Abstract
Objectives
To obtain preliminary data on the effects of high-intensity exercise on functional performance in people with Parkinson's disease (PD) relative to exercise at low and no intensity; and to determine whether improved performance is accompanied by alterations in corticomotor excitability as measured through transcranial magnetic stimulation (TMS).
Design
Cohort (prospective), randomized controlled trial.
Setting
University-based clinical and research facilities.
Participants
Thirty people with PD, 3 years or more since diagnosis, with Hoehn and Yahr stage 1 or 2.
Interventions
Subjects were randomized to high-intensity exercise using body weight–supported treadmill training, low-intensity exercise, or a zero-intensity education group. Subjects completed 24 exercise sessions over 8 weeks and had 5 education classes over 8 weeks.
Main Outcome Measures
Unified Parkinson's Disease Rating Scales (UPDRS), biomechanic analysis of self-selected, fast walking, and sit-to-stand tasks; corticomotor excitability was assessed with cortical silent period durations (CSP) in response to single-pulse TMS.
Results
A small improvement in total and motor UPDRS was observed in all groups. High-intensity group subjects demonstrated postexercise increases in gait speed, step and stride length, and hip and ankle joint excursion during self-selected and fast gait and improved weight distribution during sit-to-stand. Improvements in gait and sit-to-stand measures were not consistently observed in low- and zero-intensity groups. Importantly, the high-intensity group demonstrated lengthening in CSP.
Conclusions
The findings suggest the dose-dependent benefits of exercise and that high-intensity exercise can normalize corticomotor excitability in early PD.
Keywords: Basal ganglia, Central nervous system, Neuronal plasticity, Rehabilitation, Walking
Both basic research and clinical studies suggest that high intensity (ie, high repetition, velocity, complexity) is a characteristic of exercise that may be important in promoting activity-dependent neuroplasticity of the injured brain, including the basal ganglia.1,2 Activity-dependent neuroplasticity is defined as alterations within the CNS in response to physical activity that include such processes as neurogenesis, synaptogenesis, and molecular adaptations.3 BWSTT is currently being studied as a treatment modality for promoting activity-dependent neuroplasticity and functional recovery in stroke and spinal cord injury in part because it can be used to manipulate intensity of practice.
In the last 7 years, there have been an increasing number of studies4-10 that have examined the effect of treadmill exercise on PD, a debilitating and progressive neurodegenerative disease, characterized by motor slowness, stiffness, tremor, and balance dysfunction.11,12 Improved motor performance has been reported and treadmill speeds have gradually increased from studies in which subjects trained at self-selected velocities for comfort4,6 to speeds above overground walking velocity.
While there has been a recent attempt to examine functional performance associated with higher training intensities, few studies have employed different levels of intensity to examine exercise-induced changes in functional performance and how they may compare.7-10,13
In addition, thus far exercise studies in PD have not examined for associated CNS changes. TMS is a noninvasive method of stimulating the brain and provides a tool with which to assess the excitability of the corticospinal motor system (corticomotor excitability). Single TMS pulses are applied over the motor cortex while recording surface electromyographic responses over a contralateral target muscle. If the target muscle is preactivated (contracted), the TMS pulse induces a characteristic transient period of electromyographic silence called the CSP. Silent period durations beyond 100ms are thought to reflect long-lasting cortical inhibitory processes. Important for this study, single-pulse TMS studies have shown systematic abnormalities of CSP and other corticomotor excitability measures in people with PD. In general, these abnormalities reflect higher corticomotor excitability in PD compared with controls.14-17 As CSP represents inhibitory influences on corticomotor excitability; higher excitability would be evident as a shortened CSP duration. In fact, shortened CSP durations are among the most consistent and widely reproduced TMS finding among PD patients.18 Further, symptomatic treatment of PD with surgical or pharmacologic interventions is associated with lengthening of the CSP toward levels seen in control subjects.19-21 These studies suggest that corticomotor excitability measures, particularly CSP durations, could underlie symptomatic improvement, such as improved motor performance. Thus, not only is TMS an excellent tool to measure CSP duration and to examine possible exercise-induced changes in PD, but more importantly TMS may be used to support the existence of CNS changes in response to different exercise parameters including intensity.
The objective of this feasibility study was to obtain preliminary data on the effects of high-intensity exercise on functional performance in people with PD relative to exercise at low and no intensity; and to examine whether improved performance is accompanied by lengthening of CSP as measured through TMS.
Methods
Participants
Thirty subjects with early stage, Hoehn and Yahr stages 1 or 2 PD voluntarily consented to participate in the study. 22 Early stages of PD were chosen because (1) people with greater physical capability could engage in higher-intensity exercise and (2) neuroplastic mechanisms may be more robust and amenable to environmental influences.23,24 All subjects were required to sign an informed consent document approved by the institutional review board at USC. Subjects were recruited largely from the USC Department of Neurology Parkinson's disease and Movement Disorders Clinic as well as through newspaper and radio advertisement delivered to the greater Los Angeles area. Prior to enrollment the diagnosis of idiopathic PD was confirmed by a fellowship trained PD specialist.
The inclusion criteria for the study were the following: (1) diagnosis of PD within 3 years of study participation, (2) 18 years of age or older, (3) medical clearance from their primary care physician to participate in an exercise program, and (4) ability to walk. Potential participants were excluded if: (1) the medical or physical screening exam revealed a score of less than 24 on the MMSE25; (2) physician-determined major medical problems such as cardiac dysfunction that would interfere with participation; (3) musculoskeletal impairments or excessive pain in any joint that could limit participation in an exercise program, and (4) insufficient endurance and stamina to participate in exercise 3 times a week for 1 hour per session. For all participating subjects, all PD medications were kept stable during the course of the study.
Each subject was randomized into one of 3 groups: high-intensity exercise, low-intensity exercise, and zero-intensity (no-exercise) group. With their eyes closed, subjects were randomized by self-selecting a card corresponding to one of the 3 groups. Subjects were blinded to existing group assignments. Subjects in the 3 exercise groups received 24 sessions of exercise over 8 weeks by the same physical therapist. Heart rate and blood pressure were monitored during exercise to assess the subject's exercise tolerance and exercise intensity level. Level of intensity was defined in accordance with the Center for Disease Control and Prevention and American College of Sports Medicine guidelines by MET in which 1 MET is defined as the energy expenditure for sitting quietly.26,27 In this study, low-intensity exercise was any activity that burned less than 3 METS and high-intensity exercise was greater than 3 METs.
