Abstract
We examined antiretroviral therapy (ART) use among 501 previously diagnosed HIV-infected men who have sex with men who sought care at an STD Clinic in King County, WA during 2004–2008. Overall, 42% of men were not taking ART, 71% of whom had CD4 counts >350 cells/µL. Of those who reported unprotected anal intercourse with a partner of nonconcordant HIV status in the prior year, 48% were not taking ART (78% with CD4 counts >350 cells/µL). STD Clinics may be an important venue in which to identify persons who are not taking ART. Treating these persons could help diminish HIV transmission.
KEY TERMS: HIV Infections/drug therapy/prevention & control/transmission; Antiretroviral Therapy, Highly Active; Homosexuality, Male/statistics & numerical data; Sexual Behavior; Delivery of Health Care
INTRODUCTION
Suppression of the plasma HIV RNA level with antiretroviral therapy (ART) likely reduces the risk of HIV transmission,1, 2 and mathematical models and ecological studies suggest that expanding ART use could substantially reduce HIV incidence.3–6 Men who have sex with men (MSM) remain the group most profoundly affected by HIV in the US. At present, there are no data on the degree to which ART might decrease the risk of HIV transmission among MSM, and we are not aware of ongoing studies designed to address this issue. However, given the magnitude of the problem in MSM,7 the lack of other prevention interventions proven to affect HIV rates in that population, existing evidence on the protective effect of ART in heterosexuals, and increasing evidence that early initiation of ART is associated with clinical benefits,8–10 the impetus is strong for considering expanded ART use as an HIV prevention strategy in MSM. The potential prevention impact and cost of such a strategy will depend, in part, on the size of the HIV-infected MSM population not currently taking ART.
We examined rates of ART use in MSM with known HIV infection attending a sexually transmitted disease (STD) clinic, a population at high risk for transmitting HIV. We conducted this study in order to determine the proportion of MSM in this population who were not taking ART, stratified by CD4 count, and to assess factors associated with not taking ART. Since our analysis relied on self-reported data for ART status, CD4 count, and HIV RNA, we also aimed to evaluate the accuracy of self-report for these measures.
METHODS
We analyzed electronic clinic records from all MSM with previously diagnosed HIV infection who attended the Public Health – Seattle & King County (PHSKC) STD Clinic between January 1, 2004 and October 31, 2008. We excluded visits within one year after HIV diagnosis to avoid including behavioral data from the time preceding HIV diagnosis and to allow patients time following diagnosis to initiate ART. We retained persons missing the HIV diagnosis date in the analysis. STD Clinic staff use a standard form to collect patient-reported information in the context of clinical care, as described elsewhere.11
For each subject, we selected the most recent visit with complete self-reported information on ART status and/or CD4 count. We then selected the STD Clinic records that were missing ART status for review of the University of Washington (UW) electronic medical record system (EMR), which includes data from the Madison Clinic at Harborview Medical Center (the largest provider of HIV care in King County). We excluded persons for whom we could not find ART status in either source. We also reviewed the EMR to complete missing CD4 count and HIV RNA data for subjects off ART. To assess the accuracy of self-reported ART status, CD4 count, and HIV RNA, we randomly sampled 33% of records with complete ART status from the STD Clinic visit to compare to the UW EMR. To ascertain ART status using the EMR, we referred to HIV clinic notes most proximate to, but no more than one year from, the date of the STD Clinic visit. For CD4 count and HIV RNA, we referred to laboratory data most proximate to, but no more than one year prior to the STD Clinic visit.
