Abstract
In a recent publication, we have shown that delphinidin, an anthocyanidin induces apoptosis and cell cycle arrest in highly metastatic human prostate cancer (PCa) PC3 cells. Extending these studies, we provide additional evidence that delphinidin induces apoptosis and cell cycle arrest in androgen refractory human PCa 22Rν1 cells and that these effects are concomitant with inhibition of NFκB. We observed that delphinidin treatment to 22Rν1 cells resulted in a dose-dependent (i) G2/M phase cell cycle arrest, (ii) induction of apoptosis (iii) and inhibition of NFκB signaling. The induction of apoptosis by delphinidin was mediated via activation of caspases since a general caspase inhibitor Z-VAD-FMK significantly reversed this effect. Delphinidin treatment to cells resulted in a dose-dependent decrease in (i) phosphorylation of IKKγ (NEMO), (ii) phosphorylation of NFκB inhibitory protein IκBα, (iii) phosphorylation of NFκB/p65 at Ser536 and NFκB/p50 at Ser529, (iv) NFκB/p65 nuclear translocation, and (v) NFκB DNA binding activity. Taken together, our data show that delphinidin induces apoptosis of both androgen independent and androgen refractory human PCa cells via activation of caspases and in addition, this effect might be due to inhibition of NFκB signaling. We suggest that delphinidin could be developed as a novel agent against PCa.
Keywords: delphinidin, apoptosis, NFκB, prostate cancer
Introduction
Prostate cancer (PCa) is a major health problem worldwide and the second leading cause of male cancer related death in Western countries. In the year 2008, in the United States alone 186,320 new cases of PCa will be registered and an approximate total of 28,660 deaths are predicted.1 It is considered as an ideal disease for chemopreventive intervention preferably for naturally occurring dietary agent.2 Since PCa is a slow growing disease, it is generally detected in men over fifty years of age, usually at an advanced stage of the disease. In recent years, considerable efforts are being directed in order to establish the usefulness of naturally occurring dietary agents for chemoprevention as well as chemotherapy of PCa. Polyphenols is one such group of naturally occurring dietary agents, many of which including resveratrol and EGCG have been demonstrated to be beneficial agents interfering with several key processes involved in cancer development and progression.3,4 Among polyphenols, the anthocyanidins and their glycosylated derivatives, anthocyanins are gaining considerable popularity on the fast expanding market of food supplements as they appear to possess potentially beneficial effects against various diseases.5,6 including cancer. These anthocyanidins are abundantly present in pigmented fruits and vegetables.7,8 Delphinidin (Fig. 1A; inset), one of the major anthocyanidin present therein is a diphenylpropane-based polyphenolic ring structure harbouring compound that carries a positive charge in its central ring.9 Research from our and other laboratories around the world suggested that delphinidin possesses anti-oxidant, anti-inflammatory, anti-mutagenic and anti-angiogenic properties.10–13 It has also been documented that delphinidin inhibits epidermal growth receptor (EGFR) and platelet-derived growth factor ligand/receptor (PDGF/PDGFR) signalings and decreases invasion of breast cancer cells.14–16
Figure 1.
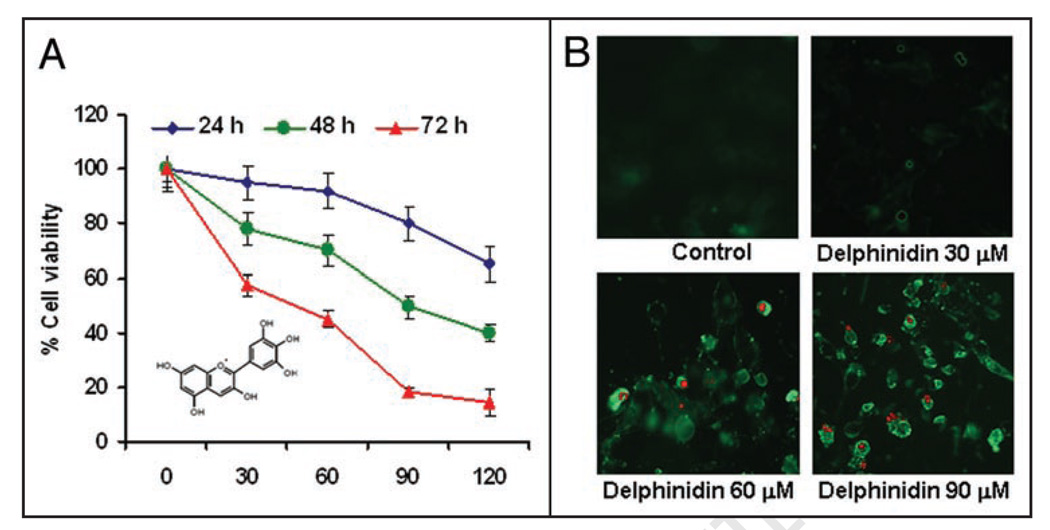
Effect of delphinidin on cell viability and apoptosis of 22Rν1 cells. (A) Human PCa cells were treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin in (0.1% DMSO) for 24, 48 and 72 hours and cell viability were determined by MTT assay as detailed in Materials and Methods. The values are represented as the percent viable cells where vehicle treated cells were regarded as 100 % viable. The data shown here is mean ± S.D of three independent experiments. The inset in (A) depicts the structure of delphinidin. (B) Representative micrographs of 22Rν1 cells undergoing apoptosis induced by treatment with specified concentrations of delphinidin for 48 hours as assessed by fluorescence microscopy. Green fluorescence of Annexin V staining represents the cells under going apoptosis and red fluorescence of PI showing the cells under going either necrosis or late apoptosis as detected by a Ziess Axiovert 100 microscope.