Procedures
High-intensity group
Subjects randomized to the high-intensity group participated in 24 sessions of BWSTT. Participants were fitted in a harness,a which was then connected to an overhead suspension systemb positioned over a treadmill. The suspension system is an overhead-motorized pneumatic lift with a digital readout displaying the amount of weight support.b BWS was initially set at 10% of the participant's body weight (to take up the “slack” in the system). However, if the participant was unable to load and support his/her weight during stance with normal gait kinematics, the BWS was increased. Subjects were trained with the assistance of 1 physical therapist and 1 aide if necessary to assist with maintaining the trunk upright. The goal of each treatment session was to have the participant reach and maintain a MET level greater than 3.0 METs and/or 75% of an AAPMHR using proper gait kinematics for stance and swing (upright posture, extending and flexing the knee, hip, and ankle and coordinating limb movements to achieve symmetrical limb cadence and equal step length). The end goal (ie, at least by session 24) was that the subject would walk on the treadmill continuously for 45 minutes within the above MET level range. However, subjects were permitted to rest if necessary.
Progression on the BWS treadmill system occurred in a number of different ways. Within the limits of an individual being able to walk with observationally “normal” gait kinematics, the following parameters were scaled up in difficulty: (1) BWS was decreased; (2) treadmill speed increased; (3) physical assistance was decreased; and (4) time on the treadmill was increased.
A physical therapist ran each treadmill session and was responsible for decisions regarding progression, monitoring upright posture, manual or verbal feedback on pelvic position, weight shift, stride characteristics, and cadence.
Low-intensity group
Subjects randomized to the low-intensity group participated in 24 sessions of PT. This group was representative of general or traditional PT for people with PD. An analysis of exercise studies in PD over a 50-year period revealed that overall the physical demand of the exercise protocols for the most part were low to moderate in intensity. Additionally, we were able to identify that the activities within the studies could be grouped into 6 categories: (1) passive ROM and stretching; (2) active ROM; (3) balance activities; (4) gait; (5) resistance training; and (6) practice of functional activities and transitional movements (ie, sit-to-stand).28,29 Each 45-minute session consisted of activities within each of these 6 categories. Activities were individualized for each subject based on specific impairments and subject goals and included but were not limited to: (1) therapist stretch of hamstrings (passive ROM); (2) active stretch of calf in standing (active ROM); (3) standing on foam, single-limb standing exercises (balance); (4) overground gait training on linoleum floor, grass (gait); (5) rubber tubing exercises, weight lifting (resistance training); and (6) transfer training, sit-to-stand, supine-to-sit (functional activities). MET levels for each activity were ascertained by either selecting activities that were listed in the Compendium of Physical Activities30 or estimating MET levels for those activities that were not listed. For example, an active ROM activity might be a doorway stretch. The estimated METs for this activity would be 1.2 because it compares with standing quietly, a listed activity in the compendium. The goal of each treatment session was to have the participant average 3.0 or less METs and/or a heart rate of 50% or less of their AAPMHR for 45 minutes, a MET level within the low-intensity exercise guidelines. Time spent in each activity was documented and average MET level was calculated at the end of each session.
Zero-intensity group
The zero-intensity intervention consisted of six 1-hour education classes taken over an 8-week period. The following topics were presented: (1) Quality of Life: What is it? (2) Improving Quality of Life in Chronic Illness and PD; (3) Stress, Appraisal, and Coping; (4) Improving Memory; (5) Nonmotor Features of PD; and (6) Treatment Advances in PD.
Subjects in all groups were allowed to continue their customary exercise routines. They were asked however, not to change their exercise routine. To monitor outside activity level, all subjects filled out a daily exercise diary that was reviewed by the treating therapist.
Data Collection
Data were collected prior to intervention and immediately after completion of exercise. Subjects began exercise or education classes within 1 to 2 weeks after the baseline assessment. All subjects took their customary medication at the same time relative to each assessment. All assessors were blinded to group assignment.
Assessments
Baseline clinical characteristics of the 3 groups of subjects were obtained and included: age, duration of PD diagnosis, UPDRS score, MMSE score, and PD medications.
UPDRS and Hoehn and Yahr staging
Disease severity was examined pre- and postintervention using the UPDRS and Hoehn and Yahr staging.22,31 The UPDRS was completed by a second PD specialist, trained in performing the UPDRS. The side of the body (left vs right) and corresponding contralateral brain hemisphere that was most affected by PD was established using the UPDRS.
Functional assessments
All testing took place at the Musculoskeletal Biomechanics Research Laboratory at USC. All tests were performed by a blinded biomechanist. Biomechanic assessments of walking and sit-to-stand were used to better understand the underlying mechanisms by which any potential changes in functional capability occurred. Reflective markers (14-mm spheres) were firmly taped to the following bony landmarks: first toe, first and fifth metatarsal heads, medial and lateral malleoli, medial and lateral epicondyles of femur, greater trochanters, iliac crests, and L5-S1. Additionally, noncolinear tracking markers were placed on the heel, lateral shank, and lateral thigh. An 8-camera (60-Hz) motion analysis systemc recorded 3-dimensional coordinates of the pelvis, thigh, shank, and foot. Ground reaction forces were obtained from force platforms.d Three-dimensional marker-coordinate processing software (Workstation)c and Visual3D Movement Analysis Softwaree were used to process the raw coordinate data and compute the bilateral segmental kinematics and kinetics for the lower extremity.
Walking test
All participants were asked to walk at a self-selected pace and as fast as possible pace along the10-m solid surface. For all the participants, 3 trials were recorded and averaged for each condition. We computed average gait velocity (in m/s); step length (the distance between 2 successive heel contacts of the opposite feet, in meters), stride length (the distance between 2 successive heel contacts of the same foot, in meters), cadence (in number of steps/min), double-limb support time (in percentage of gait cycle), and ankle, knee, and hip sagittal plane maximum joint excursions (in degrees).
