were calculated from:
Where QBi is the blood flow rate at the inlet to the dialyzer, Hct is the fractional haematocrit and the constants 0.93 and 0.72 correct for the protein content of plasma and the non-water fraction of red blood cells, respectively [9].
The product of overall mass transfer coefficient and membrane area (KoA) was calculated according to Michaels [8]:
The overall mass transfer coefficient (Ko) was calculated by dividing KoA by the membrane surface area of the dialyzer determined from the fiber numbers and dimensions listed in Table 1.
The instantaneous rate of trapping β2-microglobulin at the dialyzer membrane was calculated as the difference between the amount of solute lost from the plasma water and the amount recovered in the effluent dialysate:
Data are presented as mean ± SEM. The statistical significance of differences among dialyzers and among dialysate flow rates was examined by analysis of variance. Where a significant difference was found by analysis of variance, the significance of differences between individual dialyzers or dialysate flow rates was assessed using a Bonferroni post hoc test. P-values <0.05 were considered significant.
Results
Patients
Nine women and three men with an average age of 60 ± 4 years were enrolled in the study. They had been receiving haemodialysis for 81 ± 19 months. Their renal failure was caused by hypertension (five patients), glomerulonephritis (three patients), diabetes (one patient), systemic lupus erythematosus (one patient), polycystic kidney disease (one patient) and renal carcinoma (one patient). Blood access was via a fistula in three patients and a Gore-Tex graft in nine patients.
Dialysis treatments
Anticoagulation, obtained using a loading dose (mean 1825 IU, range 1000–2500 IU) and constant infusion (mean 1192 IU/h, range 500–2000 IU/h) of unfractionated heparin, did not differ among dialyzers. Actual blood flow rates averaged 400 ± 2 mL/min and did not differ among dialyzers or dialysate flow rates.
Urea
Because urea moves rapidly across red cell membranes, urea clearances (Table 2) were based on blood water flow rates. The quality of the clearance data was assessed by calculating the mass balance error for each determination of urea clearance. Overall, the mean absolute mass balance error was 5.2 ± 0.4%. Urea clearances for the Revaclear MAX were significantly higher than those for the Revaclear and the Optiflux F160NR (P < 0.001), while clearances for the Optiflux F200NR were intermediate and not different from those of the other three dialyzers. Urea clearances increased significantly with increasing dialysate flow rate (P < 0.001) and this increase was independent of the dialyzer.
Table 2.
Urea
| Dialyzer | Dialysate flow rate (mL/min) | Clearance (mL/min)a | KoA (mL/min)b | Ko (cm/min)b |
|---|---|---|---|---|
| Optiflux F160NR | 350 | 232 ± 3 | 720 ± 32 | 0.046 ± 0.002 |
| 500 | 252 ± 7 | 703 ± 38 | 0.045 ± 0.002 | |
| 800 | 281 ± 3 | 776 ± 23 | 0.050 ± 0.001 | |
| Optiflux F200NR | 350 | 237 ± 2 | 749 ± 20 | 0.038 ± 0.001 |
| 500 | 270 ± 4 | 847 ± 33 | 0.043 ± 0.002 | |
| 800 | 282 ± 5 | 785 ± 35 | 0.040 ± 0.002 | |
| Revaclear | 350 | 233 ± 4 | 718 ± 31 | 0.053 ± 0.002 |
| 500 | 255 ± 5 | 713 ± 33 | 0.052 ± 0.002 | |
| 800 | 276 ± 3 | 732 ± 24 | 0.054 ± 0.002 | |
| Revaclear MAX | 350 | 240 ± 3 | 789 ± 29 | 0.046 ± 0.002 |
| 500 | 270 ± 3 | 852 ± 30 | 0.050 ± 0.002 | |
| 800 | 293 ± 3 | 884 ± 30 | 0.052 ± 0.002 |
Clearances based on rate of disappearance from blood.
Calculated using the blood water flow rate (QBWi).
Data are presented as mean ± SEM for n = 12 observations. Statistical differences are described in the text.