Earlier, we reported chemopreventive and chemotherapeutic effects of pomegranate fruit juice against PCa.17,18 In fact, delphinidin is among major anthocyanidin present in pomegranate fruit extract (PFE), which we recently found to have cell growth inhibitory effect on highly aggressive PCa metastatic PC3 cells under in in vivo and in in vitro conditions. (Hafeez BB, et al., in press).19 Extending these studies further, in this study, we provide evidence that delphinidin also induces apoptosis and inhibits NFκB signaling in human PCa 22Rν1 cells.
Delphinidin Inhibits Growth and Induces Apoptosis of 22Rν1 Cells
To test the effect of delphinidin on cell viability of human PCa 22Rν1 cells, MTT assay was performed. Delphinidin treatment to cells resulted in a significant dose-dependent inhibition of cell growth at all time point tested (24, 48 and 72 hours) (Fig. 1A). The IC50 value at 48 hours post treatment of delphinidin for 22Rν1 cells was found to be 90 µM. (Fig. 1A). To further evaluate whether this cell growth inhibition in 22Rν1 cells by delphinidin is due to induction of apoptosis, we performed annexin V and propidium iodide (PI) staining in delphinidin treated cells. Data showed a significant induction of apoptosis by delphinidin at doses of 30–90 µM which was evident from the significant enhancement in annexin V staining (Fig. 1B).
Delphinidin Induces PARP Cleavage and Increases Bax/Bcl2 Ratio
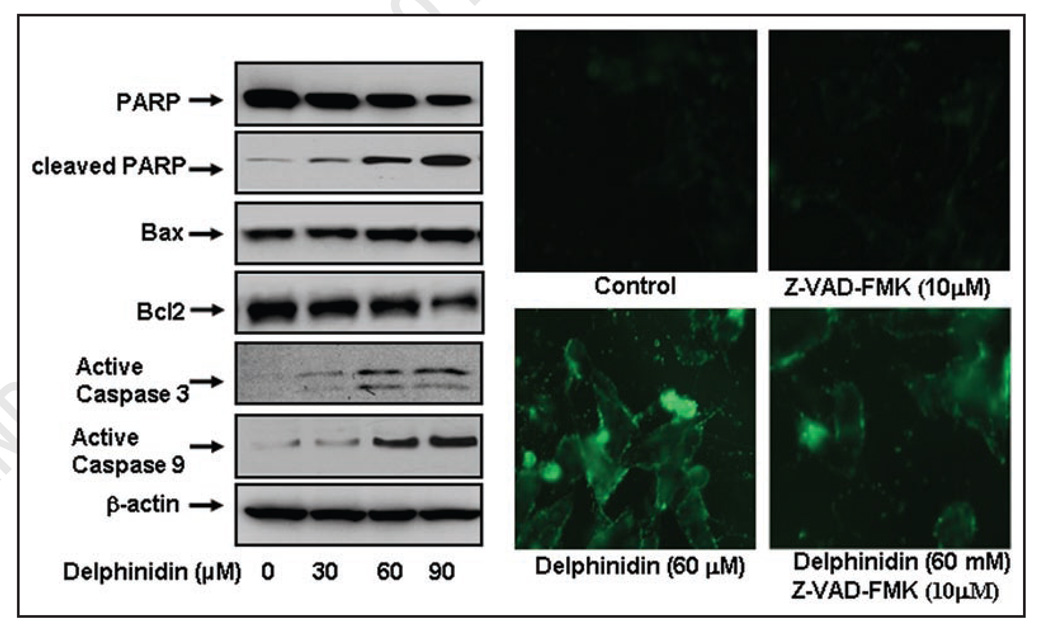
To understand the underlying mechanism by which delphinidin induces apoptosis in 22Rν1 cells, we next evaluated the effect of delphinidin on the levels of apoptotic markers. Western blot analysis showed a significant dose-dependent increase in PARP cleavage in cells treated with delphinidin, with a concomitant decrease in full length protein levels (Fig. 2A). We also observed that delphinidin treatment to cells resulted in a significant dose-dependent increase in Bax protein with concomitant with decrease in the protein level of Bcl2 resulting in substantial increase in the ratio of Bax to Bcl2 thus favoring apoptosis (Fig. 2A). These findings demonstrate that activation of PARP protein, downregulation of Bcl2 and upregulation of Bax may collectively form a molecular basis for the apoptotic action of delphinidin.
Figure 2.
Effect of delphinidin on apoptotic biomarkers in 22Rν1 cells. 22Rν1 cells were treated with vehicle alone (0.1% DMSO) or specified concentrations of delphinidin in 0.1% DMSO for 48 hours as detailed in Materials and Methods. (A) Protein levels of PARP, cleaved PARP, Bcl2, Bax and active caspase-3 and -9 in 22Rν1 cells as determined by immunoblot analysis. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody. The data are representative of three independent experiments with similar results. (B) 22Rν1 cells were treated with 10 µM concentration of a general caspase inhibitor Z-VAD-FMK for 4 hours, followed by the treatment with indicated doses of delphinidin for 48 hours. Green fluorescence of Annexin V staining represents the cells under going apoptosis.