Sit-to-stand test
The sit-to-stand task was performed using a firm, armless, adjustable-height chair. The time from initial sitting position to the final sitting position at the end of 3 repetitions was recorded at self-selected pace. All the participants performed 3 sets. A total of 9 repetitions were recorded and averaged. For biomechanic analysis, ankle, knee, and hip sagittal plane extensor net joint moments (in Nm/kg) and joint power (in W/kg) were calculated. Additionally, lower-limb symmetry was calculated as the absolute difference between right and left peak hip, knee and ankle moments and power as well as ground reaction force during standing up.
Transcranial magnetic stimulation
Corticomotor excitability using TMS was assessed before and after the 8-week intervention. Additional criteria excluded those people in which TMS would be contraindicated such as presence of a pacemaker, metal in head, pregnancy, other neurologic disorders, current use of stimulants or medications known to lower seizure threshold, and personal or family history of seizure disorder.32,33 All subjects participating in TMS signed a separate informed consent document approved by the institutional review board at USC.
Single-pulse TMS was applied with a figure-of-8 coil (9×5cm) with a Cadwell MES-10f by a blinded assessor certified as an experimenter in the TMS laboratory at USC. All TMS procedures were conducted on the more affected side first. The TMS coil was held tangentially to the skull, with the handle pointing backward and laterally; perpendicular to the central sulcus.34 Single pulses of TMS were delivered over the primary motor cortex while monitoring MEPs from the FDI muscle. The site that evoked the largest and most reliable MEP amplitudes was designated the motor hotspot. This location was marked on a Lycra cap fitted for each subject to ensure consistent targeting of this hotspot throughout the session. With the coil on the hotspot, stimulator intensity was systematically raised from 40% to 100% maximum stimulator output in 10% increments. At each intensity, 10 single TMS pulses were delivered every 5 to 10 seconds. Intensities were always delivered in ascending order. Electromyographic data were collected during isometric voluntary contraction of the FDI muscle at 10% of maximum voluntary contraction. Both subject and investigator visually monitored the level of muscle contraction and the TMS pulse was timed to occur within 1 to 2 seconds of onset of the contraction while target level of contraction was maintained. Subjects were trained to maintain contraction after each TMS pulse until instructed to relax by the investigator. Electromyographic signals were acquired using surface electrodes in a belly-tendon montage from the FDI contralateral to TMS. The signal was amplified and band-pass filtered between 1 and 1000Hz. Data were stored for later analysis in 600-ms samples, beginning 100ms before TMS onset.g
TMS Data Analysis
All data were analyzed off-line with a customized Matlab software toolh for analysis of time-series data (dataWizard).35 Each TMS trial was analyzed for CSP duration. The CSP duration was defined as the time between the TMS pulse and the first return of rectified electromyographic activity of at least 50% of pre-TMS background activity following a period of sustained silence.36 When no CSP could be discerned, CSP duration was marked as 0ms. For each subject and side, 10 CSP duration values were averaged. The relation between average CSP data and TMS intensity was fitted to a sigmoid curve.37,38 Sigmoid curves were summarized by 3 parameters: maximal CSP duration (CSPmax), maximal slope (CSPslope), and a midpoint intensity where CSP duration is half maximum (CSP50).
Statistical Analysis
Because this study was conducted as a preliminary trial to assess the responsiveness of people with early stage PD to high-intensity exercise and observe for changes in measures of brain and behavior only descriptive analyses including mean and SD were conducted. Percentage change (mean and SD) were calculated as the post value − pre value / pre value × 100 for each subject. Observed trends are reported for the individual exercise groups.
Results
Treatment Groups
All 30 subjects completed the study with no adverse events. Over the 24 exercise sessions, the high-intensity exercise group worked on average at a MET level of 4.3 with a range between 2.5 and 13.3 METs. In fact, 60% of the high-intensity exercise subjects reached 8 to 13.3 METs while running at 0% grade and speeds ranging from 8.0 to 12.8km/h (5.0–8.0mph). Heart rate for 7 of the high-intensity subjects ranged between 70% and 75% of AAPMHR within and across exercise sessions. For the remaining 3 subjects treated with β-blockers, heart rate ranged between 50% and 60% of AAPMHR. The 10 subjects in the low-intensity exercise group averaged 2.4 METs over 24 sessions. The range of intensity of exercise was between 1.8 and 2.7 METs for the low-intensity exercise group. Heart rate for the low-intensity subjects was consistently at 50% or less of AAPMHR across and within exercise sessions.
All subjects in the zero-intensity group attended all education classes. The first 4 subjects randomized to the education arm of the study attended education classes as a group. The remainder of the participants received the same education classes but they were conducted for each subject.
Clinical Characteristics
The mean baseline clinical characteristics of the 3 groups of subjects are shown in table 1. It can be seen that the groups were similar at baseline in age, Hoehn and Yahr stages, duration of PD, and MMSE. While there was variability within and between groups regarding PD medications, no PD medications were adjusted during the trial (see table 1). The baseline and postexercise means and SD in UPDRS total score and UPDRS subscores are shown in table 2 for each exercise group. Both UPDRS total and motor scores were slightly lower for each of the 3 groups at the postexercise time point.
Table 1. Clinical Characteristics of PD Subjects at Baseline.
| Characteristics | Zero Intensity | Low Intensity | High Intensity |
|---|---|---|---|
| Patients (n) | 10 | 10 | 10 |
| Sex (male/female) | 8/2 | 5/5 | 6/4 |
| Age (y) | 63.1±11.5 | 61.5±9.8 | 64.0±14.5 |
| Hoehn and Yahr stage | 1.9±0.3 | 1.9±0.3 | 1.9±0.5 |
| Duration of PD (mo) | 17.7±13.3 | 8.8±7.9 | 14.7±9.9 |
| MMSE score | 29.6±0.7 | 29.3±0.8 | 28.9±1.1 |
| Medications | |||
| Levodopa (mg) | 90.0±202.5 | 15.0±47.4 | 115.0±226.1 |
| Pramipexole (mg) | 0.5±0.7 | 0.3±0.9 | 0.5±.1.0 |
| Ropinerole (mg) | 0 | 0 | 1.6±5.1 |
| Amantadine | 30.0±94.9 | 20.0±63.2 | 30.0±94.8 |
| Selegiline | 0.5±1.6 | 1.0±3.2 | 1.0±2.1 |
NOTE. Values are mean ± SD.