We categorized patients as taking ART or not taking ART (hereafter referred to as “on ART” and “off ART,” respectively) at the time of the study visit. Patients who were off ART included those who were ART-naïve or had discontinued ART. We categorized persons off ART into CD4 count categories of ≤350, 351–500, and >500 cells/µL because these groups are often considered when deciding when to start ART. We stratified HIV RNA levels into categories of <1,500, 1,501 – 9,999, 10,000 – 99,999, and ≥100,000 copies/mL based on a study of the relationship between HIV RNA and transmission that included data from a cohort of MSM,12 with an additional stratum representing the level below which HIV transmission is unlikely in heterosexuals (<1500 copies/mL).1 We categorized age, time since HIV diagnosis, and number of anal sex partners in the past year as we have done in previous analyses.13
We used kappa statistics to compare self-reported ART status, CD4 count, and HIV RNA with that found in the EMR, categorizing those variables as we did in the analysis. We conducted descriptive analyses of the demographic, clinical, and behavioral characteristics of subjects stratified by ART status. We used Poisson regression to estimate relative risks (RR) between subject characteristics and ART use.14 For multivariate analyses, we included in the initial models all variables found to be significantly associated with the outcome measures (p<0.05) in bivariate analyses. We retained only those variables for which the p-value of the beta coefficient was <0.05 and performed a Wald test. We used Stata version 10.1 for all analyses.
RESULTS
A total of 768 HIV-infected MSM were seen for 1,191 visits to the STD Clinic during the study period. We excluded 145 (19%) men who were diagnosed with HIV within one year prior to their selected visit. Of the remaining 623 persons, 154 (25%) were missing ART status. After review of the UW EMR, 122 (20%) were still missing ART status and were excluded. Compared to men in the final study population, those excluded for missing ART status were more likely to report being out of HIV care (17% vs. 7%; p<0.001), but did not otherwise differ. For the 32 men missing self-reported ART status who had records in the EMR, the HIV clinic visits were a median of 25 days apart from the STD Clinic visits (range −321 to +118 days). The final study population comprised 501 (80%) of the 623 potentially eligible subjects.
We selected records from 155 (33%) of 469 persons who self-reported ART status for review of the UW EMR and found records for 50 (32%). The dates of clinic notes used to ascertain ART status (n=50) were a median of 54 days apart from the STD Clinic visits (range −270 to +104 days), CD4 counts (n=36) were measured a median of 83 days (range 2–323 days) prior, and HIV RNA levels (n=30) a median of 87 days (range 2–323 days) prior. Self-reported ART status matched that in the HIV clinic notes for 49 (98%) of 50 persons [kappa 0.96 (95% CI: 0.88 – 1.00)]. Self-reported CD4 count category matched laboratory data for 27 (75%) of 36 persons overall [weighted kappa = 0.77 (95% CI: 0.71 – 0.89)], and 12 (80%) of 15 persons off ART [weighted kappa = 0.87 (95% CI: 0.67 – 0.97)]. Self-reported HIV RNA category matched laboratory data for 20 (67%) of 30 persons, 15 of whom had undetectable HIV RNA [weighted kappa = 0.50 (0.16 – 0.74)]. Six of the seven (86%) persons with discordant records reported a lower HIV RNA level than that in the EMR.