Delphinidin Induces Apoptosis via Activation of Caspases
Activation of caspases is required in order to induce apoptosis. Normally, caspases are present in the pro-forms (inactive) and require site specific cleavage to become active and participate in the process of apoptosis. In fact, treatment of delphinidin significantly increased the levels of active caspases-3 and -9 (Fig. 2A). To test whether delphinidin induces apoptosis via activation of caspases, we used a general caspases inhibitor, Z-VAD-FMK. Delphinidin treatment to the cells at dose of 60 µM exhibited increase in annexin V staining, which was largely reverted in Z-VAD-FMK treated cells (Fig. 2B). These results suggest that activation of caspases may be a possible mechanism by which delphinidin induces apoptosis in 22Rν1 cells.
Delphinidin causes G2/M Phase Cell Cycle Arrest
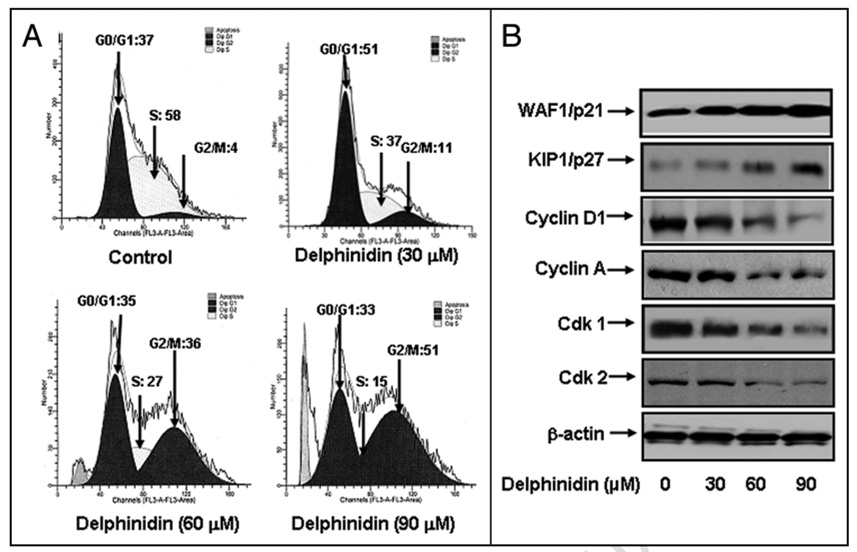
To evaluate the effect of delphinidin treatment on the distribution of cells in the cell cycle, we performed DNA cell cycle analysis. Compared to vehicle treatment, delphinidin treatment resulted in a dose-dependent accumulation of cells in the G2/M phase of the cell cycle by 11%, 36% and 51% at 30, 60 and 90 µM concentrations of delphinidin, respectively. (Fig. 3A) These data indicate that induction of apoptosis and cell growth inhibiton by delphinidin might be due to cell cycle arrest in G2/M phase.
Figure 3.
Effect of delphinidin on cell cycle and cell cycle modulatory proteins in 22Rν1 cells. Cell cycle analysis was performed by flow cytometry as detailed in Materials and Methods. (A) Cell cycle analysis in 22Rν1 cells treated with delphinidin (B) Protein levels of p21, p27, cyclin D1, cyclin A, Cdk1 and cdk2 in 22Rν1 cells as determined by immunoblot analysis. Equal loading of protein was determined by stripping and reprobing the blots with β-actin antibody. The data are representative of three independent experiments with similar results.
Delphinidin Modulates the Expression of Cell Cycle Regulatory Proteins
Genes coding for cell cycle regulators are frequently mutated in most common malignancies.20–21 We next examined the effect of delphinidin on cell cycle inhibitory proteins p27/KIP1 and p21/WAF1, which are involved in cell cycle progression. Western blot analysis demonstrated a significant induction of these proteins in a dose-dependent manner (Fig. 3B), which explains the induction of cell cycle arrest by delphinidin. We next evaluated the effect of delphinidin on the protein levels of cyclins and cdks, which are known to be regulated by KIP1/p27 and WAF1/p21. Delphinidin treatment of cells resulted in a significant dose-dependent decrease in the protein levels of cdk1 and cdk2 as well as cyclin D1 and cyclin A (Fig. 3B). These results suggest that delphinidin treatment restores proper checkpoint control via modulation of these cell cycle regulatory proteins.
