Abstract
The HGF/Met signaling pathway is deregulated in majority of cancers and is associated with poor prognosis in breast cancer. Delphinidin, present in pigmented fruits and vegetables possesses potent anti-oxidant, anti-inflammatory and anti-angiogenic properties. Here, we assessed the anti-proliferative and anti-invasive effects of delphinidin on HGF-mediated responses in the immortalized MCF-10A breast cell line. Treatment of cells with delphinidin prior to exposure to exogenous HGF resulted in the inhibition of HGF-mediated (i) tyrosyl-phosphorylation and increased expression of Met receptor, (ii) phosphorylation of downstream regulators such as FAK and Src and (iii) induction of adaptor proteins including paxillin, Gab-1 and GRB-2. In addition, delphinidin treatment resulted in significant inhibition of HGF-activated (i) Ras-ERK MAPKs and (ii) PI3K/AKT/mTOR/p70S6K pathways. Delphinidin was found to repress HGF-activated NFκB transcription with a decrease in (i) phosphorylation of IKKα/β and IκBα, and (ii) activation and nuclear translocation of NFκB/p65. Inhibition of HGF-mediated membrane translocation of PKCα as well as decreased phosphorylation of STAT3 was further observed in delphinidin treated cells. Finally, decreased cell viability of Met receptor expressing breast cancer cells treated with delphinidin argues for a potential role of the agent in the prevention of HGF-mediated activation of various signaling pathways implicated in breast cancer.
Keywords: Delphinidin, Hepatocyte growth factor, Breast cancer
Introduction
The involvement of hepatocyte growth factor (HGF)-Met signaling in breast cancer biology has been well characterized (Parr et al., 2004). Increased cell proliferation, survival, motility, and extracellular matrix degradation resulting from the pathway activation contributes to tumor growth, invasiveness, and metastasis (Parr et al., 2004). In fact, high levels of HGF and Met expression associated with invasive human breast cancer have been considered as possible indicators of earlier recurrence and shortened survival in these patients (Lindemann et al., 2007). On the contrary, HGF expression but not Met is strongly suppressed in normal breast epithelial cells. HGF and Met are therefore candidate targets for therapeutic intervention for the treatment of breast cancer (Lindemann et al., 2007).
HGF is a mesenchymal-derived cytokine, which upon binding to its receptor Met, stimulates mitogenesis, motogenesis, and morphogenesis in a wide range of cellular targets, thus contributing to oncogenesis and tumor progression (Peruzzi and Bottaro, 2006). The Met receptor is a disulfide-linked heterodimeric glycoprotein, in which the transmembraneβ-chain contains the kinase domain and a unique multifunctional docking site, which upon tyrosine phosphorylation, recruits different signal transducers and adaptors, such as Gab-1, SHC and GRB2. The latter two couple the Met receptor with the Ras-MAPK pathway, while Gab-1 binds PI3K efficiently (Forte et al., 2006). Changes in cell motility, cell shape, adhesion, resistance to apoptosis, and anchorage independent growth all contribute to the role of Met in cancer (Sattler and Salgia, 2007). Studies show that NFκB activity, along with ERK1/2 and p38 MAPK activation is required for HGF-induced proliferation (Hah and Lee, 2003). There is evidence that HGF activates PKC, ERK1/2 and p38 MAPKs simultaneously through parallel pathways and that the activation of the MAPKs does not depend on PKC (Awasthi and King, 2000). In addition, HGF-activated invasion involves kinases PI3K and PKC and results in stimulation of cell motility and concomitant overproduction of proteases, which permits cell migration through a degraded extracellular matrix (Kermorgant et al., 2001). A definite role of Stat3 in transducing survival signals downstream of Met has been identified (Zhang et al., 2002). Sustained high level of c-Src-Stat3 activation has been shown to induce HGF transcription and HGF protein expression, resulting in activation of an HGF-Met autocrine loop promoting mammary tumorigenesis, and metastasis (Elliott et al., 2002).
Dietary factors are widely believed to play an important role in determining the risk of many cancers, including those of breast. In addition, consumption of fruits and vegetables has been associated with a decreased risk of developing breast cancer (Trichopoulou et al., 1995). Interest in anthocyanidins, the flavonoid constituents that provide much of the flavor and color to these fruits and vegetables has increased immensely during the past decade as epidemiological evidence hints at their cancer preventive activity. Although biochemical and biological responses altered by various naturally occurring agents on primary cultured cells or established cell lines have been extensively studied, little information is presently available on the effect of the dietary anthocyanidins on HGF/Met signaling. We recently reported that delphinidin, a major anthocyanidin present in fruits and vegetables, protects against UVB-mediated oxidative stress and apoptosis in immortalized HaCaT cells (Afaq et al., 2007). In addition, delphinidin was found to be a potent inhibitor of epidermal growth factor receptor (EGFR) and its downstream signaling pathways in AU-565 breast cancer cells (Zaman et al., 2006). Here, we provide evidence of the inter-relationship between the signaling networks activated by HGF and the anti-invasive, anti-proliferative effect of delphinidin in immortalized human breast epithelial MCF-10A cells. Our data further show that delphinidin treatment to breast cancer cells expressing the HGF receptor Met, resulted in decreased cell viability, signifying a potential role of dietary anthocyanidins against breast cancer.
Materials and methods
Materials
Delphinidin (>98% pure) was purchased from Extrasynthase (Lyon, France). HGF was purchased from Chemicon International (Manassas, VA).
Antibodies
p-FAK, p-src, p-paxillin, GRB-2, p-Gab1, p-SHP-2, p-CrkII, p-CrkL, p-Raf1, p-MEK, MEK, p-ERK1/2, ERK1/2, p-JNK1/2, JNK1/2, PI3K/85, p-AKT, AKT, p-mTOR, mTOR, p-p70s6k, p70s6k, p-eIF4E, p-STAT3, STAT3, p-IKKα/β, p-IκBα, IκBα, and NF-κB/p65 antibodies were obtained from Cell Signaling Technology (Danvers, MA). K-Ras and Raf-1 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); p-PKCα, p-Akt, p-Met and Met antibodies were obtained from Upstate Cell Signaling Solutions (Temecula, CA).
Cell culture/treatment
MCF-10A cells were obtained from ATCC, and cultured in MEGM supplemented with cholera toxin, at 37 °C with 5% CO2 in a humid environment. The cells were serum starved for 15 h and then pre-treated with delphinidin dissolved in DMSO for 3 h at doses 5–40 µM, after which cells were harvested/ treated with HGF (40 ng/ml) for 30 min and then harvested. HCC1419 cells (ATCC; Manassas, VA) were grown in RPMI 1640 and MDA-MB-231 (ATCC) in Leibovitz's L-15 media supplemented with 10% FBS and 1% streptomycin.
Preparation of total cell lysate
After treatment of cells with delphinidin or HGF (or both), the medium was aspirated and the cells were washed twice in phosphate-buffered saline (PBS) 1 (10 mM, pH 7.4) and incubated in 0.4 ml ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid [EGTA], 1 mM ethylene diaminetetraacetic acid [EDTA], 20 mM NaF, 100 mM Na3VO4, 0.5% Nonidet P-40 [NP-40], 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF] [pH 7.4]) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA). The cells were then centrifuged at 13,000 ×g for 25 min at 4 °C, and the supernatant (total cell lysate) was collected, aliquoted and stored at −80 °C. The protein concentration was determined by the BCA protein assay kit using the manufacturer's protocol (Pierce, Rockford, IL).