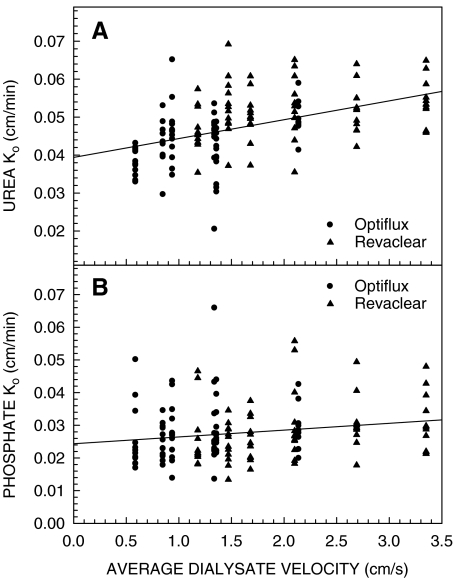
Because clearance is a function of blood and dialysate flow rates, the ability of a dialyzer to remove urea is often expressed in terms of KoA. Urea KoA and Ko values are presented in Table 2. Differences in urea KoA among dialyzers were similar to those found for urea clearances, with the Revaclear MAX having a significantly higher KoA than the Revaclear and the Optiflux F160NR (P < 0.001), and the Optiflux F200NR having a significantly higher KoA than the Revaclear (P = 0.025), but not the Optiflux F160NR. Urea KoA did not differ significantly among the three dialysate flow rates. After correcting for membrane surface area, the smaller surface area dialyzers tended to have higher urea Ko values than the larger surface area dialyzers. Urea Ko values for the Revaclear and the Optiflux F160NR were significantly higher than those for the Optiflux F200NR (P < 0.001), but not the Revaclear MAX. Urea Ko did not differ significantly among the three dialysate flow rates. The boundary layer on the dialysate side of the membrane is one component of the resistance to mass transfer incorporated in Ko [1]. The thickness of this boundary layer might be determined, in part, by the flow velocity in the dialysate compartment, which in turn is a function of the dialysate flow rate and the available cross-sectional area for flow. A higher membrane packing density results in a lower cross-sectional area for flow and a higher flow velocity. Therefore, we examined the relationship between urea Ko and the average dialysate velocity. The data in Figure 1A show that urea Ko increased significantly with increasing dialysate velocity (r2 = 0.228, P < 0.001).
Fig. 1.
Urea (panel A) and phosphate (panel B) Ko as a function of average dialysate velocity. Average dialysate velocity was calculated by dividing the dialysate flow rate by the free cross-sectional area of the dialysate compartment (cross-sectional area of the dialyzer housing − cross-sectional area of fibers).
Phosphate
Unlike urea, transfer of phosphate out of red blood cells is sufficiently slow that plasma water and red blood cell water are not in equilibrium leaving the dialyzer. For that reason, phosphate clearances calculated using blood-side flow rates do not accurately reflect dialyzer performance. To avoid this problem, phosphate clearances (Table 3) were calculated from the dialysate flow rate and outlet dialysate phosphate concentration. In general, phosphate clearances did not differ significantly among dialyzers, the only exception being a greater clearance with Optiflux F200NR than with the Revaclear (P = 0.002). Phosphate clearances increased significantly with increasing dialysate flow rate (P < 0.001), with clearances at each dialysate flow rate being significantly different from those at the other flow rates. The increase in phosphate clearance with increasing dialysate flow rate was independent of the dialyzer.
Table 3.
Phosphate
| Dialyzer | Dialysate flow rate (mL/min) | Clearance (mL/min)a | KoA (mL/min)b | Ko (cm/min)b |
|---|---|---|---|---|
| Optiflux F160NR | 350 | 168 ± 6 | 434 ± 34 | 0.028 ± 0.002 |
| 500 | 178 ± 9 | 450 ± 62 | 0.029 ± 0.004 | |
| 800 | 194 ± 8 | 458 ± 33 | 0.029 ± 0.002 | |
| Optiflux F200NR | 350 | 178 ± 8 | 504 ± 58 | 0.026 ± 0.003 |
| 500 | 190 ± 6 | 491 ± 29 | 0.025 ± 0.002 | |
| 800 | 208 ± 6 | 560 ± 40 | 0.029 ± 0.002 | |
| Revaclear | 350 | 153 ± 6 | 334 ± 24 | 0.025 ± 0.002 |
| 500 | 176 ± 8 | 422 ± 48 | 0.031 ± 0.004 | |
| 800 | 186 ± 8 | 415 ± 35 | 0.030 ± 0.003 | |
| Revaclear MAX | 350 | 167 ± 7 | 434 ± 48 | 0.026 ± 0.003 |
| 500 | 179 ± 6 | 435 ± 31 | 0.026 ± 0.002 | |
| 800 | 200 ± 6 | 520 ± 39 | 0.030 ± 0.002 |
Clearances based on rate of appearance in dialysate.