Table 2. UPDRS.
| UPDRS | Zero Intensity | Low Intensity | High Intensity |
|---|---|---|---|
| UPDRS total | |||
| Baseline | 36.1±9.5 | 39.4±9.3 | 35.9±13.3 |
| Postexercise | 32.9±10.6 | 34.2±8.0 | 33.8±14.6 |
| UPDRS mental | |||
| Baseline | 0.8±0.79 | 1.6±1.2 | 1.4±2.1 |
| Postexercise | 1.1±0.99 | 1.7±1.5 | 1.4±1.8 |
| UPDRS ADLs | |||
| Baseline | 7.7±3.8 | 7.3±2.9 | 7.0±3.3 |
| Postexercise | 6.8±4.0 | 5.8±2.7 | 7.6±3.9 |
| UPDRS motor | |||
| Baseline | 27.6±7.3 | 30.5±8.7 | 27.6±10.3 |
| Postexercise | 24.9±8.8 | 26.7±7.5 | 24.8±9.0 |
NOTE. Values are mean ± SD.
Abbreviation: ADLs, activities of daily living.
Motor Performance
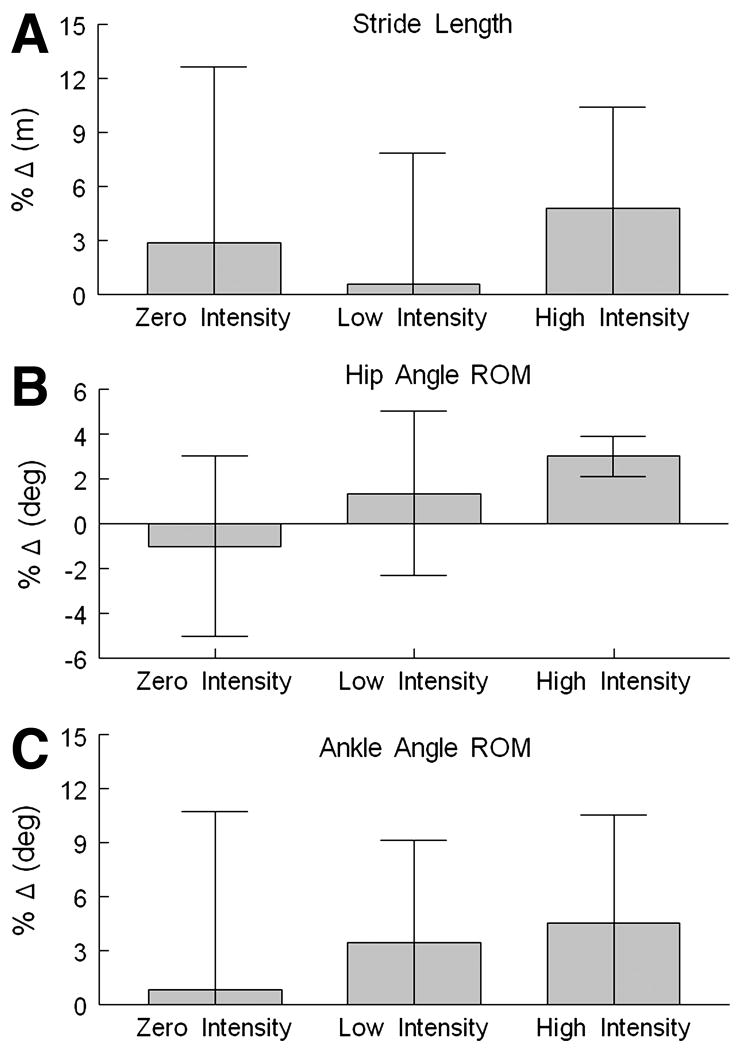
All groups showed improvement in some gait performance measures (tables 3, 4). However, the high-intensity group showed consistent improvement in most gait parameters. Specifically, the high-intensity exercise group demonstrated pre to post increases in self-selected gait speed (4.4% increase), stride length (4.7%) (fig 1A), and step length (5.8% increase). Hip (fig 1B) and ankle (fig 1C) joint excursion increased by 7.5% and 4.6%, respectively, after 24 sessions of BWSTT. Additionally, double-limb support time decreased by 6.3% (ie, increased single-limb support). Similar to the results of the self-selected speed condition, in the fast walking assessment the high-intensity exercise group demonstrated within group postintervention increases in stride length (4.8 %), step length (5.6 %), hip (7.4%), and ankle (8.5%) joint ROM.
Table 3. Kinematic Variables During Walking.
| Outcome Measure | Postexercise | Postexercise | Postexercise |
|---|---|---|---|
| Velocity (m/s) | |||
| Baseline | 1.39±0.17 | 1.40±0.18 | 1.46±0.20 |
| Postexercise | 1.41±0.17 | 1.42±0.17 | 1.52±0.19 |
| Step length (m) | |||
| Baseline | 0.68±0.11 | 0.71±0.08 | 0.73±0.10 |
| Postexercise | 0.71±0.11 | 0.72±0.07 | 0.77±0.08 |
| Stride length (m) | |||
| Baseline | 1.37±0.23 | 1.42±0.15 | 1.48±0.18 |
| Post Exercise | 1.41±0.23 | 1.44±0.14 | 1.54±0.16 |
| Step width (m) | |||
| Baseline | 0.12±0.02* | 0.10±0.02 | 0.11±0.02 |
| Postexercise | 0.11±0.02 | 0.10±0.02 | 0.11±0.02 |
| Cadence (steps/min) | |||
| Baseline | 120.33±9.26 | 120.57±11.60 | 120.66±10.40 |
| Postexercise | 121.09±8.60 | 118.94±10.20 | 120.85±8.50 |
| Double-limb support time (% of gait cycle) | |||
| Baseline | 24.04±6.17 | 19.53±4.49 | 21.20±3.35 |
| Postexercise | 21.22±4.03 | 19.87±3.58 | 19.68±2.58 |
| Hip ROM (deg) | |||
| Baseline | 40.1±5.3 | 39.6±4.4 | 41.22±5.5 |
| Postexercise | 39.1±4.2 | 41.0±4.6 | 44.25±6.2 |
| Knee ROM (deg) | |||
| Baseline | 64.0±4.1 | 63.4±5.1 | 66.0±5.8 |
| Postexercise | 64.6±4.2 | 64.5±4.5 | 64.8±4.8 |
| Ankle ROM (deg) | |||
| Baseline | 24.2±5.2 | 27.6±2.5 | 29.0±3.4 |
| Postexercise | 24.4±5.5 | 28.6±3.4 | 30.3±3.3 |
NOTE. Values are mean ± SD.