Preparation of cytosolic and nuclear lysates
Following treatment of cells with delphinidin or HGF (or both), the medium was aspirated and the cells were washed twice in PBS (10 mM, pH 7.4). The cells were incubated in 0.4 ml ice-cold lysis buffer (HEPES (10 mM, pH 7.9), KCl (10 mM), EDTA (0.1 mM), EGTA (0.1 mM), DTT (1 mM), PMSF (1 mM)) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA) for 15 min, after which 12.5 µl of 10% Nonidet P-40 was added and the contents were mixed on a vortex and then centrifuged for 1 min (14,000 g) at 4 °C. The supernatant was saved as cytosolic lysate and stored at −80 °C. The nuclear pellet was resuspended in 50 µl of ice-cold nuclear extraction buffer (HEPES (20 mM, pH 7.9), NaCl (0.4 M), EDTA (1 mM), EGTA (1 mM), DTT (1 mM), PMSF (1 mM)) with freshly added protease inhibitor cocktail for 30 min with intermittent mixing. The tubes were centrifuged for 5 min (14,000 g) at 4 °C, and the supernatant (nuclear extract) was stored at −80 °C. The protein concentration was determined by the BCA protein assay kit using the manufacturer's protocol (Pierce, Rockford, IL).
Cell lysis and fractionation
For preparation of cytosolic and membrane-bound proteins, cells were harvested, washed in 1PBS and suspended in 50 mM phosphate buffer (pH 7.4) containing 150 mM NaCl, 1% Triton X-100 and a mixture of protease inhibitors, and homogenized in Dounce homogenizer. Post-nuclear supernatants were centrifuged for 2 h at 100,000 g in a Beckman TLA-100.1 rotor at 4 °C and the cytosolic fraction was collected and adjusted to 1% Triton X-100. The membrane pellet was solubilized in lysis buffer containing 1% Triton X-100 and was cleared by centrifuging at 15,000 ×g for 20 min.
Western blot analysis
For Western blot analysis, 25–30 µg of protein was resolved over 6–16% PAGE and transferred to a nitrocellulose membrane. The blot containing the transferred protein was blocked in blocking buffer (5% nonfat dry milk, 1% Tween 20; in 20 mM TBS, pH 7.6) for 1 h at room temperature followed by incubation with appropriate monoclonal/ polyclonal primary antibody in blocking buffer for 1 h to overnight at 4 °C. This was followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase (Amersham Biosciences, UK) for 1 h and several washes and finally detection by chemiluminescence (ECL kit, Amersham Biosciences, UK) and autoradiography using XAR-5 film obtained from Eastman Kodak Co. (Rochester, NY).
Cell migration assay
Cell migration assay was performed using CHEMICON® QCM™ 24-well migration assay kit. Cells treated with delphinidin (5–40 µM) were loaded onto the chamber. Conditioned media was added to each of the wells with or without HGF and cells were allowed to invade through the membrane by incubating at 37 °C for 14 h after which the invaded cells on the bottom of the insert membrane were detached, lysed and subsequently detected by CyQUANTâ GR dye using a fluorescence plate reader at 480/520 nm filter set.
Luciferase activity
MCF-10A cells were seeded at a concentration of 1.5×105 cells per well in six-well plates and co-transfected with 2 µg NF-κB-driven luciferase reporter constructs and 1 µg Renilla plasmid (Promega, Madison, WI) using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA). After 48 h, cells were treated and luciferase activity was measured using the reporter luciferase assay system (Promega) and normalized to Renilla activity. All experiments were repeated at least three times to prove their reproducibility.
Immunocytochemistry
MCF-10A cells were seeded in 2 chamber tissue culture glass slides and treated with/without delphinidin and HGF. For NFκB/p65 staining, cells were fixed in 2% paraformaldehyde, permeabilized in cold methanol (−20 °C), and blocked with 2% donkey serum, incubated first with NFκB/p65 antibody [1:50 in 5% donkey serum (1PBS)] o/n and then with donkey anti-rabbit Rhodamine RedTM-X-conjugated antibody [1:50]. For PKCα staining, cell were fixed, permeabilized and blocked as described, incubated with PKCα antibody [1:50] o/n followed by incubation with anti-rabbit FITC tagged secondary antibody for 1 h. Samples were mounted using Prolong antifade kit (Invitrogen), and observed using a Zeiss Axiophot DM HT microscope. Images were captured with an attached camera linked to a computer. Images and figures were composed using ADOBE PHOTOSHOP 7.0 (Adobe Systems, Mountain View, CA).
Statistical analysis
Results were analyzed using a two-tailed Student's t test to assess statistical significance. Values of P<0.05 were considered statistically significant.
Results
HGF treatment to MCF-10A cells resulted in increased phosphorylation of the Met receptor
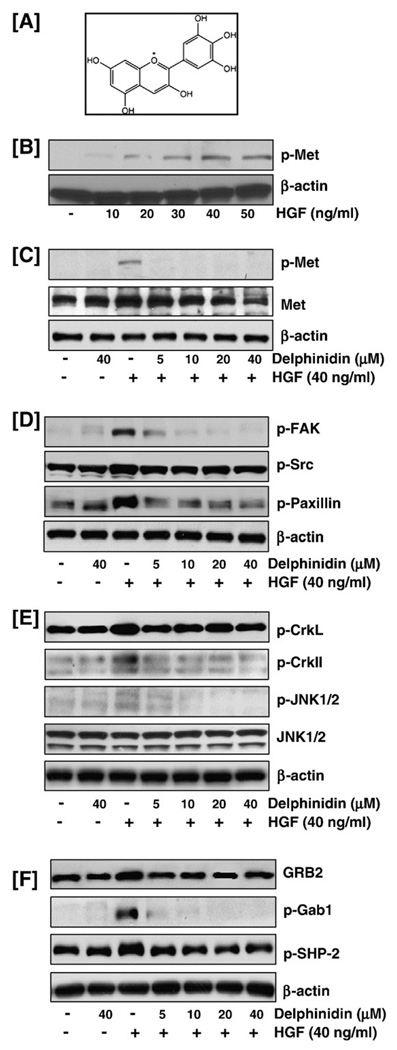
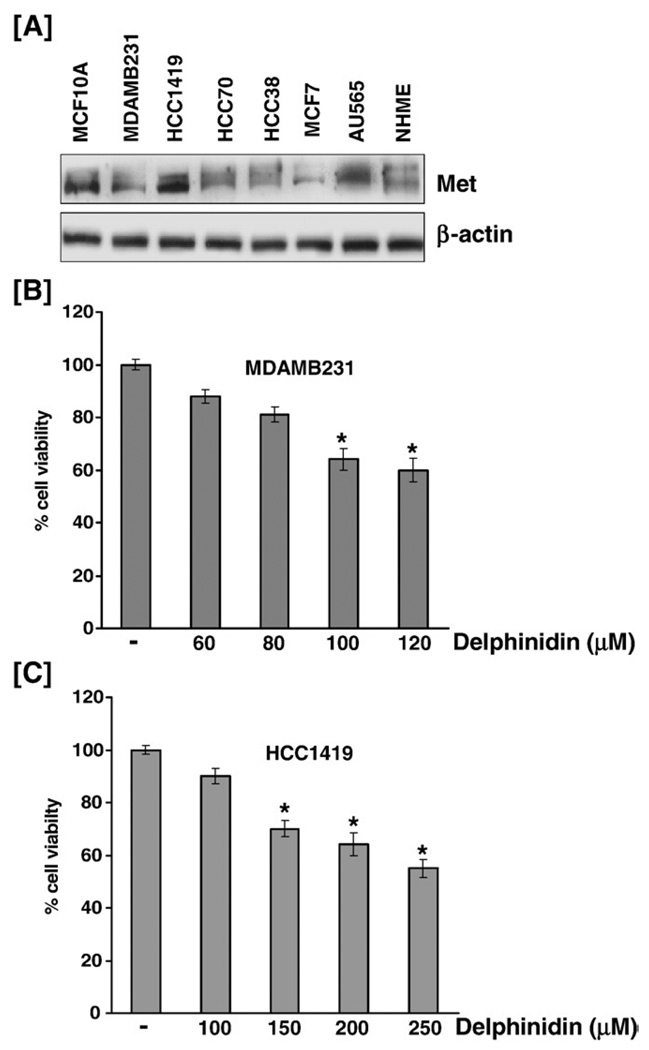
We first established the effect of varying doses of HGF on the phosphorylation of the Met receptor in immortalized MCF-10A breast epithelial cells. MCF-10A cells were treated with different concentrations of HGF (10–50 ng/ml) for 30 min, and whole cell lysates were prepared. Immunoblot analysis showed that induction of MCF-10A cells with HGF for 30 min resulted in a dose-dependent increase in the phosphorylation of Met, up to 40 ng/ml where after the effect was sustained at 50 ng/ml (Fig. 1B). Since the phosphorylation status at the higher doses of 40 and 50 ng/ml was almost similar, we therefore selected a dose of 40 ng/ml of HGF for further studies.
