Abstract
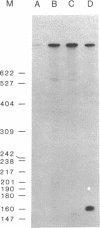
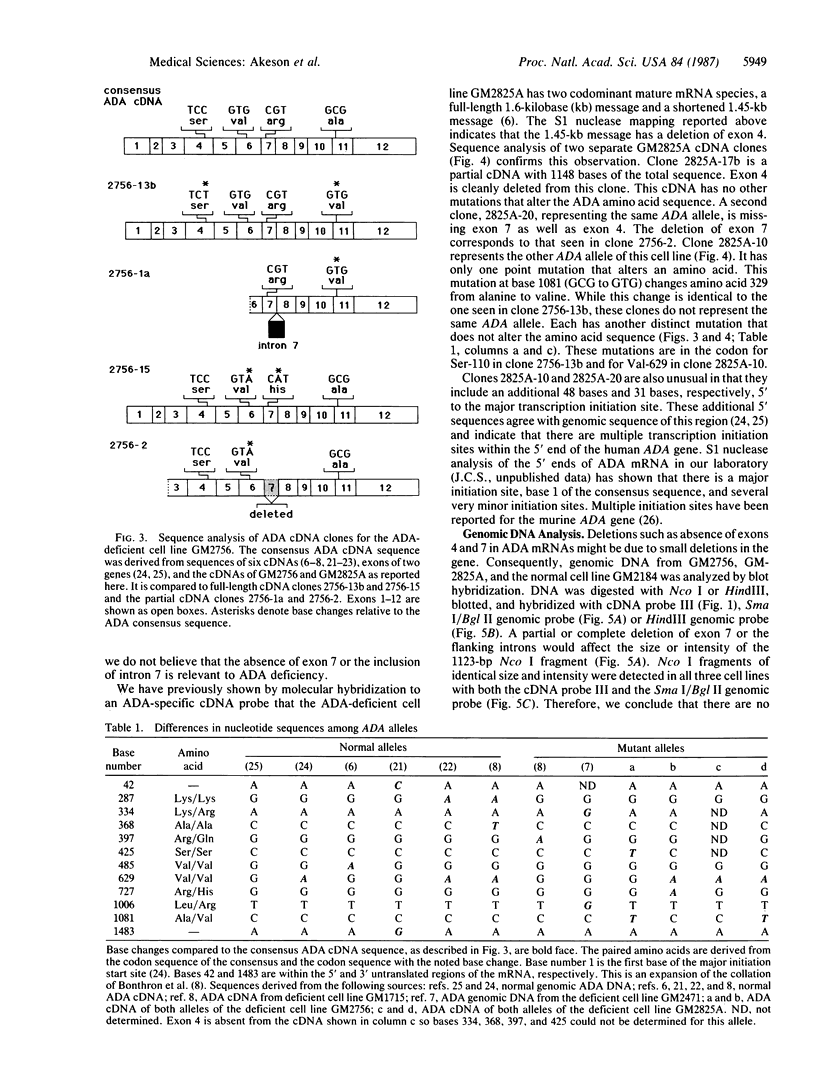
Adenosine deaminase (ADA; adenosine aminohydrolase, EC 3.5.4.4) deficiency is one cause of the genetic disease severe combined immunodeficiency. To identify mutations responsible for ADA deficiency, we synthesized cDNAs to ADA mRNAs from two cell lines, GM2756 and GM2825A, derived from ADA-deficient immunodeficient patients. Sequence analysis of GM2756 cDNA clones revealed a different point mutation in each allele that causes amino acid changes of alanine to valine and arginine to histidine. One allele of GM2825A also has a point mutation that causes an alanine to valine substitution. The other allele of GM2825A was found to produce an mRNA in which exon 4 had been spliced out but had no other detrimental mutations. S1 nuclease mapping of GM2825A mRNAs showed equal abundance of the full-length ADA mRNA and the ADA mRNA that was missing exon 4. Several of the ADA cDNA clones extended 5' of the major initiation start site, indicating multiple start sites for ADA transcription. The point mutations in GM2756 and GM2825A and the absence of exon 4 in GM2825A appear to be directly responsible for the ADA deficiency. Comparison of a number of normal and mutant ADA cDNA sequences showed a number of changes in the third base of codons. These changes do not affect the amino acid sequence. Analyses of ADA cDNAs from different cell lines detected aberrant RNA species that either included intron 7 or excluded exon 7. Their presence is a result of aberrant splicing of pre-mRNAs and is not related to mutations that cause ADA deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Wiginton D. A., Hutton J. J. Characterization of normal and mutant adenosine deaminase messenger RNAs by translation and hybridization to a cDNA probe. Hum Genet. 1984;68(2):169–172. doi: 10.1007/BF00279309. [DOI] [PubMed] [Google Scholar]
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bonthron D. T., Markham A. F., Ginsburg D., Orkin S. H. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J Clin Invest. 1985 Aug;76(2):894–897. doi: 10.1172/JCI112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddona P. E., Shewach D. S., Kelley W. N., Argos P., Markham A. F., Orkin S. H. Human adenosine deaminase. cDNA and complete primary amino acid sequence. J Biol Chem. 1984 Oct 10;259(19):12101–12106. [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Minowada J. Isoenzyme studies in human leukemia-lymphoma cell lines--1. Carboxylic esterase. Leuk Res. 1985;9(2):209–229. doi: 10.1016/0145-2126(85)90084-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Johnson L. F. Molecular cloning of DNA sequences complementary to mouse thymidylate synthase messenger RNA. J Biol Chem. 1984 Jun 10;259(11):7206–7211. [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia D. E., Al-Ubaidi M. R., Yeung C. Y., Bigo H. A., Wright D., Kellems R. E. Molecular cloning of the murine adenosine deaminase gene from a genetically enriched source: identification and characterization of the promoter region. Mol Cell Biol. 1986 Dec;6(12):4458–4466. doi: 10.1128/mcb.6.12.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mesnard J. M., Geldreich A., Xiong C., Lebeurier G., Hirth L. Expression of a putative plant viral gene in Escherichia coli. Gene. 1984 Nov;31(1-3):39–47. doi: 10.1016/0378-1119(84)90193-8. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Solnick D. Alternative splicing caused by RNA secondary structure. Cell. 1985 Dec;43(3 Pt 2):667–676. doi: 10.1016/0092-8674(85)90239-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Minowada J. Terminal transferase immunofluorescence, enzyme markers and immunological profile of human leukemia-lymphoma cell lines representing different levels of differentiation. Leuk Res. 1983;7(3):331–338. doi: 10.1016/0145-2126(83)90097-8. [DOI] [PubMed] [Google Scholar]
- Valerio D., Dekker B. M., Duyvesteyn M. G., van der Voorn L., Berkvens T. M., van Ormondt H., van der Eb A. J. One adenosine deaminase allele in a patient with severe combined immunodeficiency contains a point mutation abolishing enzyme activity. EMBO J. 1986 Jan;5(1):113–119. doi: 10.1002/j.1460-2075.1986.tb04184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Hutton J. J. Sequence of human adenosine deaminase cDNA including the coding region and a small intron. Nucleic Acids Res. 1984 Mar 12;12(5):2439–2446. doi: 10.1093/nar/12.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Hutton J. J. Immunoreactive protein in adenosine deaminase deficient human lymphoblast cell lines. J Biol Chem. 1982 Mar 25;257(6):3211–3217. [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]