Table 4. Kinematic Variables During Fast Walking.
| Outcome Measure | Zero Intensity | Low Intensity | High Intensity |
|---|---|---|---|
| Velocity (m/s) | |||
| Baseline | 1.96±0.38 | 1.92±0.23 | 1.91±0.32 |
| Postexercise | 2.04±0.40 | 1.94±0.19 | 2.00±0.34 |
| Step length (m) | |||
| Baseline | 0.81±0.17 | 0.82±0.11 | 0.84±0.11 |
| Postexercise | 0.82±0.13 | 0.85±0.09 | 0.88±0.11 |
| Stride length (m) | |||
| Baseline | 1.60±0.31 | 1.65±0.22 | 1.66±0.21 |
| Postexercise | 1.64±0.26 | 1.65±0.12 | 1.74±0.24 |
| Step width (m) | |||
| Baseline | 0.12±0.03 | 0.10±0.02 | 0.10±0.03 |
| Postexercise | 0.11±0.02 | 0.10±0.02 | 0.11±0.02 |
| Cadence (steps/min) | |||
| Baseline | 147.45±18.47 | 141.37±13.91 | 138.65±11.50 |
| Postexercise | 151.11±20.23 | 142.12±14.26 | 138.71±9.87 |
| Double-limb support time (% of gait cycle) | |||
| Baseline | 17.36±4.40 | 16.12±4.51 | 16.27±4.14 |
| Postexercise | 13.59±3.82 | 15.80±3.22 | 15.48±2.87 |
| Hip ROM (deg) | |||
| Baseline | 45.0±5.3 | 45.2±5.9 | 47.2±6.5 |
| Postexercise | 44.6±4.9 | 46.7±5.0 | 50.4±6.3 |
| Knee ROM (deg) | |||
| Baseline | 64.0±7.8 | 62.0±6.0 | 67.1±6.7 |
| Postexercise | 60.2±13.4 | 61.7±7.2 | 66.9±5.6 |
| Ankle ROM (deg) | |||
| Baseline | 26.3±4.3 | 28.3±5.3 | 30.3±4.7* |
| Postexercise | 26.4±4.4 | 29.5±5.7 | 32.5±3.3 |
NOTE. Values are mean ± SD.
Fig 1.
The percentage change (post- − pre-exercise / pre-exercise × 100) in motor performance measures are shown for each of the 3 exercise groups: zero, low, and high intensity. (A) Percentage change in stride length (in meters) for each group. Stride length is the distance from right-heel contact to the following right-heel contact. (B) Percentage change in hip angle ROM (in degrees) or hip joint excursion for each group. (C) Percentage change in ankle angle ROM (in degrees) or ankle joint excursion for each group.
The average time to accomplish 3 repetitions of sit-to-stand decreased slightly for all groups. We were unable detect any pre-post differences in lower-extremity symmetry (left versus right lower extremity) at the hip, knee, and ankle joints during sit-to-stand because of the high variability of these measures. However, while both the zero- and low-intensity groups demonstrated increased asymmetry of ground reaction force (82.4%, 5.1% respectively), the high-intensity group demonstrated a 33.3% increase in symmetry suggesting more equal distribution of body weight between the lower extremities during the sit-to-stand task.
Transcranial Magnetic Stimulation
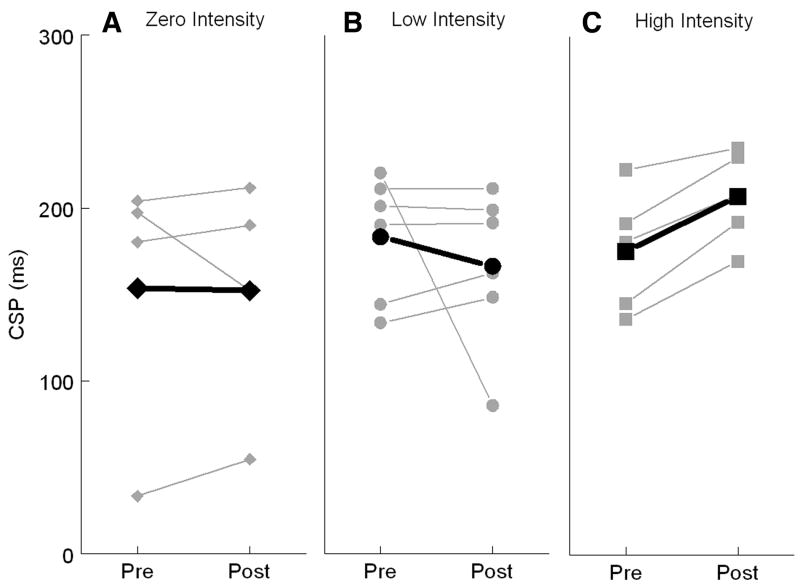
A subset of subjects from each group participated in TMS testing (4 in zero-intensity, 7 in low-intensity, 5 in high-intensity). Both the more- and less-affected hemispheres were tested. No subject had any side effects from TMS. There was no differential effect of intensity of exercise on CSPslope or CSP50. However, for CSPmax, the high-intensity group demonstrated an average increase in maximal CSP duration for the more affected hemisphere (32ms) compared with a 17-ms decrease for the low-intensity group and no change for the no exercise group (black lines in fig 2). Increases in CSP duration were seen in all subjects that had undergone high-intensity exercise (mean ± SD, pre: 175.4±35.3; post: 207.3±27.1). The same pattern for the high-intensity group was observed for the less affected hemisphere with an average 19.4-ms increase in CSP after the exercise intervention. The zero-intensity group showed an average 6.5-ms decrease with no change for the low-intensity group. There was 1 subject each in the zero- and low-intensity groups who showed pronounced shortening of CSPmax. On close inspection, the data from these 2 subjects was deemed valid and the discernible shortening seen potentially reflects the variability between subjects that are within a 3-year range of PD diagnosis.