Calculated using the plasma water flow rate (QPWi).
Data are presented as mean ± SEM for n = 12 observations. Statistical differences are described in the text.
Calculation of KoA and Ko requires the blood and dialysate flow rates. Choosing the appropriate blood flow rate for phosphate is difficult because there may be some phosphate transfer between cells and plasma water as blood transits the dialyzer. Following the recommendation of Gotch and colleagues [9], we used plasma water flow rate to calculate phosphate KoA and Ko values (Table 3) in spite of recovering 11 ± 1% more phosphate in the dialysate than was lost from plasma water. The logistics of collecting samples over 45 min prevented separation of plasma and red blood cells immediately following sample collection, and as a result there was likely to have been some re-equilibration of phosphate between red blood cells and plasma water that accounted for the 11% difference. Differences in phosphate KoA among dialyzers were similar to those found for clearances, with the Optiflux F200NR having a significantly higher KoA than the Revaclear (P = 0.002). After correcting for membrane surface area, there were no differences in phosphate Ko among the four dialyzers. Neither phosphate KoA nor Ko differed among the three dialysate flow rates, although phosphate Ko did increase significantly with increasing dialysate velocity (r2 = 0.033, P = 0.029) (Figure 1B).
β2-Microglobulin
Clearances of β2-microglobulin based on plasma water flow rate are presented in Table 4. Clearances of β2-microglobulin were significantly greater for the Revaclear and Revaclear MAX than for the Optiflux F160NR and F200NR dialyzers (P < 0.001); however, there was no difference between the Revaclear and the Revaclear MAX or between the Optiflux F160NR and F200NR. In no case did the β2-microglobulin clearance depend on dialysate flow rate. For all dialyzers, clearances based on plasma water were significantly greater than clearances based on dialysate. This difference, which was independent of dialysate flow rate, reflects trapping of β2-microglobulin by the membrane. Overall, 46 ± 3% of β2-microglobulin removal by the Optiflux dialyzers was attributable to trapping at the membrane, whereas 28 ± 2% of β2-microglobulin removal was attributable to trapping at the membrane for the Revaclear dialyzers (P < 0.001).
Table 4.
β2-Microglobulin
| Dialyzer | Dialysate flow rate (mL/min) | Clearance (mL/min)a | KoA (mL/min)b | Ko (cm/min)b |
|---|---|---|---|---|
| Optiflux F160NR | 350 | 54 ± 4 | 27 ± 2 | 0.0017 ± 0.0001 |
| 500 | 53 ± 5 | 28 ± 2 | 0.0018 ± 0.0001 | |
| 800 | 50 ± 6 | 31 ± 5 | 0.0020 ± 0.0003 | |
| Optiflux F200NR | 350 | 46 ± 5 | 27 ± 2 | 0.0014 ± 0.0001 |
| 500 | 42 ± 3 | 25 ± 2 | 0.0013 ± 0.0001 | |
| 800 | 50 ± 5 | 21 ± 1 | 0.0011 ± 0.0001 | |
| Revaclear | 350 | 75 ± 4 | 61 ± 2 | 0.0045 ± 0.0002 |
| 500 | 74 ± 5 | 57 ± 2 | 0.0042 ± 0.0002 | |
| 800 | 76 ± 5 | 56 ± 3 | 0.0041 ± 0.0002 | |
| Revaclear MAX | 350 | 65 ± 5 | 65 ± 4 | 0.0038 ± 0.0002 |
| 500 | 80 ± 4 | 62 ± 3 | 0.0037 ± 0.0002 | |
| 800 | 73 ± 4 | 64 ± 3 | 0.0038 ± 0.0002 |
Clearances based on rate of disappearance from blood.
Calculated using the plasma water flow rate (QPWi).