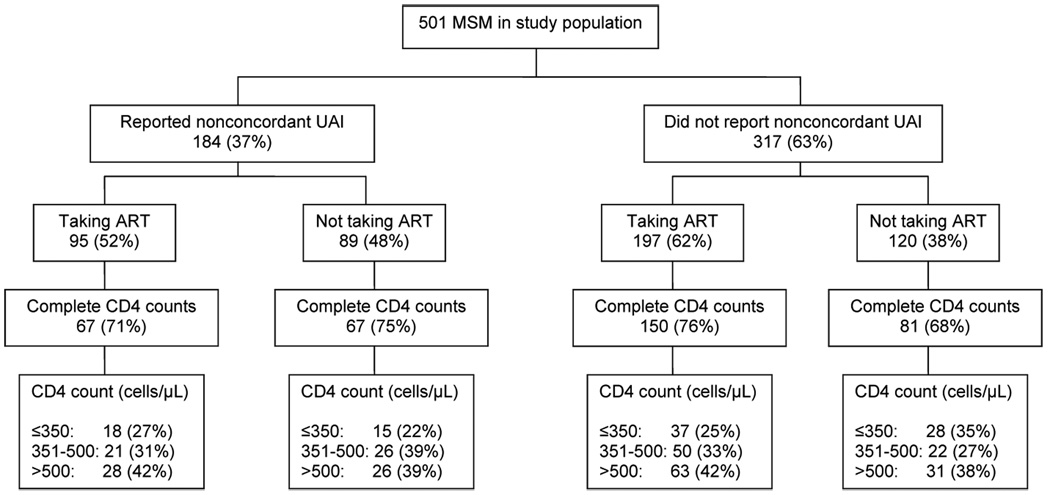
As shown in the figure, 209 (42%) subjects were off ART, including 89 (48%) of 184 men who reported unprotected anal intercourse (UAI) with a partner of negative or unknown HIV status (nonconcordant UAI) in the preceding year. The Table summarizes the characteristics of the study population, stratified by ART use. The HIV diagnosis date was more likely to be missing in persons off ART [RR 1.80 (95% CI: 1.36 – 2.37)], but did not vary by other characteristics (data not shown). Of the 148 persons off ART with complete CD4 counts, 43 (29%) had CD4 counts ≤350, 48 (32%) had 351–500, and 57 (39%) had >500 cells/µL. In multivariate analysis, only shorter time since HIV diagnosis and being out of HIV medical care were significantly associated with being off ART. Characteristics of persons off ART in the three CD4 count strata were similar (data not shown).
FIGURE. Antiretroviral Therapy (ART) Status and CD4 Count Distribution Among 501 HIV-infected Men who have Sex with Men (MSM) Attending the Public Health – Seattle & King County STD Clinic.
Nonconcordant UAI = Unprotected anal intercourse with a partner of negative or unknown HIV status in the prior year
TABLE.
Demographic, Clinical, and Behavioral Characteristics of 501 HIV-infected Men who have Sex with Men Attending the Public Health – Seattle & King County STD Clinic, Stratified by Antiretroviral Therapy (ART) Use
| Total Population (N = 501) No. (% total) |
On ART (n = 292) No. (% each variable) |
Off ART (n = 209) No. (% each variable) |
Univariate RR1 Off ART versus On ART |
Multivariate RR1 Off ART versus On ART |
|
|---|---|---|---|---|---|
| Age (years) | |||||
| 20–29 | 59 (12) | 22 (37) | 37 (63) | REF | REF |
| 30–39 | 164 (33) | 87 (53) | 77 (47) | 0.75 (0.58 – 0.97) | 1.14 (0.83 – 1.58) |
| 40–49 | 210 (42) | 133 (63) | 77 (37) | 0.58 (0.45 – 0.76) | 1.10 (0.79 – 1.53) |
| ≥ 50 | 68 (14) | 50 (74) | 18 (26) | 0.42 (0.27 – 0.66) | 0.68 (0.36 – 1.30) |
| Race | |||||
| White | 377 (75) | 215 (57) | 162 (43) | REF | |
| African-American | 53 (11) | 29 (55) | 24 (45) | 1.05 (0.77 – 1.45) | |
| Native American | 6 (1) | 4 (67) | 2 (33) | 0.78 (0.25 – 2.42) | |
| Asian/Pacific Islander | 15 (3) | 8 (53) | 7 (47) | 1.09 (0.62 – 1.89) | |