Fig. 1.
Effect of delphinidin on Met and its downstream effector molecules. (A) Structure of delphinidin. (B) HGF treatment resulted in increased phosphorylation of the HGF receptor Met: MCF-10A cells, serum starved for 12 h, were treated with different concentrations of HGF (10–50 ng/ml) for 30 min, and whole cell lysates were prepared. Immunoblot analysis showed that induction of MCF-10A cells with HGF for 30 min resulted in a dose-dependent increase in the phosphorylation of Met. (C) Delphinidin inhibited HGF-induced phosphorylation and activation of Met. (D) Delphinidin inhibited HGF-induced phosphorylation of FAK, Src and paxillin. (E) Delphinidin inhibited HGF-induced phosphorylation of CrkII, CrkL and JNK1/2 proteins. (F) Delphinidin inhibited HGF-induced activation of GRB2 and phosphorylation of Gab1 and SHP-2: MCF-10A cells, serum starved for 12 h and treated without or with delphinidin (5–40 µM) and stimulated with 40 ng/ml HGF for 30 min in serum-free media were harvested and total cell lysates were prepared for western blot analysis as detailed in 'Materials and methods'. Equal protein loading was confirmed by β-actin. The data shown here are from representative experiments repeated three times with similar results.
Delphinidin inhibited HGF-induced phosphorylation and activation of Met
Next, to determine the effect of delphinidin on HGF-mediated signaling, MCF-10A cells were treated with increasing concentrations of delphinidin (5–40 µM) for 3 h and then exposed to exogenous HGF (40 ng/ml) for 30 min. Our data showed that the significant phosphorylation of Met protein observed with HGF stimulation was effectively suppressed by delphinidin treatment (Fig. 1C). In addition, the total Met protein levels were also found to be downregulated with increasing doses of delphinidin treatment (Fig. 1C).
Delphinidin inhibited HGF/Met-mediated recruitment and activation of downstream effector molecules
In order to assess the effect of delphinidin on the downstream signaling events induced by HGF/Met, MCF-10A cells pre-treated with delphinidin and induced with HGF were analyzed by immunoblot analysis. HGF/Met mediated recruitment and activation of focal adhesion kinase (FAK) and c-Src result in the formation of a dual kinase complex that promotes cell motility, cell cycle progression and cell survival. Data presented in Fig. 1D show that delphinidin inhibited HGF-induced phosphorylation of FAK and Src proteins thereby potentially hindering the signals transmitted through the activation of the FAK-Src complex. The FAK-Src complex binds to and can phosphorylate various adaptor proteins such as paxillin, phosphorylation of which is known to alter cell adhesion of Met transformed cells (Mitra and Schlaepfer, 2006). Delphinidin treated cells showed decreased phosphorylation of paxillin when compared to the HGF-induced cells suggestive of an inhibitory effect of delphinidin on cell motility and adhesion (Fig. 1D). CrkII and CrkL are required for the breakdown of adherent junctions, spreading of epithelial colonies, and the formation of lamellipodia in response to HGF (Lamorte et al., 2002). Interaction between phosphorylated Gab1, a major substrate of Met and Crk mediates activation of the JNK pathway downstream of Met (Watanabe et al., 2006). Our data demonstrated that HGF stimulation of MCF-10A cells resulted in increased phosphorylation of the Crk proteins and delphinidin inhibited this phosphorylation. In addition, delphinidin also abolished the HGF-induced increase in the phosphorylation of JNK protein (Fig. 1E). The tyrosine specific phosphatase SHP-2, a positive regulator of ERK activity, is recruited to Gab1 following HGF stimulation. GRB2 is known to trigger the Ras-Raf-ERK pathway through SOS (Wada et al., 1998). Delphinidin pre-treated MCF-10A cells showed decreased phosphorylation of SHP-2 with concomitant inhibition of phosphorylation of Gab1 and activation of GRB2, demonstrating that delphinidin has the potential of blocking the activity of signaling complexes, required for its function downstream from Met (Fig. 1F).
Delphinidin inhibited HGF-induced phosphorylation and activation of Ras-ERK MAPK pathway
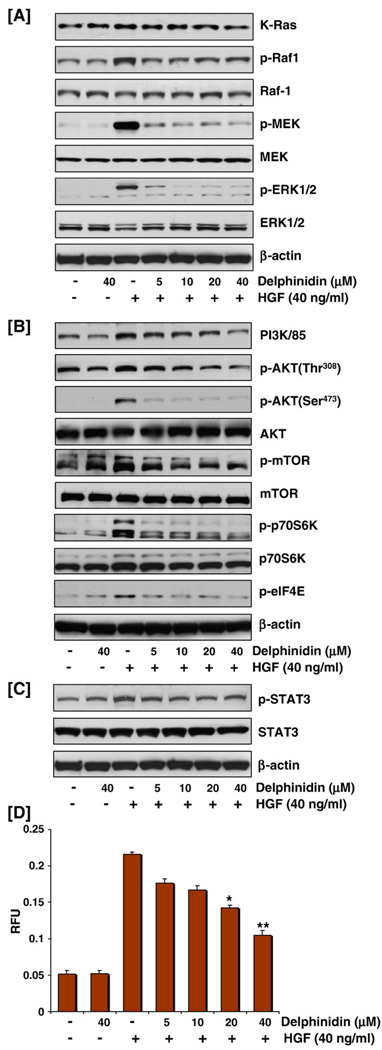
Ras-Raf-MEK-ERK cascade is a critical prosurvival signaling mechanism. H-Ras targets Raf-ERK cascade by integrating and transmitting extracellular signals from growth factor receptors to Raf. Upon activation, Raf phosphorylates MEK, which in turn activates ERK, leading to the propagation of signals to modulate a series of cellular survival events (Du et al., 2004). Indeed, significant phosphorylation of Raf-1, MEK1/2, and ERK1/2 was observed on stimulation with HGF. These responses were abolished by delphinidin at doses of 5–40 µm suggesting that delphinidin can inhibit HGF-induced prosurvival signals in MCF-10A cells (Fig. 2A).