Fig 2.
Pre- and postexercise measures of maximal CSP duration (in milliseconds) for subjects in the (A) zero-intensity, (B) low-intensity, and (C) high-intensity groups. Four subjects within the zero-intensity group participated in the TMS studies compared with 6 subjects in the low-intensity and 5 subjects in the high-intensity exercise groups. The thick black lines represent the average pre- and post-CSP for each group.
Discussion
In this study, we found that people with PD participating in high-intensity BWSTT improve spatiotemporal gait parameters, kinematics of gait performance, and lower-extremity symmetry of ground reaction force in sit-to-stand task. This improvement was not consistently observed across measures in the other exercise intensity groups. Additionally, every subject undergoing high-intensity BWSTT demonstrated a lengthening of CSP. Lengthened CSP was not consistently observed in subjects in the zero- and low-intensity groups suggesting that high-intensity exercise may induce activity-dependent neuroplasticity as measured through changes in corticomotor excitability. Finally, all subjects in the high-intensity group completed the exercise program, demonstrating that subjects with PD can be challenged at and can tolerate a very high-intensity treadmill exercise program.
All subjects showed improvement in gait velocity during both self-selected and fast-paced walking, which was most evident in the high-intensity group. Similar to our results some improvement in gait velocity during self-selected speed walking has been reported in other studies examining treadmill exercise effects in PD. The modest improvement in gait velocity after treadmill exercise reported in all these studies including ours may be related to the fact that subjects were already walking at normal gait velocities before the exercise programs.39-42 Perhaps a more notable observation than changes in gait velocity was that the subjects in the high-intensity exercise group demonstrated improved gait performance manifest as changes in gait strategy. Thus, the high-intensity subjects walked within the range of normal gait speed, but did so by taking longer steps and moving forward over their limbs through a larger ROM (see tables 3, 4). These changes in gait performance driven by high-intensity practice were not observed to the same degree in the low- and zero-intensity groups and represent a pattern that is in notable contrast to the problematic gait pattern of people with PD (ie, short steps and limited advancement forward over the lower limbs). Similar findings were reported by Pohl et al,9 in which relatively high-intensity treadmill training compared to conventional gait therapy was shown to have effects on gait pattern, specifically stride length and double-limb support time.
From our study, we learned that increased gait speed may not be a sufficient enough measure to determine the success of a treadmill intervention in early stage PD and use of a wide range of gait parameters may be necessary to observe improvement in essential characteristics of gait. In addition to gait measures used in our study, measures of swing and stance time variability (coefficient of variation) have been shown to be particularly sensitive at detecting abnormalities in early PD.43 Herman et al8 recently observed that these measures of variability may be modified by an exercise intervention.
Additionally, the high-intensity group demonstrated increased symmetry of ground reaction forces in the sit-to-stand task. This suggests that high-intensity treadmill exercise may lead to improvement in other nongait motor tasks and that the treadmill training may assist in the ability to load the lower limbs equally. While treadmill studies in PD have examined other standing or upright axial tasks in addition to gait performance, to our knowledge this study is the first to report a benefit in another distinct motor task that may be due to intense gait training exercise.6,7,10,13
In our study, there did not appear to be detectable postintervention differences in the UPDRS between the exercise groups, which was in contrast to the more consistent improvement in functional performance tests observed in the high-intensity group. Possible reasons to account for differences in outcome measures may be due to (1) the large degree of variability in the UPDRS among people within 3 years of diagnosis, along with the small sample size, and (2) greater sensitivity of functional and objective measures compared with the more subjective UPDRS in capturing changes in motor performance.
An important objective of this study was to examine if high-intensity exercise may be accompanied by CNS changes. Using TMS, we found lengthening of maximal CSP in all subjects in the high-intensity exercise group that was not consistently observed in subjects in either the zero- and low-intensity groups. This finding suggests that intensity may be an important exercise parameter for facilitating activity-dependent neuroplasticity in association with improved motor performance.
Among TMS studies examining corticomotor excitability in PD patients, CSP durations are among the most consistent abnormalities reported with generally shorter duration associated with greater parkinsonian symptoms.44 CSP durations are usually shorter in PD patients compared with controls and, within PD patients, are shorter on the more affected side compared with the less affected side.14,45 Both CSP durations and parkinsonian symptoms are sensitive to dopaminergic medication. Similar to the observed effects of high-intensity exercise, CSP durations are prolonged in PD patients after taking levodopa,45-47 apomorphine,48 and pergolide,19,49 drugs known to provide effective symptomatic relief of motor symptoms. As clinical improvement accompanies dopaminergic treatment, this clinical improvement therefore corresponds with an increase in CSP duration.45
The level of excitability within the motor cortex is a balance between excitation and inhibition. The late part of the silent period duration is thought to reflect long-lasting cortical inhibitory processes.50 As such, the duration of the CSP has been used as an index of the strength and time course of these processes. Studies have shown a loss of cortical excitation and inhibition balance in PD with higher motor system excitability in patients with PD at rest compared with controls most likely the result of reduced intracortical inhibition.14,15,44 Under tonic muscle contraction, shortened CSP in PD also suggests an enhanced excitability.14 Inhibition as measured through CSP may be important for suppressing competing motor networks thereby facilitating cortical-basal ganglia loops specified for an intended motor action.44 Loss of inhibition as the result of loss of inhibitory cortical mechanisms (ie, shortened CSP) as is seen in PD may impair the focus of neuronal activity onto the appropriate pathways and enhance unspecific motor program transmission. Therefore interventions such as intense exercise that lengthens CSP may help to restore normal motor processing.