Delphinidin Inhibits NFκB Signaling at Multiple Levels
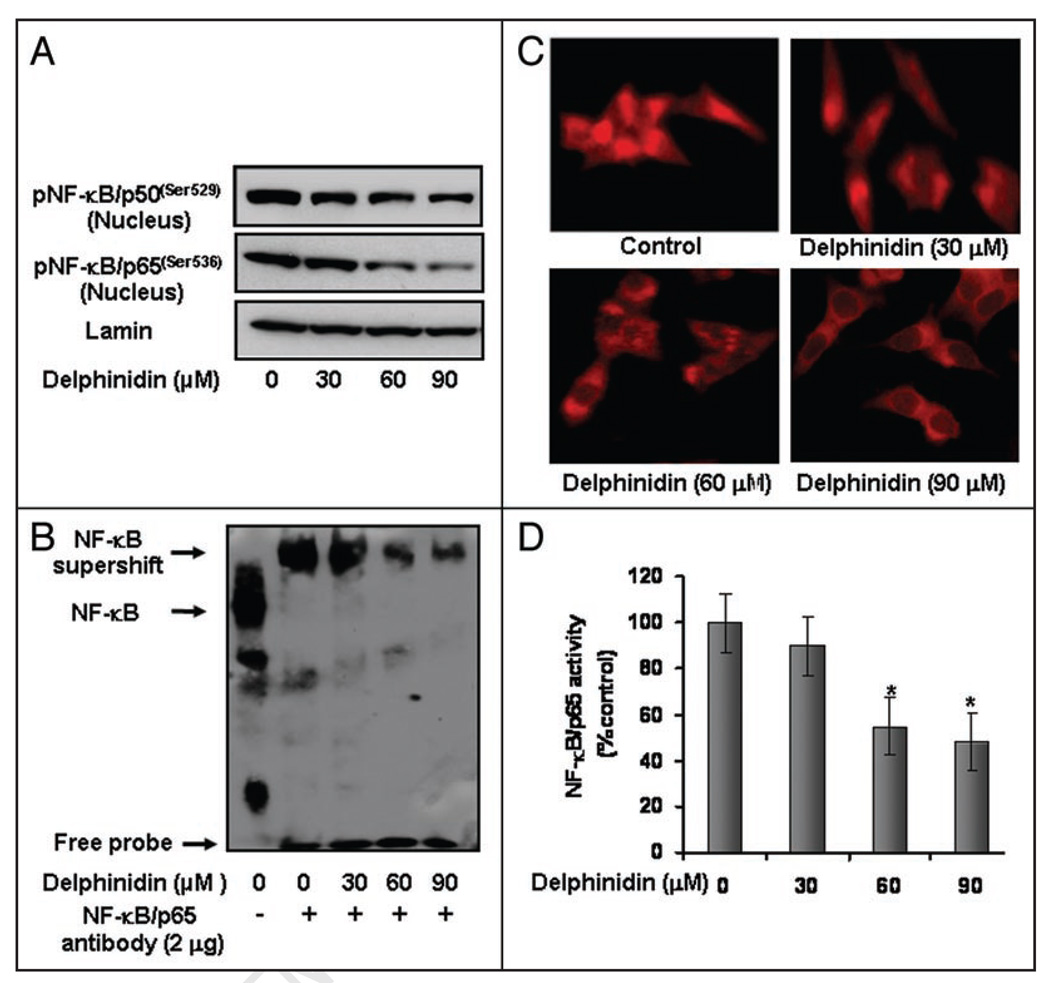
NFκB overexpression has been detected in many cancer types including prostate.22,23 It has been demonstrated that activation of NFκB blocks apoptosis and accelerates cell proliferation.24 We tested whether delphinidin treatment inhibits constitutive NFκB activation in these PCa cells. Employing Western blot analysis, we observed that delphinidin treatment to cells resulted in decrease in active phosphorylation of NFκB/p50 at Ser529 and NFκB/p65 at Ser536 in the nuclear fraction (Fig. 4A). We further confirmed the inhibition of NFκB/p65 DNA binding activity in delphinidin treated cells by performing electrophoretic mobility shift assay and subsequent inhibition of NFκB nuclear shuttling by immunocytochemistry. Delphinidin treatment of cells resulted in a dose-dependent decrease in NFκB/p65 DNA binding activity (Fig. 4B). Delphinidin treated cells exhibited marked decrease in NFκB nuclear localization further suggesting inhibition of NFκB nuclear translocation (Fig. 4C). Delphinidin treatment to cells showed no nuclear immunostaining with anti-p65 antibody at dose of 60–90 µM whereas control cells showed an intense nuclear fluorescence (Fig. 4C). However, delphinidin treatment at doses of 30 µM showed weak expression of NFκB/p65 in the nucleus (Fig. 4C). We further confirmed these data by employing a specific NFκB/p65 enzyme-linked immunosorbent assay, and observed that treatment of cells with delphinidin resulted in a significant inhibition of nuclear translocation of NFκB/p65 in a dose-dependent manner (Fig. 4D). These results corresponded with the decrease in NFκB DNA binding activity data and further support inhibition of nuclear translocation of NFκB/p65 subunit into the nucleus by delphinidin.
Figure 4.
Effect of delphinidin on phospho-NFκB/p50 and p65 and NFκB DNA binding activity in 22Rν1 cells. Nuclear extracts were prepared from cells treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin in 0.1% DMSO for 48 hours (A) Protein levels of phospho-NFκB/p50(ser529) and phospho-NFκB/p65(ser536) in nuclear lysates of 22Rν1 cells as determined by Western blot analysis. Equal loading of protein was assessed by stripping and reprobing the blots with lamin antibody. (B) NFκB DNA binding activity in 22Rν1 cells as detailed in Materials and Methods. Lane 1 represents NFκB-DNA complex. Lane 2 depicts the shift in NFκB-DNA complex due to binding with NFκB/p65 specific antibody. Lane 3–5 indicates change in the NFκB/p65 oligo-immune complex due to treatment with delphinidin. (C) Immunocytochemistry of NFκB/p65 in 22Rν1 cells. (D) Specific ELISA for NFκB/p65 was performed in nuclear lysates of 22Rν1 cells treated with vehicle alone (0.1% DMSO) and specified concentrations of delphinidin.