Fig. 2.
Effect of delphinidin on cell signaling molecules involved in proliferation and invasion: (A) Delphinidin inhibited HGF-induced activation of Ras/Raf/MEK/ERK pathway through decreased phosphorylation of Raf, MEK and ERK (B) Delphinidin inhibited HGF-induced increase in the protein expression of PI3K85 and phosphorylation of AKT, mTOR, p70S6K and eIF4E. (C) Delphinidin inhibited HGF-induced phosphorylation of STAT3: MCF-10A cells, serum starved for 12 h and treated without or with delphinidin (5–40 µM) and stimulated with 40 ng/ml HGF for 30 min in serum-free media were harvested and total cell lysates were prepared for western blot analysis as detailed in 'Materials and methods'. Equal protein loading was confirmed by β-actin. The data shown here are from representative experiments repeated three times with similar results. (D) Delphinidin inhibited HGF-induced cell migration: Cell migration assay was performed using CHEMICON® QCM™ 24-well migration assay kit. MCF-10A cells treated with delphinidin (5–40 µM) were loaded onto the chamber. Conditioned media was added to each of the wells and cells were allowed to invade through the membrane by incubating at 37 °C for 14 h after which the invaded cells on the bottom of the insert membrane are detached, lysed and subsequently detected by CyQUANTâ GR dye using a fluorescence plate reader at 480/520 nm filter set. Data represent mean value±SE of three independent experiments; *P<0.05, HGF versus HGF treated with delphinidin, **P<0.001, HGF versus HGF treated with delphinidin.
Delphinidin inhibited HGF-induced phosphorylation and activation of PI3K/AKT pathway
HGF-driven survival requires the engagement of the PI3K/AKT in addition to the ERK MAPK transduction pathways (Fassetta et al., 2006). We therefore investigated the effect of delphinidin on the PI3K/AKT pathway in HGF-stimulated MCF10 cells. HGF induced rapid phosphorylation of AKT at serine and threonine residues, with a concomitant increase in the expression of the regulatory subunit of PI3K(p85) protein (Fig. 2B). It is further evident from the data that phosphorylation of AKT at both residues and therefore its activation as well as that of PI3K was almost completely suppressed by delphinidin at the selected doses. In addition, HGF-induced mTOR and p70S6K phosphorylation was also decreased in delphinidin pre-treated cells (Fig. 2B). These data suggest that delphinidin can significantly modulate the PI3K/AKT-mediated survival pathway thereby compromising cell proliferation and survival.
Delphinidin inhibited HGF-induced phosphorylation of STAT3
STAT3 activation by HGF is independent of PI3K or MAPKs and alters gene expression leading to changes in cellular shape and motility and is potentially involved in the control of invasive cell behavior (Zhang et al., 2002). HGF stimulation of MCF-10A cells evoked activation of STAT3 as evidenced by its phosphorylation (Fig. 2C), and delphinidin-mediated ablation of STAT3 expression may partly be responsible for a reduction of Met-driven migration and invasion (Fig. 2D), indicating an important role of delphinidin in the control of tumor progression.
The QCM™ fluorimetric cell migration assay was used to study the effect of delphinidin on HGF-stimulated cell migration. This assay employs a homogenous fluorescence detection format in which invaded cells on the bottom of the insert membrane are dissociated and subsequently lysed and detected by the CyQUANTâ GR dye. The advantage of this model over others is that this assay allows quantitative determination of cell migration. Data presented in Fig. 2D show that treatment of MCF-10A cells with HGF stimulated migration across the polycarbonate membrane and pre-treatment of MCF-10A cells with delphinidin blocked the ability of HGF/Met to induce cell motility and migration.
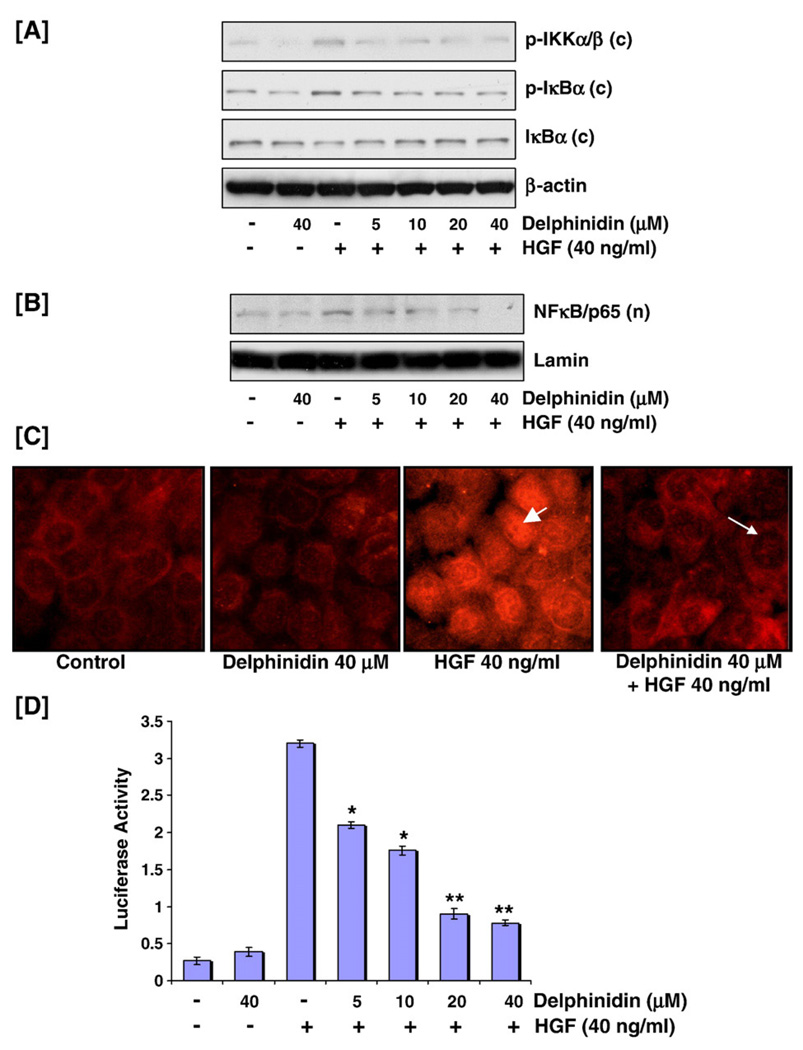
Delphinidin inhibited HGF-induced phosphorylation and activation of NF-κB/p65 pathway
It has been suggested that HGF-mediated activation of NF-κB and in turn cellular proliferation involves the AKT signaling pathway that is also dependent on Src (Fan et al., 2005). In our system, delphinidin treatment resulted in a decrease in HGF-mediated phosphorylation of IKKα/β and IκBα, and activation and nuclear translocation of NF-κB/p65, as shown by western blot analysis (Figs. 3A, B). Immunocytochemical analysis further elucidated this effect. In untreated cells or cells treated with delphinidin alone, cytoplasmic immunostaining was seen with anti-p65 antibody whereas cells treated with HGF showed an intense nuclear fluorescence (Fig. 3C). Predictably, delphinidin pretreated cells exposed to HGF showed a significant diminution in nuclear p65 immunostaining (Fig. 3C). Next, we investigated whether delphinidin can suppress HGF-induced NF-κB promoter activity. Transfection with a luciferase reporter plasmid containing an NF-κB-inducible promoter sequence showed increased reporter activity after HGF exposure that was specifically inhibited by delphinidin pre-treatment (Fig. 3D). Our data show that delphindin is capable of suppressing NF-κB signaling in MCF-10A cells, activation of which is considered essential for HGF-mediated proliferation (Muller et al., 2002).