Data are presented as mean ± SEM for n = 12 observations. Statistical differences are described in the text.
KoA and Ko values for β2-microglobulin are presented in Table 4. These values are based on dialysate-side clearances since they are not confounded by solute removal due to trapping at the membrane. β2-Microglobulin KoA and Ko were significantly greater for the two Revaclear dialyzers than for the two Optiflux dialyzers (P < 0.001). There was no difference in KoA between the Revaclear and the Revaclear MAX or between the Optiflux F160NR and the Optiflux 200NR. However, after allowing for the differences in the membrane surface area, β2-microglobulin Ko was significantly greater for the Revaclear than for the Revaclear MAX (P = 0.001) and Ko for the Optiflux F160NR was significantly greater than for the Optiflux F200NR (P < 0.001). Neither KoA nor Ko depended significantly on dialysate flow rate.
Discussion
In their original report, Leypoldt and colleagues suggested that the increase in KoA associated with increasing dialysate flow rate could result from a reduced dialysate boundary layer thickness or improved flow distribution in the dialysate compartment [2]. Soon thereafter, dialyzers became available with spacer yarns in the fiber bundle or fibers with undulations to improve dialysate flow distribution. Imaging studies showed that both strategies improved flow distribution and were associated with improved small molecule clearances [5]. Leypoldt and colleagues confirmed that the introduction of fiber undulations in Optiflux dialyzers improved small molecule clearance in vitro, but found that it did not abolish the dependence of KoA on dialysate flow rate for urea and creatinine [6]. From these observations, they concluded that the increase in KoA at higher dialysate flow rates results from a decrease in dialysate boundary layer thickness rather than better flow distribution in the dialysate compartment.
Our results, showing that KoA for urea and phosphate was statistically independent of dialysate flow rate during clinical use of dialyzers containing fibers with undulations, contrast with those of Leypoldt and colleagues [6]. One possible explanation for our failure to reproduce the in vitro findings for dialyzers with undulating fibers in the clinical setting might be that blood-side resistance plays a greater role in determining KoA when fibers are perfused with blood, which is more viscous and complex than the aqueous solution used by Leypoldt et al. in their in vitro studies. This possibility is supported by observations made with the Baxter CA170 dialyzer. When the dialysate flow rate was increased from 500 to 800 mL/min, in vitro, Leypoldt and colleagues reported a 15% increase in urea KoA [2], whereas we found only a 7% increase when the same flow rates were used during clinical dialysis [3]. Moreover, when other dialyzers studied by Leypoldt et al. were used for clinical dialysis, Depner et al. found only a 5.5 ± 1.5% increase in urea KoA when the dialysate flow rate was increased from 500 to 800 mL/min [10]. A second possible explanation relates to the use of different dialysis machines in the two studies. The Phoenix machine used in our study delivers a steady flow of dialysate to the dialyzer, while the dialysate flow in the Fresenius 2008E machine used by Leypoldt et al. is pulsatile because of the machine’s balancing chamber technology. Pulsatile flow has been associated with enhanced clearances [11], presumably by disrupting boundary layers or enhancing convective mass transfer through the creation of a push–pull phenomenon.
Although KoA for urea and phosphate was independent of dialysate flow rate, we did observe a significant increase in urea Ko and, to a lesser extent, phosphate Ko with increasing dialysate velocity (Figure 1). This observation is consistent with a reduction in the thickness of the dialysate-side boundary layer as the dialysate flow rate and, hence, the flow velocity in the dialysate compartment increases. We could only estimate the average flow velocity in the dialysate compartment based on the dialysate flow rate and the free cross-sectional area of the dialysate compartment. In practice, the dialysate-side boundary layer is a function of the local flow velocity, which is also affected by the flow distribution in the dialysate compartment. Under-perfusion of some regions of the dialysate compartment can lead to a loss of effective membrane area [12]. The use of fibers with undulations has been shown to improve dialysate flow distribution compared to straight fibers [5]. Moreover, Yamamoto and colleagues have shown that the design of the baffles at the inlet to the dialysate compartment also influences both dialysate flow distribution and dialyzer performance [13,14]. Our inability to reproduce our previous finding of a statistically significant increase in urea KoA with increasing dialysate flow rate [3] is consistent with better dialysate flow distribution through the use of undulating fibers in the current study compared to straight fibers in our previous study, together with possible improvements in baffle design.