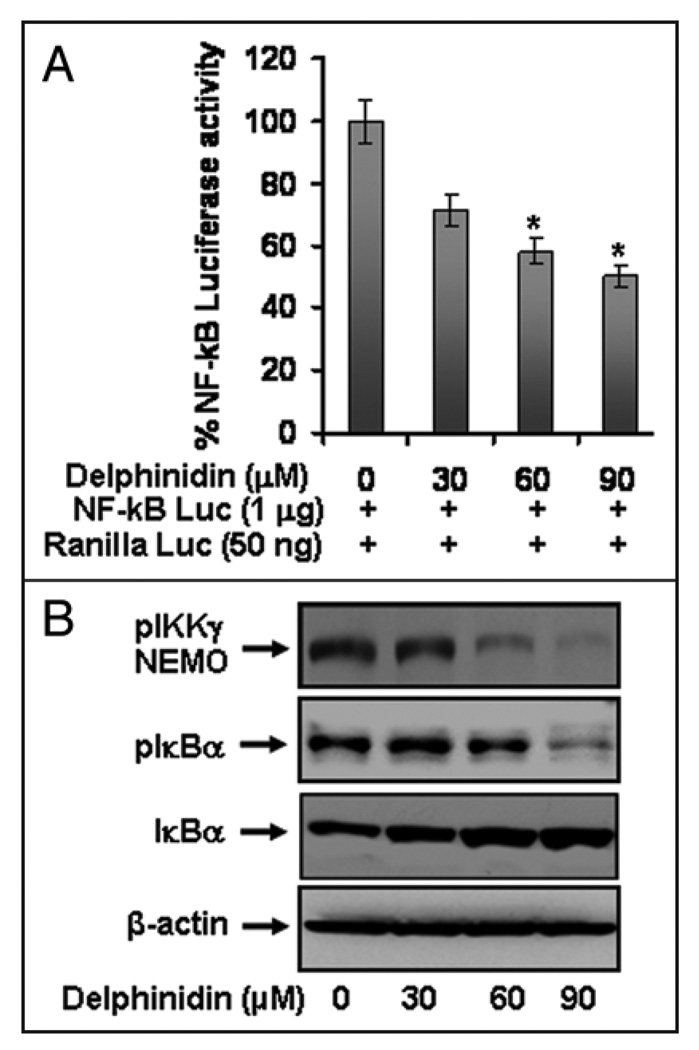
Delphinidin Inhibits NFκB Mediated Transcription Activation and Phospho-IκBα and its Upstream Kinase IKKγNEMO)
To further examine the effects of delphinidin on NFκB activation, we performed NFκB mediated promoter activity using NFκB binding sites containing luciferase reporter plasmid. Delphinidin treatment to cells resulted in a significant dose-dependent decrease in luciferase activity (Fig. 5A), suggesting its effect at transcriptional level. Delphinidin treatment resulted in a dose-dependent decrease in the phosphorylation of NFκB inhibitory protein IκBα with a concomitant increase in total IκBα protein (Fig. 5B). IκB is regulated by upstream kinase IKKγ, which phosphorylates IκB. We observed that delphinidin treatment to cells resulted in a significant inhibition of pIKKγ protein (Fig. 5B) but no such effect was observed on the protein level of other catalytic subunit IKKα and β. These results suggest that delphinidin inhibits NFκB signaling via inhibition of regulatory upstream kinase IKKγ.
Figure 5.
Effect of delphinidin on NFκB/p65 transcriptional activation and the protein levels of pIKKγ, pIκBα, total IκBα in 22Rν1 cells. (A) Effect of delphinidin treatment on NFκB promoter activity. Cells were transiently co-transfected with NFκB luciferase plasmid and Renilla luciferase plasmids for 12 hours and cells were treated with vehicle only or specified concentrations of delphinidin for 24 hours and harvested as detailed in Materials and Methods. (B) Protein levels of pIKKγ, pIκBα and total IκBα in cells treated with vehicle alone (0.1% DMSO) alone and specified concentrations of delphinidin in (0.1% DMSO) as determined by Western blot analysis. Equal loading of protein was confirmed by stripping and reprobing the blots with β-actin antibody.
Discussion
Pomegranate fruit extract contains several anthocyanins and hydrolyzable tannins. In an effort to identify the active ingredient responsible for its anti-cancer effects, we found that delphinidin was the most abundant constituent present therein.25 Recently, we observed that delphinidin induces apoptosis in highly aggressive prostate cancer PC3 cells and inhibits tumor growth in a xenograft mouse model (Hafeez BB, et al., in press).19 Because essentially all PCa subsequently becomes resistant to anti-hormone therapy and transits to an androgen refractory state, we tested the effect of delphinidin on 22Rν1 human PCa cells. Notably this cell line is derived from a xenograft that was serially propagated in mice after castration-induced regression and relapse of the parental, androgen-dependent CWR22 xenograft. In the present study, we observed that delphinidin induced apoptosis and inhibited NFκB signaling in 22Rν1 cells. We observed that the primary mode of delphinidin-mediated inhibition of cell growth is through the induction of apoptosis which resulted from activation of Poly (ADP-ribose) polymerase (PARP) protein cleavage. During induction of apoptosis DNA fragmentation leads to activation of PARP, therefore, cleavage of PARP protein is considered as an important biomarker of apoptosis. We observed significant increase of cleaved PARP protein in delphinidin treated cells suggesting delphinidin induces apoptosis via DNA fragmentation in 22Rν1 cells. Caspases are involved in apoptosis induction via cleavage in PARP proteins.26,27 We found that the induction of apoptosis by delphinidin is regulated primarily by the activation of caspases since pre-treatment with Z-VAD-FMK, a caspase inhibitor, significantly prevented delphinidin mediated apoptosis.
Bcl2 is an upstream effector molecule in the apoptotic pathway and has been identified as a potent suppressor of apoptosis and is generally overexpressed in most cancers including PCa, thereby escaping apoptosis and undermining therapy.28–30 Bcl2 forms a heterodimer with the apoptotic protein Bax and thereby neutralizes its apoptotic effects. Therefore, alteration in the ratio of Bax/Bcl2 is a decisive factor that plays an important role to determine whether cells will undergo apoptosis. We observed that delphinidin significantly modulated the ratio of these proteins in these cells suggesting the involvement of intrinsic apoptotic pathway by which delphinidin induces apoptosis.