Fig. 3.
Effect of delphinidin on HGF-induced activation of NF-κB/p65 pathway. (A) Delphinidin inhibited HGF-induced phosphorylation of IKKα/β and IκBα: MCF-10A cells, serum starved for 12 h and treated without or with delphinidin (5–40 µM) and stimulated with 40 ng/ml HGF for 30 min in serum-free media were harvested and cytosolic lysates were prepared for western blot analysis as detailed in 'Materials and methods'. Equal protein loading was confirmed by β-actin. The data shown here are from representative experiments repeated three times with similar results. (B, C) Delphinidin inhibited HGF-induced nuclear translocation of NFκB/p65: Nuclear extracts were prepared, as detailed earlier, for immunoblot analysis. Equal protein loading was evaluated by lamin. For immunocytochemical analyses MCF-10A cells were seeded in tissue culture slides and treated with HGF with/without delphinidin. Briefly, cells were fixed in 2% paraformaldehyde, and incubated with NF-κB/p65 antibody followed by incubation with donkey anti-rabbit Rhodamine Red TM-X-conjugated antibody. Samples were mounted using Prolong antifade kit and observed using a Zeiss Axiophot DM HT microscope. NF-κB/p65 translocation was detected in the nuclei of cells exposed to HGF alone (arrowhead) whereas delphinidin treatment effectively inhibited this translocation as determined by significant diminution in nuclear p65 immunostaining (arrow). (D) Delphinidin inhibited HGF-induced NFκB/p65 promoter activity: MCF-10A cells were seeded at a concentration of 1.5 × 105 cells per well in six-well plates and co-transfected with NF-κB-driven luciferase reporter construct and Renilla plasmid. After 48 h, cells were treated with HGF without or with delphinidin (5–40 µM) as detailed earlier. Luciferase activity was measured using luciferase reporter assay and normalized with respect to Renilla activity. Data represent mean value±SE of three independent experiments where *P<0.05, HGF versus HGF treated with delphinidin, **P<0.001, HGF versus HGF treated with delphinidin.
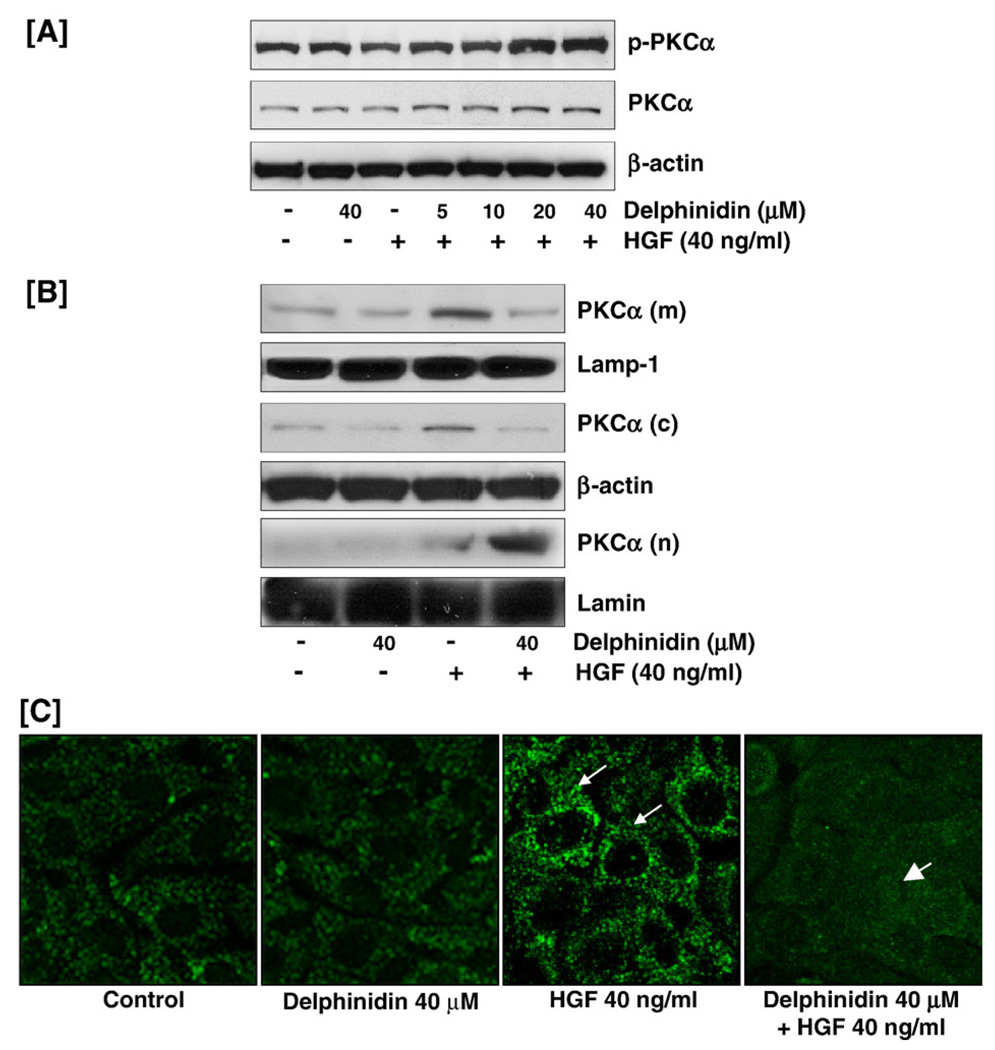
Delphinidin inhibited HGF-mediated membrane translocation and activation of PKCα
Since PKCα has been shown to be involved in the regulation of tumor growth and metastatic spread of breast cancer cells, we next determined the effect of delphinidin in the differential regulation of PKCα expression and distribution in MCF-10 cells. Initial experiments performed through western blot analysis of whole cell lysates showed a decrease in phosphorylation of PKCα with HGF treatment that was reversed by delphinidin treatment (Fig. 4A). To investigate this further, different cellular fractions were isolated as PKCα is thought to reside in the cytoplasm in an inactive conformation and to translocate to the plasma membrane or cytoplasmic organelles upon cell activation by different stimuli (Martelli et al., 2006). Western blot analysis showed that HGF activation of the Met receptor resulted in the translocation of PKCα from the cytosol to the membrane (Fig. 4B). Delphinidin appeared to reverse this effect so that PKCα was translocated from the membrane to the nuclei of the delphinidin treated cells. Immunofluorescent microscopy further showed that HGF-mediated translocation of PKCα to the membrane was inhibited by pre-treatment of the cells by delphinidin (Fig. 4C). This is important as delphinidin-mediated inactivation of PKCα may be responsible for reduced cell proliferation and neoplastic transformation of HGF-treated cells.