| Multiple/Other | 9 (2) | 6 (67) | 3 (33) | 0.78 (0.31 – 1.97) | |
| Missing | 41 (8) | 30 (73) | 11 (27) | ||
| Hispanic ethnicity | 41 (8) | 30 (73) | 11 (27) | 0.61 (0.37 – 1.03) | |
| Years since HIV diagnosis | |||||
| 1 to <5 | 106 (21) | 41 (39) | 65 (61) | REF | REF |
| 5 to 10 | 83 (17) | 57 (69) | 26 (31) | 0.50 (0.36 – 0.69) | 0.50 (0.36 – 0.70) |
| >10 | 168 (34) | 131 (78) | 37 (22) | 0.40 (0.29 – 0.54) | 0.40 (0.29 – 0.55) |
| Missing | 144 (29) | 63 (44) | 81 (56) | ||
| In HIV Medical Care | |||||
| Yes | 429 (86) | 265 (62) | 164 (38) | REF | REF |
| No | 33 (7) | 5 (15) | 28 (85) | 2.22 (1.84 – 2.68) | 1.99 (1.57 – 2.52) |
| Missing | 39 (8) | 22 (56) | 17 (44) | ||
| STD diagnosis at clinic visit | |||||
| Gonorrhea | 63 (13) | 26 (41) | 37 (59) | 1.50 (1.18 – 1.90) | 1.11 (0.85 – 1.45) |
| Chlamydial infection | 52 (10) | 26 (50) | 26 (50) | 1.23 (0.91 – 1.65) | |
| Syphilis | 58 (12) | 35 (60) | 23 (40) | 0.94 (0.67 – 1.32) | |
| Nonconcordant UAI2 | 184 (37) | 95 (52) | 89 (48) | 1.28 (1.04 – 1.57) | 1.05 (0.84 – 1.32) |
| Number of male anal sex partners3 | |||||
| 0–1 | 45 (9) | 27 (60) | 18 (40) | REF | |
| 2–9 | 220 (44) | 134 (61) | 86 (39) | 0.98 (0.66 – 1.45) | |
| ≥ 10 | 185 (37) | 100 (54) | 85 (46) | 1.15 (0.78 – 1.70) | |
| Missing | 51 (10) | 31 (61) | 20 (39) | ||
| Methamphetamine use3 | 126 (25) | 58 (46) | 68 (54) | 1.44 (1.17 – 1.77) | 1.16 (0.89 – 1.52) |
| Injection drug use3 | 49 (10) | 19 (39) | 30 (61) | 1.55 (1.20 – 1.99) | 1.23 (0.88 – 1.73) |
Bold typeface signifies p≤0.05
Unprotected anal intercourse with a partner of negative or unknown HIV status in the preceding year
In the preceding year
More men off ART (43%) reported nonconcordant UAI in the preceding year than did men on ART [33%; RR 1.31 (95% CI: 1.04 – 1.64)]. Of 64 men off ART who reported nonconcordant UAI and had complete HIV RNA data, 13 (20%) had <1,500, 11 (17%) had 1,501 – 9,999, 30 (47%) had 10,000 – 99,999, and 10 (16%) had ≥100,000 copies/mL. In multivariate analysis, the 40 men who were off ART, reported nonconcordant UAI, and had HIV RNA >10,000 copies/mL were more likely than men in the remainder of the population to report methamphetamine use in the prior year (p=0.008) and less likely to have been diagnosed with HIV >10 years (p=0.006).