Several studies have implicated cell cycle arrest as the primary determinant in the induction of apoptosis.31,32 Therefore, inhibition of the cell cycle has been appreciated as a target for the management of cancer.33,34 It has been reported that G2/M accumulation prevents cancer cells from repairing DNA damage, forcing them into M phase. Thus, the G2 checkpoint has emerged as an attractive therapeutic target for cancer therapy.35 Our study indicates that delphinidin exerts strong growth inhibitory effects on 22Rν1 cells by arresting cells in G2/M phase of cell cycle. It has been well documented that cell cycle is primarily regulated by complexes containing cdks and cyclins, which are critical for progression of cell cycle and their inactivation leads to cell cycle arrest.36,37 The observed inhibitory effects of delphinidin particularly on cyclin D1, cyclin A, cdk1 and cdk2 in PCa cells suggests its interference in cell cycle progression and therefore induction of apoptosis. Our data demonstrate that delphinidin arrests PCa 22Rν1 cells in G2/M phase via modulating cell cycle regulatory molecules suggesting yet another important molecular mechanism through which delphinidin inhibits the growth of PCa cells.
Inhibition of tumorigenesis often involves repression of oncogenic signal transduction pathways, leading to cell cycle arrest and consequently apoptosis. It has been demonstrated that activation of NFκB confers resistance to apoptosis induced by various chemotherapeutic agents.38 Other studies suggest that activation of NFκB signaling induces inflammation and therefore leads to carcinogenesis.39,40 In prostatectomy specimens of PCa with relapsed tumor NFκB was found to be concentrated in the nuclear fraction.41 Therefore, effective inhibition of NFκB could be critical in providing a targeted pathway for PCa prevention and treatment. Delphinidin treatment led to a dose dependent decrease in the DNA binding potential of NFκB, thereby making it transcriptionally incompetent to drive the expression of target genes. Also, NFκB nuclear exclusion induced by delphinidin further synergizes with its inability to bind to DNA in order to repress its function. In the absence of stimulatory signals, NFκB resides in the cytoplasm in the form of hetrodimeric complex with its inhibitory proteins IκBα. Stimulation to the cells with various stimuli activates the IκB kinase (IKK) complex, which is composed of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ/NEMO). Activated IKK phosphorylates NFκB bound IκB proteins, and targets them for polyubiquitination and rapid degradation by creating binding site for SCFβTRCP ubiquitin ligase complex.42 Our results suggest that delphinidin inhibits NFκB signaling through a sequence of events at multiple levels that include inhibition of phosphorylation of IKKγ (NEMO), followed by inhibition of phosphorylation of IκBα and restoration of its subsequent degradation.
The present study demonstrates the effect of delphinidin on cells cycle arrest, induction of apoptosis, activation of caspases and NFκB signaling in 22Rν1 cells. In summary, our present findings, together with earlier observations, suggest that delphinidin is capable of killing resistant cells and further strengthens the wide chemotherapeutic potential of delphinidin and its subsequent development as a potential anti-cancer agent against human PCa.
Materials and Methods
Cell lines and reagents
22Rν1 cells were obtained from American Type Culture Collection (Manassas, VA) and were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% antibiotics. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humidified incubator. Delphinidin (>99% pure) was obtained from Extrasynthese (GENAY-Cedex, France). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was procured from Sigma (Saint Louis, MO). Bcl2 cdk1, cdk2, KIP1/p27 and lamin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cyclin D1, Cyclin A, active caspases-3, -9, pNFκB/p65S(ser536), pNFκB/p50(ser529), IκBα, pIκBα, pIKKγ, IKKα and WAF1/p21 antibodies were procured from Cell Signaling Technology (Beverly, MA). Full length PARP, NFκB/p65, and Bax antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Cleaved PARP antibody was procured from Promega (Madison, WI). The general caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluromethylketone (Z-VAD-FMK) was obtained from R&D Systems, Inc., (Minneapolis, MN). Rhodamine Red™-X-conjugated antibody was procured from Jackson Immuno Research Laboratories Inc., (West Grove, PA). Anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Amersham Pharmacia Life Sciences (Arlington Heights, IL). Novex precast 12% Tris-Glycine gels were obtained from Invitrogen (Carlsbad, CA). Matrigel was procured from BD Bioscience (San Jose, CA).
Treatment of cells
Cells were grown to 50–70% confluence and then treated with freshly prepared delphinidin solution in DMSO. The final concentration of DMSO used was 0.1% (v/v) for each treatment. Control cells were treated with 0.1% DMSO served as vehicle group. After 48 hours of treatment with delphinidin (30–90 µM), the cells were harvested, and cell lysates were prepared and stored at −80°C for subsequent use. For caspase inhibition experiments, the 22Rν1 cells were pre-treated with 10 µM Z-VAD-FMK, a general caspase inhibitor, and 4 hours before the addition of delphinidin (90 µM) in the culture media.