Fig. 4.
Effect of delphinidin on PKCα. (A) Delphinidin reversed HGF-induced decrease in PKCα phosphorylation phosphorylation: MCF-10A cells, serum starved for 12 h and treated without or with delphinidin (5–40 µM) and stimulated with 40 ng/ml HGF for 30 min in serum-free media were harvested and total cell lysates were prepared for western blot analysis as detailed in 'Materials and methods'. Equal protein loading was confirmed by β-actin. (B, C) Delphinidin inhibited HGF-induced activation and membrane translocation of PKCα: Membrane, cytosolic and nuclear extracts of MCF-10A cells treated with delphinidin/HGF were prepared, as detailed in 'Materials and methods', for western blot analysis. Equal protein loading was evaluated by lamp-1 (membrane), β-actin (cytosol) and lamin (nuclear) for the three fractions. For immunocytochemical analyses MCF-10A cells were seeded in tissue culture slides and treated with/without delphinidin and HGF, fixed in 2% paraformaldehyde, and incubated with p-PKCα antibody followed by incubation with anti-rabbit FITC tagged secondary antibody for 1 h. Samples were mounted using Prolong antifade kit and observed using a Zeiss Axiophot DM HT microscope. p-PKCα translocation was detected in the cell membranes exposed to HGF alone (arrow) whereas delphinidin treatment effectively inhibited this translocation as determined by significant diminution in nuclear p65 immunostaining (arrowhead). The data shown here are from representative experiments repeated three times with similar results.
Delphinidin treatment to breast cancer cells expressing the Met receptor resulted in a decrease in cell viability
The above mentioned experiments led us to believe that delphinidin may be an important regulator of cell proliferation and invasion in cancer cells expressing the HGF receptor. For this effect, we first determined the endogenous levels of protein expression of phosphorylated (data not shown) and unphosphorylated Met in exponentially growing human breast cancer cells and normal mammary epithelial cells (Fig. 5A). We next selected breast cancer cell lines representing clinically relevant subtypes of human breast cancer in which Met was expressed at high levels. We found that treatment of MDA-MB-231 cells and the highly invasive HCC1419 cell line with delphinidin resulted in a dose-dependent inhibition of cell growth when assessed at 48 h post-treatment (Figs. 5B, C). This is significant as it adds credence to our initial data that shows that delphinidin can be an effective agent for the suppression of activated HGF/Met signaling.
Fig. 5.
Delphinidin inhibited the growth of breast cancer cells. (A) Met expression in various breast cancer cell lines: Cells, cultured in recommended media were harvested and total cell lysates were prepared for western blot analysis as detailed in 'Materials and methods'. (BC) Delphinidin treatment to MDA-MB-231 and HCC1419 cells resulted in a dose-dependent decrease in cell viability: Cells were treated with specified concentrations of delphinidin for 48 h and cell viability was determined by MTT assay as detailed in 'Materials and methods'. Data represent mean value of percent viable cells±SE of three independent experiments, where vehicle-treated cells were regarded as 100% viable. P<0.05 was considered statistically significant.
Discussion
Signals from tumor microenviroment and cancer cells are transformed into a biological response through transmembrane receptors. Agents that can target tumor at one or more of these levels: the microenvironment, receptor-based signals, and signal transducers can play a crucial role against cancer. Of these, receptor-based signal inhibition seems to be the most promising approach to develop cancer therapeutics (Mazzone and Comoglio, 2006). It is known that several types of cancer cells secrete molecules that enhance HGF production, the only natural ligand for the Met tyrosine kinase receptor, a product of the c-Met proto-oncogene. Activation of Met-mediated oncogenic signaling occurs most commonly through aberrant paracrine HGF stimulation and consequent receptor overexpression or through autocrine HGF production, receptor mutation, or gene amplification or rearrangement. Genetic alterations leading to an overexpression of HGF are associated with the development of epithelial neoplasia and invasive carcinoma (Matsumoto and Nakamura, 2006). Indeed, it has been shown that increased expression of HGF and its receptor, Met, occurs in invasive human breast cancer, particularly at the migrating tumor front, and that high levels of HGF and Met expression correlate with poor survival of breast cancer patients (Qiao et al., 2000).
Increased cell proliferation, survival, motility, and extracellular matrix degradation resulting from pathway activation contributes to tumor growth, invasiveness, and metastasis. Following ligand binding and autophosphorylation, Met transmits intercellular signals using a unique multisubstrate docking site present within the C-terminal end of the receptor. Met docking site is responsible for the recruitment of a wide spectrum of downstream signal transducers including PI3K, the GRB2/SOS complex, the nonreceptor tyrosine kinase Src, the adaptors Shc and Gab-1, and the transcriptional factor STAT3. Also documented is the role of FAK in promoting HGF-induced cell migration (Lai et al., 2000). In addition, many metastatic human cancers have elevated levels of FAK (Mazzone and Comoglio, 2006). Thus, the current understanding of oncogenesis mediated by Met signaling supports at least three possibilities of intervention: antagonism of ligand/receptor interaction, inhibition of tyrosine kinase catalytic activity, and blockade of receptor/effector interactions. Although, it is at present not clear if the inhibitory effect of delphinidin is indeed mediated through antagonism of ligand binding nonetheless the inhibitory effect on the kinase catalytic function is apparent through its suppression of phosphorylation of the Met receptor with a concomitant dose-dependent decrease in the total protein levels (Fig. 1B). A compound with four hydroxyl groups (3, 3′, 4′ and 5′), delphinidin has recently been shown to be a potent inhibitor of vascular endothelial growth factor receptor activity (Lamy et al., 2006). The presence of vicinal hydroxyl substituents on the B-ring of delphinidin seems to be crucial for interaction with EGFR, as tyrosine kinase activity associated with this cell surface receptor is inhibited by delphinidin in human breast cancer cells (Oak et al., 2006). This is significant as inhibition of downstream effectors of the activated receptors by delphinidin may disturb vital biological cues and hinder the progression of carcinogenesis. In this context, our data demonstrated that delphinidin was able to block the interactions between the activated HGF receptor Met and the downstream intracellular effectors at varying levels (Fig. 1).