Taken together, our data suggest that the blood-side resistance to mass transfer is limiting for small solutes in dialyzers incorporating features to promote good flow distribution in the dialysate compartment, such as undulating fibers. Although strategies designed to increase dialysate velocity, such as increasing the fiber packing density for a given membrane surface area and fiber length, appear to have some effect on dialysate-side mass transfer resistance for small solutes, the resulting reduction in overall mass transfer resistance appears to be small in the setting of clinical dialysis where blood-side resistance is greatest.
Revaclear dialyzers provided more β2-microglobulin removal than Optiflux dialyzers in spite of a reduced membrane surface area. Also, trapping at the membrane contributed significantly more to removal of β2-microglobulin by the Optiflux membrane than by the Revaclear membrane in agreement with our previous findings [15]. For all dialyzers, β2-microglobulin clearance was independent of dialysate flow rate, which is expected since large molecule clearances depend more on membrane surface area and permeability than on the blood and dialysate flow rates. To compare β2-microglobulin permeability of the membranes, KoA values were calculated based on the dialysate-side clearance. While β2-microglobulin KoA for the Revaclear dialyzers was significantly greater than for the Optiflux dialyzers, KoA did not increase with increasing membrane surface area within each dialyzer type. After correcting for membrane surface area, Ko for the dialyzer with the smaller membrane surface area was significantly greater than that for the dialyzer with the larger membrane surface area within each dialyzer type. One possible explanation for this difference is that the smaller number of fibers in the dialyzers with the smaller membrane surface area results in a higher blood compartment pressure drop at a given blood flow rate and, therefore, a greater degree of internal filtration and back-filtration that enhances large molecule removal, similar to what has been reported for decreasing fiber diameter [16]. This reasoning suggests that increasing membrane surface area by increasing the length of the fibers, rather than by increasing their number, might result in a higher KoA for large solutes.
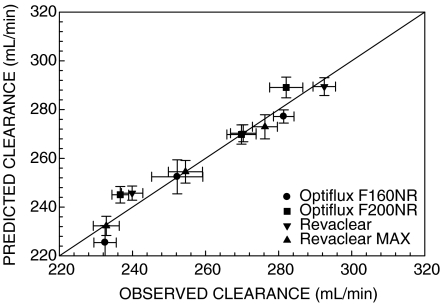
Overall, our data suggest that increasing dialysate flow rates in dialyzers with fiber undulations to enhance dialysate flow distribution is likely to be of limited benefit. The Michaels equation was used to predict blood water urea clearances at dialysate flow rates of 350, 500, and 800 mL/min using the urea KoA determined from the experimental data obtained at a dialysate flow rate of 500 mL/min. Figure 2 compares this predicted clearance to the measured clearance. The data cluster around the identity line, illustrating that increasing or decreasing dialysate flow rate has essentially no effect on clearance other than the change predicted assuming a constant KoA. Based on a constant KoA, a blood flow rate of 400 mL/min and a haematocrit of 35%, the Michaels equation predicts that reducing the dialysate flow rate from 800 mL/min to 700, 600 or 500 mL/min would reduce the urea clearance by about 2, 4 and 7%, respectively. The magnitude of these changes suggests that the impact on delivered Kt/Vurea of reducing the dialysate flow rate from 800 to 600 mL/min, which would reduce concentrate consumption by 25%, is likely to be small. The comparable reductions in phosphorus clearance would be about 1, 2 and 4%, assuming clearance from plasma water, while there would be no reduction in β2-microglobulin clearance.
Fig. 2.
Predicted urea clearance versus measured urea clearance. Urea clearance predicted using the Michaels equation and a constant KoA (derived from experimental data for QD = 500 mL/min) did not differ from the measured clearances at dialysate flow rates of 350 and 800 mL/min. Data are presented as mean ± SEM for n = 12. The solid line is the line of identity.