Cell viability assay
The effect of delphinidin on cell viability of 22Rν1 cells was determined by MTT assay. Cells were plated at a density of 1 × 104 cells per well in 200 µl of complete culture medium and treated with delphinidin (30–120 µM) in 96-well microtiter plates for 48 hours. After incubation for specified time at 37°C in a humidified incubator, MTT (5 mg/ml in PBS; diluted in 10 ml of serum free media) was added to each well and incubated for 2 hours, after which the plate was centrifuged at 1000 rpm for 5 min at 4°C. After careful removal of the medium, 0.1 ml buffered DMSO was added to each well. The absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect of delphinidin on cell growth inhibition was assessed as percent cell viability, where vehicle-treated cells were taken as 100% viable.
Detection of apoptosis by fluorescence microscopy
The Annexin-V-FLUOS staining kit (Roche Diagnostic Corporation, Indianapolis, IN) was used for the detection of apoptotic bodies following the vendor’s protocol. This kit uses a dual-staining protocol in which the cells show green fluorescence of Annexin-V (apoptotic cells) and red fluorescence of propidium iodide (necrotic cells or late apoptotic cells). 22Rν1 cells were grown to about 60% confluence and then treated with delphinidin as described above. The fluorescence was detected by a Ziess-Axiophot DM HT microscope (Zeiss-Axiophot, Jena, Germany). Images were captured with an attached camera.
DNA cell cycle analysis
50–60% confluent 22Rν1 cells were synchronized by overnight serum starvation and treated with delphinidin (30–180 µM) for 48 hours in complete medium. The cells were trypsinized, washed twice with chilled PBS and centrifuged. The cell pellet was resuspended in 50 µl cold PBS to which cold methanol (450 µl) was added and the cells were incubated for 1 hour at 4°C. The cells were centrifuged at 1000 rpm. for 5 min, pellet washed twice with chilled PBS, suspended in 500 µl PBS and incubated with 5 µl RNase (20 µg/ml final concentration) at 37°C for 30 min. The cells were chilled over ice for 10 min and incubated with propidium iodide (50 µg/ml final concentration) for 1 hour and analyzed by flow cytometry. Flow cytometry was performed with a FACScan (Becton Dickinson, Heidelberg Germany). A minimum of 10,000 cells/sample were counted and the DNA histograms were further analyzed by using ModiFitLT software (Verily Software House, Topsham, ME) for cell cycle analysis.
Immunoblot analysis
The total cell lysates were prepared, Western blot analysis was performed as described earlier.43 The protein concentration was determined by using BCA protein assay kit.
Enzyme-linked immuno-sorbent assay (ELISA)
After treatment with delphinidin (30–180 µM) for 48 hours, cells were harvested and nuclear lysates were prepared as described earlier.18 The commercially available kit for NFκB/p65 was purchased from Active Motif (Carlsbad, CA) contains the specific oligonucleotides with the specific consensus sequence for NFκB/p65 binding. 5 µg of nuclear lysate protein from each group was taken for quantification of NFκB activity. Experiment was performed according to manufacturer’s instructions. Optical density was taken at 450 nm by using ELISA reader (Multiscan MCC/340, Fisher Scientific, Pittsburgh, PA).
Electrophoretic mobility shift assay (EMSA)
EMSA for NFκB was performed using a lightshift™ chemiluminescent EMSA kit (Pierce, Rockford, IL) following manufacturer’s protocol. NFκB binding site containing oligonucleotide (called NFκB oligo) was biotin labeled using the Biotin 3' endlabeling kit (Pierce Biotechnology, Rockford, IL). The sequence of NFκB oligo was 5'-AGT TGA GGG GAC TTT CCC AGG C-3'; 3'-TCA ACT CCC CTG AAA GGG TCC G-5'. Briefly, 5 µg of nuclear extract was incubated with 20 µg of NFκB/p65 antibody at room temperature for 30 minutes followed by further incubation with 1 pmol of biotin labeled NFκB oligo for 30 min at 37°C. The DNA protein complex formed was resolved on 6% DNA retardation gel, transferred to a nylon membrane. After transfer was completed, DNA was cross linked to the membrane at 120 mJ/cm2 using a UV cross-linker. The biotin end-labled DNA was detected using the Streptovidin-Horseradish Peroxides Conjugate and lightShift™ Chemiluminiscent Substrate according to manufacturer’s instructions. The membrane was exposed to X-ray film (XAR-5 Amersham Life Science Inc., Arlington Heights, IL) and developed using a Kodak film processor.
Immunocytochemical staining of NFκB/p65
22Rν1 cells were seeded in two chambered tissue culture glass slides and treated with delphinidin as described previously. Cells were washed with PBS and fixed in 2% paraformaldehyde in PBS for 10 min at room temperature and then permeabilized in cold methanol (−20°C). After that cells were washed three times with PBS and blocked with 2% donkey serum in 1X PBS for 1 hour and incubated with NFκB/p65 antibody [(1:50 in 5% donkey serum (1X PBS)] overnight at 4°C. After three washes with PBS, cells were incubated with 1:50 of donkey anti-rabbit Rhodamine Red™-X-conjugated antibody for 1 hour at room temperature. Slides were mounted using Prolong antifade kit (Invitrogen, Eugene, OR) and photographed using a Zeiss Axiophot DMHT microscope (Zeiss-Axiophot, Jena, Germany).
Luciferase assay
2 × 106 22Rν1 cells were electroporated with human NFκB luciferase reporter plasmid (pNFκB-TA-Luc, 1 µg), from (Clontech Laboratories, Inc., Mountain View, CA) along with 50 ng of renilla luciferase reporter plasmid pRL-TK (Promega, Madison, WI), which was used as internal control to normalize transfection efficiency using AMAXA nucleofection kit. Parallel to that, cells were also transfected with empty pTA-Luc reporter vector, 1 µg). After electroporation, 30,000 cells were distributed per well of a 24 well plate and allowed to grow for 16 hours followed by delphinidin treatment for another 24 hours with above described concentrations. Dual luciferase assay reagent kit was procured from Promega (Promega, Madison, WI) and luciferase activity was measured according to manufacturer’s protocol.