Concomitant activation of multiple pathways accounts for the complexity of the resulting biological response to HGF. The Ras-ERK MAPK pathway has been shown to be mostly associated with HGF mitogenic and morphogenic effect, whereas the PI3K/AKT pathway seems to be mainly related to motogenic and antiapoptotic effects (Birchmeier et al., 2003). Our data indicate that delphinidin has the potential to halt cellular growth via targeting overlapping signaling cascades in HGF-stimulated cells. As observed in Figs. 2A and B delphinidin inhibited the activation of PI3K/AKT pathway as well as abrogated the phosphorylation of MAPKs in a sequential manner. In addition, delphinidin was able to inhibit the nuclear translocation of NF-κB/p65 and prevent the degradation of IκBα/β in activated MCF-10A cells (Fig. 3). HGF has been shown to be involved not only in stimulating cell growth but also in modifying enzyme activities and the expression and distribution of proteins. PKCα is involved in the regulation of several important biological processes such as cell proliferation and differentiation, neoplastic transformation, and apoptosis. PKC-dependent NF-κB activation is required for matrix metalloproteinase-9 induction in hepatocellular carcinoma cells (Awasthi and King, 2000). PKCα levels may especially be increased in breast cancer patients with low or negative estrogen receptor levels (Lahn et al., 2004). Activation of PKCα by ErbB2 through Src has been shown to promote breast cancer cell invasion that can be blocked by combined treatment with PKCα and Src inhibitors (Tan et al., 2006). There is evidence that loss of PKC function contributes to the anti-proliferative action of a number of anticancer compounds (Musashi et al., 2000). Our data show that delphinidin inhibited the activation of PKCα through blocking HGF-mediated membrane translocation of the enzyme thereby inhibiting cell proliferation and invasion (Fig. 4).
The involvement of Met in human tumors has been definitively established and can be achieved through several mechanisms, including Met interaction with unrelated membrane receptors, such as integrins, plexins, CD44, FAS and other receptor tyrosine kinases. There is evidence that epidermal growth factor, cooperates with HGF to promote a highly invasive phenotype via the increased secretion of MMP-9 in human ovarian cancer cells (Zhou et al., 2007). Therefore, it would be naive to consider Met as the only regulator of invasive growth. Several cytokines and growth factors can induce proliferation, differentiation, chemotaxis, migration, and protection from apoptosis. Thus, there is a need for the development of agents that are able to inhibit key molecules at each of steps that are involved in the complex and multistep process of carcinogenesis. Additionally, inhibition of signaling axes at different levels may provide a strategy to prevent and overcome resistance that ultimately occurs with most of the compounds used in clinical practice. Delphinidin seems to act as a multifunctional anticancer agent through its inhibitory effect on several aspects of both tumor growth and tumor angiogenesis. Indeed delphinidin was shown to be a potent inhibitor of glioblastoma cell migration. This effect is though to be mediated through a decrease in the expression of both urokinase-type plasminogen activator receptor and the low-density lipoprotein receptor-related protein, at the transcriptional levels (Lamy et al., 2007). Moreover, there is evidence that induction of apoptosis by anthocyanidins is a pivotal mechanism of their cancer chemopreventive functions (Yeh and Yen, 2005). Consistent with this, cell viability studies demonstrated that delphinidin treatment to highly invasive HCC1419 and MDA-MB-231 breast cancer cells resulted in a decrease in the viability of these cells (Fig. 5).
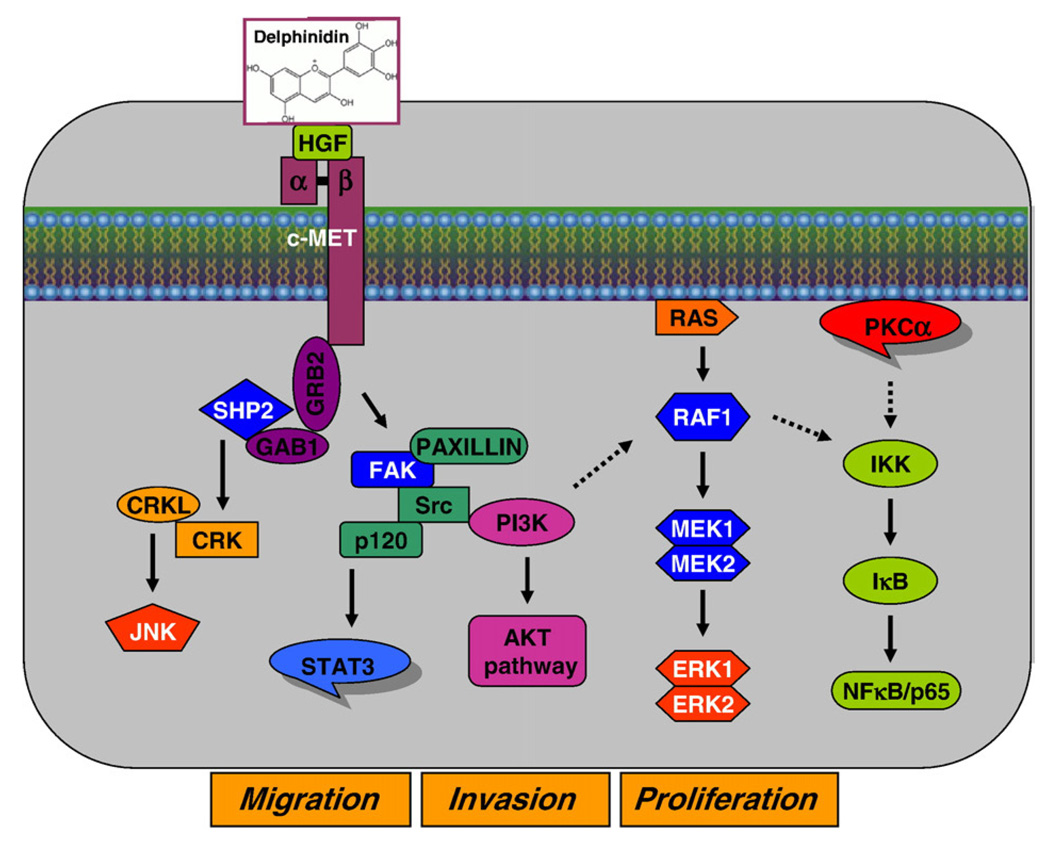
In conclusion, we have demonstrated that delphinidin can inhibit HGF-induced early biochemical effects, such as receptor tyrosine phosphorylation and upregulation, activation of the major signaling pathways, as well as delayed biological responses, namely block of proliferation and cell migration (Fig. 6). We showed that these effects were dependent on HGF stimulation, as induction of MCF-10A cells with exogenous HGF resulted in these responses and could be inhibited by pre-treatment of cells with delphinidin. Finally, we presented evidence that delphinidin treatment resulted in growth inhibition of HGF expressing HCC1419 and MDA-MB-231 breast cancer cells. Our studies reveal the possible benefits of delphinidin in preventing tumor development and malignant progression through inhibition of several major pathways involved in breast oncogensis. This is important as HGF/Met signaling in addition to oncogenesis has also been implicated in chemo- and radio-resistance of tumors (Matsumoto and Nakamura, 2006). Combination of HGF/Met inhibitors with the current cancer drugs could result in a more effective but less toxic therapy.
Fig. 6.
Schematic representation of the inhibitory effect of delphinidin against HGF-mediated signaling in immortalized human breast epithelial MCF-10A cells.
Acknowledgments
This study was supported by the United States Public Health Service Grants RO1 CA 78809, RO1 CA 101039, RO1 CA 120451 and P50 DK065303.