In summary, increasing the dialysate flow rate from 500 to 800 mL/min had only a modest impact on dialyzer performance, limited to the theoretical increase predicted by the Michaels equation for a constant KoA. This finding suggests that increasing the dialysate flow rate beyond 500–600 mL/min may provide only a marginal benefit in terms of delivered Kt/V, although this possibility needs to be confirmed in a randomized clinical trial. The data also suggest that increasing membrane surface area may not necessarily increase the removal of large solutes if the increase in surface area is achieved by increasing the number of fibers.
Acknowledgments
This study was supported by a grant from Gambro Renal Products.
Conflict of interest statement. None declared.
References
- 1.Colton CK, Lowrie EG. Hemodialysis: physical principles and technical considerations. In: Brenner BM, Rector FC Jr, editors. The Kidney. Philadelphia: WB Saunders; 1981. [2nd edn]. pp. 2425–2489. [Google Scholar]
- 2.Leypoldt JK, Cheung AK, Agodoa LY, et al. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. Kidney Int. 1997;51:2013–2017. doi: 10.1038/ki.1997.274. [DOI] [PubMed] [Google Scholar]
- 3.Ouseph R, Ward RA. Increasing dialysate flow rate increases dialyzer urea mass transfer - area coefficients during clinical use. Am J Kidney Dis. 2001;37:316–320. doi: 10.1053/ajkd.2001.21296. [DOI] [PubMed] [Google Scholar]
- 4.Hauk M, Kuhlmann MK, Riegel W, et al. In vivo effects of dialysate flow rate on Kt/V in maintenance hemodialysis patients. Am J Kidney Dis. 2000;35:105–111. doi: 10.1016/S0272-6386(00)70308-8. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Brendolan A, Crepaldi C, et al. Blood and dialysate flow distributions in hollow-fiber hemodialyzers analyzed by computerized helical scanning technique. J Am Soc Nephrol. 2002;13:S53–S61. [PubMed] [Google Scholar]
- 6.Leypoldt JK, Cheung AK, Chirananthavat T, et al. Hollow fiber shape alters solute clearances in high flux hemodialyzers. ASAIO J. 2003;49:81–87. doi: 10.1097/00002480-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 8.Michaels AS. Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans Am Soc Artif Intern Organs. 1966;12:387–392. [PubMed] [Google Scholar]
- 9.Gotch FA, Panlilio F, Sergeyeva O, et al. Effective diffusion volume flow rates (Qe) for urea, creatinine, and inorganic phosphorus (Qeu, Qecr, QeiP) during hemodialysis. Semin Dial. 2003;16:474–476. doi: 10.1046/j.1525-139x.2003.16102.x. [DOI] [PubMed] [Google Scholar]
- 10.Depner TA, Greene T, Daugirdas JT, et al. Dialyzer performance in the HEMO study: in vivo KoA and true blood flow determined from a model of cross-dialyzer urea extraction. ASAIO J. 2004;50:85–93. doi: 10.1097/01.mat.0000104824.55517.6c. [DOI] [PubMed] [Google Scholar]
- 11.Runge TM, Briceño JC, Sheller ME, et al. Hemodialysis: evidence of enhanced molecular clearance and ultrafiltration volume by using pulsatile flow. Int J Artif Organs. 1993;16:645–652. [PubMed] [Google Scholar]
- 12.Noda I, Brown-West DG, Gryte CC. Effect of flow maldistribution on hollow fiber dialysis - experimental studies. J Membr Sci. 1979;5:209–225. [Google Scholar]
- 13.Yamamoto K, Matsukawa H, Yakushiji T, et al. Technical evaluation of dialysate flow in a newly designed dialyzer. ASAIO J. 2007;53:36–40. doi: 10.1097/01.mat.0000245525.83936.79. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Matsuda M, Hirano A, et al. Computational evaluation of dialysis fluid flow in dialyzers with variously designed jackets. Artif Organs. 2009;33:481–486. doi: 10.1111/j.1525-1594.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- 15.Ouseph R, Hutchison CA, Ward RA. Differences in solute removal by two high-flux membranes of nominally similar synthetic polymers. Nephrol Dial Transplant. 2008;23:1704–1712. doi: 10.1093/ndt/gfm916. [DOI] [PubMed] [Google Scholar]
- 16.Ronco C, Brendolan A, Lupi A, et al. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int. 2000;58:809–817. doi: 10.1046/j.1523-1755.2000.00230.x. [DOI] [PubMed] [Google Scholar]