DISCUSSION
We found that 42% of previously diagnosed, HIV-infected MSM who attended our STD Clinic were not taking ART, and that almost half (48%) of previously diagnosed, HIV positive men who reported nonconcordant UAI were not taking ART. Most (71%) of the men who were off ART did not meet CD4 count criteria for initiating ART according to treatment guidelines active during the study period (≤350 cells/µL). However, 63% of those off ART with CD4 counts >350 cells/µL who reported nonconcordant UAI had HIV RNA levels >10,000 copies/mL and thus could likely transmit HIV. We found that many subjects understated their HIV RNA levels, suggesting that the proportion of the population off ART with HIV RNA >10,000 copies/mL is probably even larger. This group is at high risk for transmitting HIV due to a combination of behavioral risk, lack of viral suppression and, in some instances, concurrent STD. The importance of untreated MSM with previously diagnosed HIV in ongoing HIV transmission is uncertain, but this group may account for an important and growing proportion of new cases.15–17
In contrast to HIV treatment guidelines active during the study period, current guidelines recommend ART initiation for persons with CD4 counts ≤500 cells/µL, and half of the guideline panel members favored recommending initiation for those with CD4 counts >500 cells/µL.10 To the extent these guidelines are applied, most of the men off ART in our study would be eligible for receiving ART. Although not the primary consideration in the clinical context, increased ART use might benefit MSM on the population level by decreasing HIV transmission. The potential benefits of increased ART use must be considered against the potential risks of adverse drug effects, antiretroviral resistance, and behavioral disinhibition. Our finding that men off ART were more likely to report being out of HIV care, while not surprising, highlights the need to establish means of re-linking patients to HIV care when they seek care in venues like STD clinics. Since we excluded many men who reported being out of HIV care due to missing ART status, this study underestimates the proportion of men seen in our clinic who report being out of HIV care. STD Clinics could be an important venue through which to promote engagement with HIV care and consideration of ART use in MSM at risk for transmitting HIV. We are instituting an automated system, linked to our STD Clinic medical record that identifies HIV-infected patients without recent HIV care visits to ensure that clinicians refer them to outreach workers who can assist them in re-linking to HIV care.
This study has important limitations. We relied on patient-reported data. Although we found substantial agreement between self-report and HIV clinic records for CD4 count category and ART status, supporting the validity of our approach, we were only able to examine EMR data from a minority of persons. We found only moderate agreement for HIV RNA category. We attempted to exclude patients within one year after HIV diagnosis, but we were not able to determine the diagnosis date for about a quarter of the population. Temporary discontinuations of ART would overestimate the proportion of the population off ART, but this probably occurred infrequently. Finally, we used data from a single clinic, so our results may not be generalizable.
In summary, we found that a substantial proportion of HIV-infected MSM at risk for transmitting HIV are not taking ART. This group is likely important in HIV transmission. In combination with ongoing HIV prevention interventions, efforts to increase ART use and improve engagement in HIV care could potentially reduce HIV transmission in MSM, and STD Clinics could play an important role in such interventions.
ACKNOWLEDGEMENTS
The authors thank Katherine Thomas for statistical advice and Fred Koch for database management.
Financial support: Public Health – Seattle & King County, National Institutes of Health (T32 AI-07140 and K23 AI-65243).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 18th meeting of the International Society for STD Research/British Association for Sexual Health & HIV; London, UK; June 28 – July 1, 2009.
REFERENCES
- 1.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 2.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 5.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 6.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376 doi: 10.1016/S0140-6736(10)60936-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PS, Hamouda O, Delpech V, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol. 2009;19:423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed April 12, 2010];Department of Health and Human Services. 2009 December 1;:1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 11.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008;49:212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 12.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007 Oct 30;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden MR, Wood RW, Buskin SE, Fleming M, Harrington RD. Ongoing risk behavior among persons with HIV in medical care. AIDS Behav. 2007;11:726–735. doi: 10.1007/s10461-007-9244-5. [DOI] [PubMed] [Google Scholar]
- 14.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 15.Golden MR, Hogben M, Potterat JJ, Handsfield HH. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis. 2004;31:709–712. doi: 10.1097/01.olq.0000145847.65523.43. [DOI] [PubMed] [Google Scholar]
- 16.Cassels S, Menza TW, Goodreau SM, Golden MR. HIV serosorting as a harm reduction strategy: evidence from Seattle, Washington. AIDS. 2009;23:2497–2506. doi: 10.1097/QAD.0b013e328330ed8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden MR, Goodreau S, Hughes J, Dombrowski JC, Kent J, Stekler J. Estimation of the proportion of new HIV cases among men who have sex with men transmitted from persons in different stages of HIV infection, diagnosis, and treatment [Paper# 1001]; Presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco: 2010. [Google Scholar]