References
- Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- Awasthi V, King RJ. PKC, p42/p44 MAPK, and p38 MAPK are required for HGF-induced proliferation of H441 cells. Am. J. Physiol., Lung Cell. Mol. Physiol. 2000;279:L942–L949. doi: 10.1152/ajplung.2000.279.5.L942. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat. Rev., Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Du J, Cai SH, Shi Z, Nagase F. Binding activity of H-Ras is necessary for in vivo inhibition of ASK1 activity. Cell Res. 2004;14:148–154. doi: 10.1038/sj.cr.7290214. [DOI] [PubMed] [Google Scholar]
- Elliott BE, Hung WL, Boag AH, Tuck AB. The role of hepatocyte growth factor (scatter factor) in epithelial-mesenchymal transition and breast cancer. Can. J. Physiol. Pharm. 2002;80:91–102. doi: 10.1139/y02-010. [DOI] [PubMed] [Google Scholar]
- Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–1766. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- Fassetta M, D'Alessandro L, Coltella N, Di Renzo MF, Rasola A. Hepatocyte growth factor installs a survival platform for colorectal cancer cell invasive growth and overcomes p38 MAPK-mediated apoptosis. Cell. Signal. 2006;18:1967–1976. doi: 10.1016/j.cellsig.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Binding activity of H-Ras is necessary for in vivo inhibition of ASK1 activity. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- Hah N, Lee ST. An absolute role of the PKC-dependent NF-kappaB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2003;305:428–433. doi: 10.1016/s0006-291x(03)00788-5. [DOI] [PubMed] [Google Scholar]
- Kermorgant S, Aparicio T, Dessirier V, Lewin MJ, Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001;22:1035–1042. doi: 10.1093/carcin/22.7.1035. [DOI] [PubMed] [Google Scholar]
- Lahn M, Kohler G, Sundell K, Su C, Li S, Paterson BM, Bumol TF. Protein kinase C alpha expression in breast and ovarian cancer. Oncology. 2004;67:1–10. doi: 10.1159/000080279. [DOI] [PubMed] [Google Scholar]
- Lai JF, Kao SC, Jiang ST, Tang MJ, Chan PC, Chen HC. Involvement of focal adhesion kinase in hepatocyte growth factor-induced scatter of Madin–Darby canine kidney cells. J. Biol. Chem. 2000;275:7474–7480. doi: 10.1074/jbc.275.11.7474. [DOI] [PubMed] [Google Scholar]
- Lamorte L, Royal I, Naujokas M, Park M. Crk adapter proteins promote an epithelial–mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol. Biol. Cell. 2002;13:1449–1461. doi: 10.1091/mbc.01-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy S, Blanchette M, Michaud-Levesque J, Lafleur R, Durocher Y, Moghrabi A, Barrette S, Gingras D, Beliveau R. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis. 2006;27:989–996. doi: 10.1093/carcin/bgi279. [DOI] [PubMed] [Google Scholar]
- Lamy S, Lafleur R, Bédard V, Moghrabi A, Barrette S, Gingras D, Béliveau R. Anthocyanidins inhibit migration of glioblastoma cells: structure–activity relationship and involvement of the plasminolytic system. J. Cell. Biochem. 2007;100:100–111. doi: 10.1002/jcb.21023. [DOI] [PubMed] [Google Scholar]
- Lindemann K, Resau J, Nahrig J, Kort E, Leeser B, Annecke K, Welk A, Schafer J, Vande Woude GF, Lengyel E, Harbeck N. Differential expression of c-Met, its ligand HGF/SF and HER2/neu in DCIS and adjacent normal breast tissue. Histopathology. 2007;51:54–62. doi: 10.1111/j.1365-2559.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Nyakern M, Manzoli FA. Nuclear protein kinase C. Biochim. Biophys. Acta. 2006;1761:542–551. doi: 10.1016/j.bbalip.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int. J. Cancer. 2006;119:477–483. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- Mazzone M, Comoglio PM. The Met pathway: master switch and drug target in cancer progression. FASEB J. 2006;20:1611–1621. doi: 10.1096/fj.06-5947rev. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Muller M, Morotti A, Ponzetto C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol. Cell. Biol. 2002;22:1060–1072. doi: 10.1128/MCB.22.4.1060-1072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int. J. Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini-Kerth VB. Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br. J. Pharmacol. 2006;149:283–290. doi: 10.1038/sj.bjp.0706843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin. Cancer Res. 2004;10:202–211. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin. Cancer Res. 2006;12:3657–3660. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- Qiao H, Saulnier R, Patryzkat A, Rahimi N, Raptis L, Rossiter J, Tremblay E, Elliott B. Cooperative effect of hepatocyte growth factor and fibronectin in anchorage-independent survival of mammary carcinoma cells: requirement for phosphatidylinositol 3-kinase activity. Cell Growth Differ. 2000;11:123–133. [PubMed] [Google Scholar]
- Sattler M, Salgia R. c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr. Oncol. Rep. 2007;9:102–108. doi: 10.1007/s11912-007-0005-4. [DOI] [PubMed] [Google Scholar]
- Tan M, Li P, Sun M, Yin G, Yu D. Upregulation and activation of PKC alpha by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKC alpha and Src inhibitors. Oncogene. 2006;25:3286–3295. doi: 10.1038/sj.onc.1209361. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Katsouyanni K, Stuver S, Tzala L, Gnardellis C, Rimm E, Trichopoulos D. Consumption of olive oil and specific food groups in relation to breast cancer risk in Greece. J. Natl. Cancer Inst. 1995;87:110–116. doi: 10.1093/jnci/87.2.110. [DOI] [PubMed] [Google Scholar]
- Wada S, Sasaki Y, Horimoto M, Ito T, Ito Y, Tanaka Y, Toyama T, Kasahara A, Hayashi N, Hori M. Involvement of growth factor receptor-bound protein-2 in rat hepatocyte growth. J. Gastroenterol. Hepatol. 1998;13:635–642. doi: 10.1111/j.1440-1746.1998.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tsuda M, Makino Y, Ichihara S, Sawa H, Minami A, Mochizuki N, Nagashima K, Tanaka S. Adaptor molecule Crk is required for sustained phosphorylation of Grb2-associated binder 1 and hepatocyte growth factor-induced cell motility of human synovial sarcoma cell lines. Mol. Cancer Res. 2006;4:499–510. doi: 10.1158/1541-7786.MCR-05-0141. [DOI] [PubMed] [Google Scholar]
- Yeh CT, Yen GC. Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J. Agric. Food Chem. 2005;53:1740–1749. doi: 10.1021/jf048955e. [DOI] [PubMed] [Google Scholar]
- Zaman N, Afaq F, Khan N, Syed DN, Mukhtar H. Delphinidin, a major anthocyanin in pigmented fruits and vegetables is a potent inhibitor of epidermal growth factor receptor and its downstream signaling pathway. Proc. Am. Assoc. Cancer Res. 2006;47:2266. [Google Scholar]
- Zhang YW, Wang LM, Jove R, Vande Woude GF. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Pon YL, Wong AS. Synergistic effects of epidermal growth factor and hepatocyte growth factor on human ovarian cancer cell invasion and migration: role of ERK1/2 and p38 MAPK. Endocrinology. 2007;148:5195–5208. doi: 10.1210/en.2007-0361. [DOI] [PubMed] [Google Scholar]