The mechanism underlying the lengthening of the silent period duration in people with PD undergoing high-intensity exercise is unclear. However it is known that CSP is mainly mediated by GABA-B receptors.44 GABA is the major inhibitory neurotransmitter in the basal ganglia, and abnormalities of GABAergic transmission are key aspects of the pathophysiology of movement disorders that involve the basal ganglia.51 Additionally, it has been identified that voluntary exercise can increase levels of BDNF. BDNF has neurotrophic and neuroprotective properties, can enhance brain plasticity and appears to be a prime candidate for mediating the long-term benefits of exercise on the brain.52,53 BDNF enhances neuronal function by promoting synaptogenesis and neurogenesis.54 Evidence has shown that BDNF modulates the level of functional inhibition in an activity-dependent manner by regulating the number of GABAergic interneurons.55 While the role of BDNF in modulating GABA-mediated inhibitory transmission is not fully understood, it is possible that the lengthening of CSP in this study is related to an exercise-induced increase in BDNF.56
In our study, we found that the increase in CSP duration for the high-intensity group was observed in an intrinsic hand muscle while the intense exercise involved a lower-extremity task, specifically treadmill training. This finding suggests that CSP may serve as a marker for a more “generalized” change in the CNS as a function of a primarily lower-extremity intervention. This is not the first report of postintervention and specifically post treadmill training effects on corticomotor excitability using TMS in people with neuropathology. Changes in corticomotor excitability and associated improvements in walking function were found after intensive treadmill training both in people with spinal cord injury57,58 and stroke.59 However, to our knowledge, this is the first demonstration of exercise-induced changes in corticomotor excitability using TMS in individuals with PD, a progressive neurologic disorder. Animal models of PD have also supported activity-dependent neuroplasticity after intensive treadmill training as measured through changes in dopamine handling and neurotransmission, including increased dopamine release, decreased uptake and an increase in the postsynaptic dopamine D2-receptor subtype within the basal ganglia.2,60
Study Limitations
An important limitation of this study is the small sample size and large variability in disease severity, and baseline motor performance. As a result of this variability we were not able to demonstrate group differences using inferential statistics. Nevertheless, we were able to show benefits of high-intensity exercise in motor performance and corticomotor excitability. This trend of changes we observed warrants further study with a larger sample size to allow for a more discerning statistical analysis and determination of the relationship between changes in corticomotor excitability and motor performance.
Conclusions
The interest to promote neuroplasticity in PD as a means for eliciting improvement in motor performance has underscored the importance of identifying those exercise parameters that are essential for promoting activity-dependent neuroplasticity. Findings from our study suggest a potential role of intensity of exercise in driving activity-dependent neuroplasticity and functional improvement in people with PD and warrants further investigation.
To our knowledge, this is the first study to demonstrate that people with PD can engage in very high levels of exercise intensity up to 13.3 METs and the first to report improvement in both measures of brain and behavior in people with PD as the result of high-intensity exercise. By understanding the effects of exercise on neuroplasticity, novel, nonpharmacologic therapeutic modalities may be designed to delay or reverse disease progression in idiopathic PD.
Acknowledgments
Supported by the Kinetics Foundation and National Institute of Neurological Disorders and Stroke (grant no. K23-NS045764).
List of Abbreviations
- AAMHR
age-appropriate maximal heart rate
- BDNF
brain-derived neurotrophic factor
- BWS
body-weight support
- BWSTT
body weight–supported treadmill training
- CNS
central nervous system
- CSP
cortical silent period
- FDI
first dorsal interosseous
- GABA
γ-aminobutyric acid
- MEP
motor evoked potential
- MET
metabolic equivalent
- MMSE
Mini-Mental State Examination
- PD
Parkinson's disease
- PT
physical therapy
- ROM
range of motion
- SD
standard deviation
- TMS
transcranial magnetic stimulation
- UPDRS
Unified Parkinson's Disease Rating Scale
- USC
University of Southern California
Footnotes
Presented in part to the Society for Neuroscience, October 17, 2006, Atlanta, GA; American Physical Therapy Association Combined Sections, February 1–5, 2006, San Diego, CA; the World Parkinson Congress, February 22–26, 2006, Washington, DC; and the National Parkinson Foundation Collaboration for Care Leadership Conference, October 20–22, 2005, San Francisco, CA.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
Robertson Harness, PO Box 90086, Henderson, NV 89009-0086.
Vigor Equipment Inc, 4915 Advance Way, Stevensville, MI 49127.
Vicon, 14 Minns Business Park, West Way, Oxford, Oxfordshire OX2 0JB, UK.
Model OR6-6-1; Advanced Mechanical Technology Inc, 176 Waltham St, Watertown, MA 02472-4800.
C-motion Inc, 20030 Century Blvd, Ste 104, Germantown, MD 20874.
Cadwell Laboratories, 909 N Kellogg St, Kennewick, WA 99336.
LabView; National Instruments Corp, 11500 N Mopac Expwy, Austin, TX 78759-3504.