Statistical analysis
Results were analyzed using a two-tailed Student’s t test to assess statistical significance. Values of p < 0.05 were considered statistically significant.
Acknowledgements
This work was supported by United States PHS grants RO1 CA 78809 and RO1CA120451.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;9:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 4.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 5.Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J Appl Physiol. 2006;100:1164–1170. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- 6.Vuorela S, Kreander K, Karonen M, Nieminen R, Hämäläinen M, Galkin A, et al. Preclinical evaluation of rapeseeds, raspberry and pine bark phenolics for health related effects. J Agric Food Chem. 2005;53:5922–5931. doi: 10.1021/jf050554r. [DOI] [PubMed] [Google Scholar]
- 7.Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappaB in normal human epidermal keratinocytes. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 8.Mazza G. Anthocyanins in grapes and grape products. Crit Rev Food Sci Nutr. 1995;35:341–371. doi: 10.1080/10408399509527704. [DOI] [PubMed] [Google Scholar]
- 9.Hou DX, Fujii M, Terahara N, Yoshimoto M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J Biomed Biotechnol. 2004;2004:321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin and pelargonidin. J Agric Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 11.Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. 2005;70:417–425. doi: 10.1016/j.bcp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Azevedo L, Alves de Lima PL, Gomes JC, Stringheta PC, Ribeiro DA, Salvadori DM. Differential response related to genotoxicity between eggplant (Solanum melanogena) skin aqueous extract and its main purified anthocyanin (delphinidin) in vivo. Food Chem Toxicol. 2007;45:852–858. doi: 10.1016/j.fct.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Lamy S, Blanchette M, Michaud-Levesque J, Lafleur R, Durocher Y, Moghrabi A, et al. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis. 2006;27:989–996. doi: 10.1093/carcin/bgi279. [DOI] [PubMed] [Google Scholar]
- 14.Afaq F, Zaman N, Khan N, Syed DN, Zaid MA, Mukhtar H. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin present in pigmented fruits and vegetables. Int J Cancer. 2008;123:1508–1515. doi: 10.1002/ijc.23675. [DOI] [PubMed] [Google Scholar]
- 15.Lamy S, Beaulieu E, Labbé D, Bédard V, Moghrabi A, Barrette S, et al. Delphinidin, a dietary anthocyanidin, inhibits platelet derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis. 2008;29:1033–1041. doi: 10.1093/carcin/bgn070. [DOI] [PubMed] [Google Scholar]
- 16.Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008 doi: 10.1016/j.taap.2008.03.023. (PMID:18499206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci USA. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik A, Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;4:371–373. doi: 10.4161/cc.5.4.2486. [DOI] [PubMed] [Google Scholar]
- 19.Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, et al. A Dietary Anthocyanidin Delphinidin Induces Apoptosis of Human Prostate Cancer PC3 Cells in vitro and in vivo: Involvement of NFκB signaling. Cancer Res. doi: 10.1158/0008-5472.CAN-08-2232. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastan MB, Canman CE, Leonard CJ. p53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995;14:3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- 21.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JS, Jennings TA, Nazeer T, Sheehan CE, Fisher HA, Kauffman RA, et al. Prognostic factors in prostate cancer. Am J Clin Pathol. 2003;120:85–100. doi: 10.1309/PW69K48RRFJLXKBD. [DOI] [PubMed] [Google Scholar]
- 23.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Gu L, Zhu N, Woods WG, Findley HW. Transfection of a dominant-negative mutant NFκB inhibitor (IκBm) represses p53-dependent apoptosis in acute lymphoblastic leukemia cells: interaction of IκBm and p53. Oncogene. 2003;22:8137–8144. doi: 10.1038/sj.onc.1206911. [DOI] [PubMed] [Google Scholar]
- 25.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NFkappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson DW. Caspase structure, proteolytic substrates and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 28.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 29.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Revelos K, Petraki C, Gregorakis A, Scorilas A, Papanastasiou P, Koutsilieris M. Immunohistochemical expression of Bcl2 is an independent predictor of time-to-biochemical failure in patients with clinically localized prostate cancer following radical prostatectomy. Anticancer Res. 2005;25:3123–3133. [PubMed] [Google Scholar]
- 31.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 32.Vermeulen K, Berneman ZN, Van Bockstaele DR. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:165–175. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald ER, El-Deiry WS. Cell cycle control as a basis for cancer drug development. Int J Oncol. 2000;16:871–886. [PubMed] [Google Scholar]
- 34.Owa T, Yoshino H, Yoshimatsu K, Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem. 2000;8:1487–1503. doi: 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- 35.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–528. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devault A, Cavadore JC, Fesquet D, Labbé JC, Lorca T, Picard A, et al. Concerted roles of cyclin A, cdc25+ mitotic inducer, and type 2A phosphatase inactivating the cyclin B/cdc2 protein kinase at the G2/M phase transition. Cold Spring Harb Symp Quant Biol. 1991;56:503–513. doi: 10.1101/sqb.1991.056.01.057. [DOI] [PubMed] [Google Scholar]
- 37.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 38.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNFalpha-induced apoptosis by NFkappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 39.Karin M, Greten FR. NFkappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 40.Karin M. Nuclear factor kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 41.Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 43.Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NFκB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]