The MathWorks Inc, 3 Apple Hill Dr, Natick, MA 01760-2098.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher BE, Petzinger GM, Nixon K, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 3.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–82. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 4.Miyai I, Fujimoto Y, Ueda Y, et al. Treadmill training with body weight support: its effect on Parkinson's disease. Arch Phys Med Rehabil. 2000;81:849–52. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 5.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83:1370–3. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 6.Toole T, Maitland CG, Warren E, Hubmann MF, Panton L. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. NeuroRehabilitation. 2005;2:307–22. [PubMed] [Google Scholar]
- 7.Cakit BD, Saracoglu M, Genc H, Erdem HR, Inan L. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson's disease. Clin Rehabil. 2007;21:698–705. doi: 10.1177/0269215507077269. [DOI] [PubMed] [Google Scholar]
- 8.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil. 2007;88:1154–8. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Pohl M, Rockstroh G, Ruckriem S, Mrass G, Mehrholz J. Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson's disease. Arch Phys Med Rehabil. 2003;84:1760–6. doi: 10.1016/s0003-9993(03)00433-7. [DOI] [PubMed] [Google Scholar]
- 10.Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88:63–76. doi: 10.2522/ptj.20060351. [DOI] [PubMed] [Google Scholar]
- 11.Nutt JG, Wooten GF. Diagnosis and initial management of Parkinson's disease. N Engl J Med. 2005;353:1021–7. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- 12.Clarke CE. Parkinson's disease. BMJ. 2007;335:441–5. doi: 10.1136/bmj.39289.437454.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff J. Effect of gait speed on gait rhythmicity in Parkinson's disease: variability of stride time and swing time respond differently. J Neuro Eng Rehabil. 2005;2:1–7. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson's disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41:1449–56. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- 15.Valls-Sole J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson's disease. Neurology. 1994;44:735–41. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- 16.Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–9. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- 17.Dioszeghy P, Hidasi E, Mechler F. Study of central motor functions using magnetic stimulation in Parkinson's disease. Electromyogr Clin Neurophysiol. 1999;39:101–5. [PubMed] [Google Scholar]
- 18.Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson's disease. Brain Res Brain Res Rev. 2002;38:309–27. doi: 10.1016/s0165-0173(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 19.Strafella AP, Valzania F, Nassetti SA, et al. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson's disease: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1198–202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- 20.Young MS, Triggs WJ, Bowers D, Greer M, Friedman WA. Stereotactic pallidotomy lengthens the transcranial magnetic cortical stimulation silent period in Parkinson's disease. Neurology. 1997;49:1278–83. doi: 10.1212/wnl.49.5.1278. [DOI] [PubMed] [Google Scholar]
- 21.Dauper J, Peschel T, Schrader C, et al. Effects of subthalamic nucleus (STN) stimulation on motor cortex excitability. Neurology. 2002;59:700–6. doi: 10.1212/wnl.59.5.700. [DOI] [PubMed] [Google Scholar]
- 22.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 23.Kieburtz K. Designing neuroprotection trials in Parkinson's disease. Ann Neurol. 2003;53:S100–9. doi: 10.1002/ana.10484. [DOI] [PubMed] [Google Scholar]
- 24.Kieburtz K. Issues in neuroprotection clinical trials in Parkinson's disease. Neurology. 2006;66:S50–7. doi: 10.1212/wnl.66.10_suppl_4.s50. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AH, Cable NT, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sports Sci. 2004;22:703–25. doi: 10.1080/02640410410001712421. [DOI] [PubMed] [Google Scholar]
- 27.Gibbons RJ, Balady GJ, Bricker JT, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) [published erratum in: J Am Coll Cardiol 2006;48:17] J Am Coll Cardiol. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 28.Deane KH, Ellis-Hill C, Jones D, et al. Systematic review of paramedical therapies for Parkinson's disease. Mov Disord. 2002;17:984–91. doi: 10.1002/mds.10197. [DOI] [PubMed] [Google Scholar]
- 29.Deane KH, Jones D, Ellis-Hill C, Clarke CE, Playford ED, Ben-Shlomo Y. A comparison of physiotherapy techniques for patients with Parkinson's disease. Cochrane Database Syst Rev. 2001;(1):CD002815. doi: 10.1002/14651858.CD002815. [DOI] [PubMed] [Google Scholar]
- 30.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Fahn S, Elton RL, Fahn S, Marsden CD, Calne DB, Goldstein M. Unified Parkinson's Disease Rating Scale: recent developments in Parkinson's disease. Florham Park: Macmillan Healthcare Information; 1987. pp. 153–63. [Google Scholar]
- 32.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 33.Anand S, Hotson J. Transcranial magnetic stimulation: neurophysiological applications and safety. Brain Cogn. 2002;50:366–86. doi: 10.1016/s0278-2626(02)00512-2. [DOI] [PubMed] [Google Scholar]
- 34.Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–6. [PubMed] [Google Scholar]
- 35.Koski L, Schrader LM, Wu AD, Stern JM. Normative data on changes in transcranial magnetic stimulation measures over a ten hour period. Clin Neurophysiol. 2005;116:2099–109. doi: 10.1016/j.clinph.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114:938–44. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 37.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 38.Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, Mills KR. Silent period to transcranial magnetic stimulation: construction and properties of stimulus–response curves in healthy volunteers. Exp Brain Res. 2005;163:21–31. doi: 10.1007/s00221-004-2134-4. [DOI] [PubMed] [Google Scholar]
- 39.Lopopolo RB, Greco M, Sullivan D, Craik RL, Mangione KK. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: a meta-analysis. Phys Ther. 2006;86:520–40. [PubMed] [Google Scholar]
- 40.Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: Evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79:317–22. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- 41.Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, Jonkers I. Quantitative gait analysis in Parkinson's disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86:1007–13. doi: 10.1016/j.apmr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarin M, Rizzone M, Bergamasco B, et al. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson's disease. Exp Brain Res. 2005;160:517–27. doi: 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- 43.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease. Eur J Neurosci. 2006;24:1815–20. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- 44.Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson's disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol. 2005;116:244–53. doi: 10.1016/j.clinph.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson's disease and drug-induced parkinsonism. Brain. 1994;117(Pt 2):317–23. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- 46.Nakashima K, Wang Y, Shimoda M, Sakuma K, Takahashi K. Shortened silent period produced by magnetic cortical stimulation in patients with Parkinson's disease. J Neurol Sci. 1995;130:209–14. doi: 10.1016/0022-510x(95)00029-2. [DOI] [PubMed] [Google Scholar]
- 47.Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995;37:181–8. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- 48.Manfredi L, Garavaglia P, Beretta S, Pellegrini G. Increased cortical inhibition induced by apomorphine in patients with Parkinson's disease. Neurophysiol Clin. 1998;28:31–8. doi: 10.1016/S0987-7053(97)89576-7. [DOI] [PubMed] [Google Scholar]
- 49.Ziemann U, Bruns D, Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neurosci Lett. 1996;208:187–90. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]
- 50.Brasil-Neto JP, Cammarota A, Valls-Sole J, Pascual-Leone A, Hallett M, Cohen LG. Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurol Scand. 1995;92:383–6. doi: 10.1111/j.1600-0404.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 51.Galvan A, Wichmann T. GABAergic circuits in the basal ganglia and movement disorders. Progress Brain Res. 2007;160:287–312. doi: 10.1016/S0079-6123(06)60017-4. [DOI] [PubMed] [Google Scholar]
- 52.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 53.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30(2):75–9. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mole Cell Neurosci. 2006;31:481–92. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardoni R, Ghirri A, Salio C, Prandini M, Merighi A. BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Dev Neurobiol. 2007;67:960–75. doi: 10.1002/dneu.20401. [DOI] [PubMed] [Google Scholar]
- 57.Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–9. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–55. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 59.Forrester LW, Hanley DF, Macko RF. Effects of treadmill exercise on transcranial magnetic stimulation-induced excitability to quadriceps after stroke. Arch Phys Med Rehabil. 2006;87:229–34. doi: 10.1016/j.apmr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]