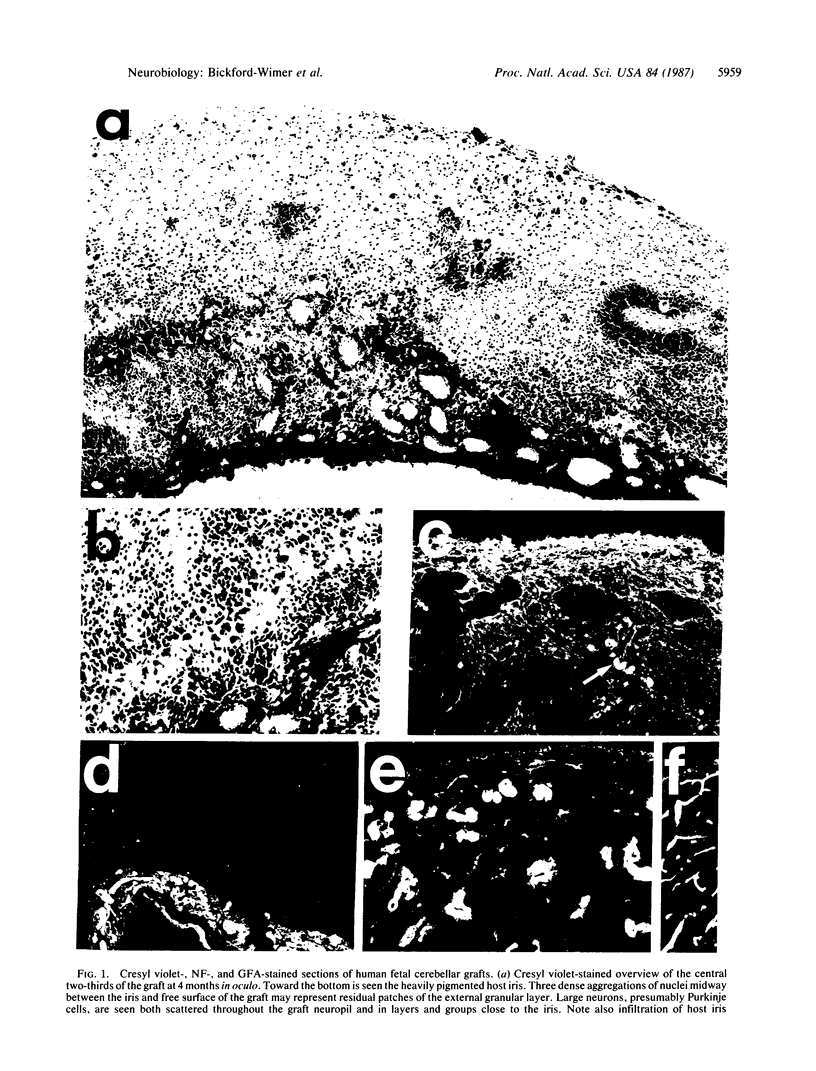
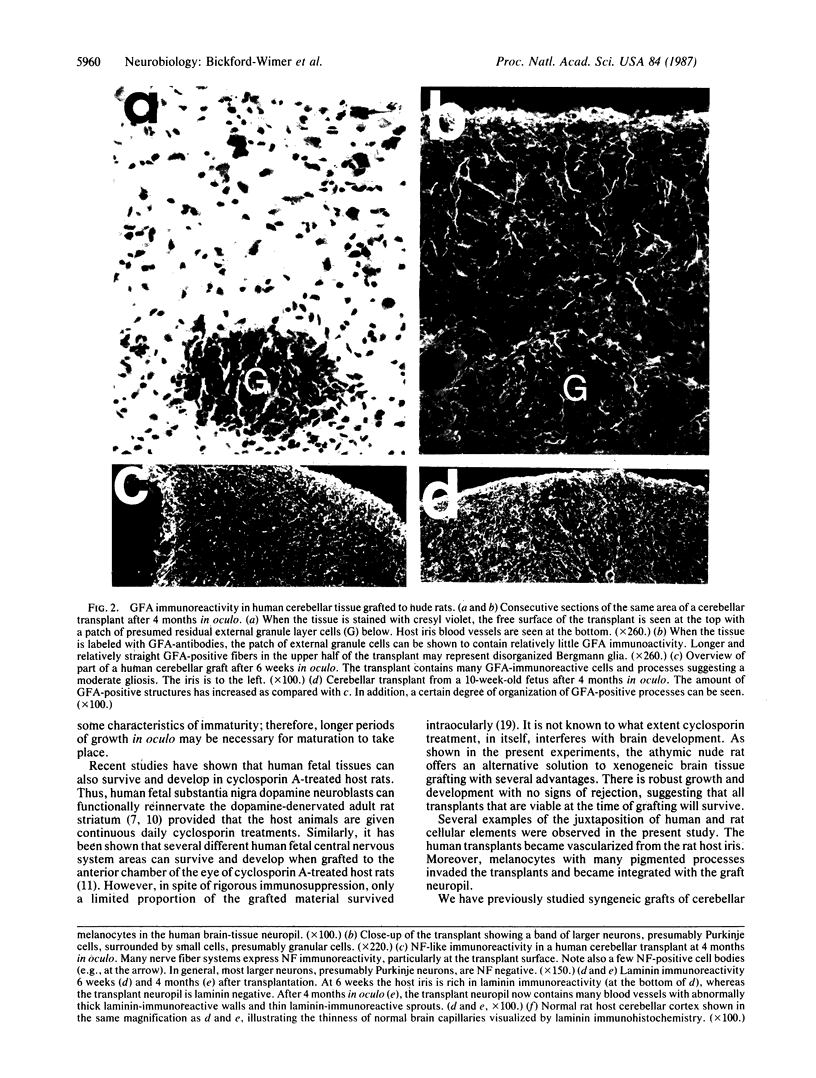
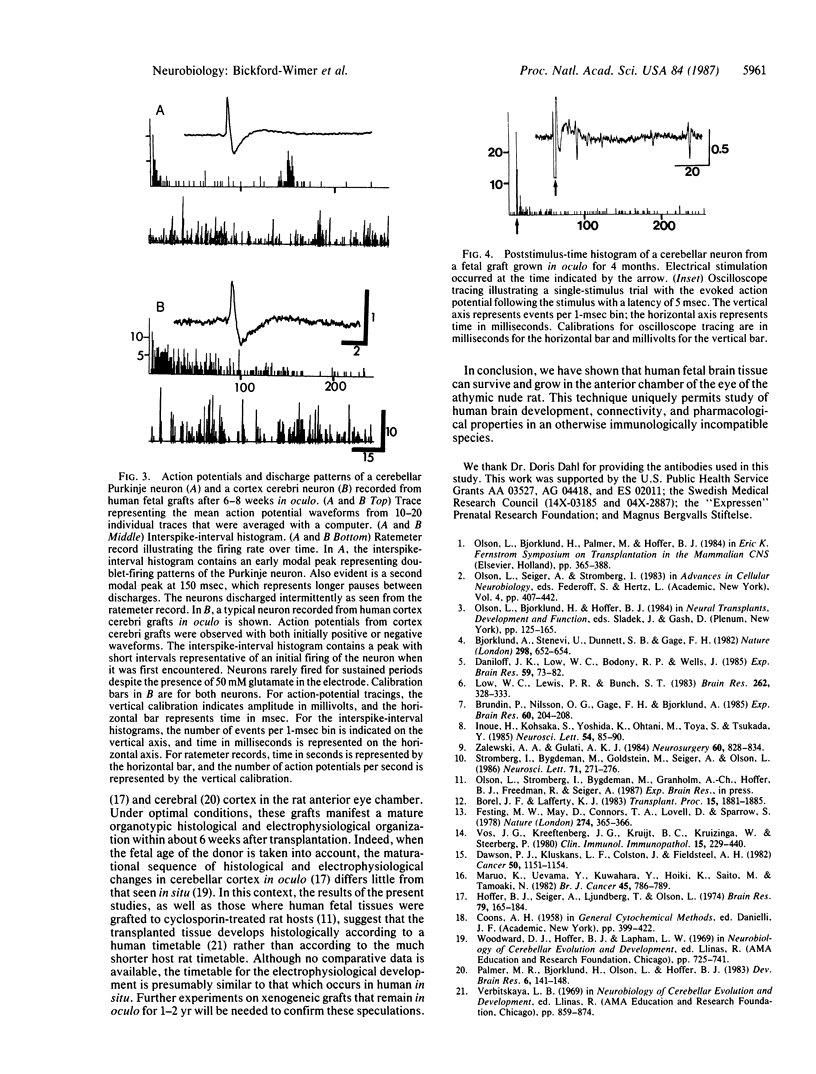
Abstract
Human fetal tissue fragments from cortex cerebri and cerebellum were grafted to the anterior chamber of the eye of adult athymic nude rats. The grafts were obtained from tissue fragments recovered after elective routine abortions, performed in weeks 8-11 of gestation. Both cerebellar and cortex cerebri grafts survived and developed in the anterior chamber of the eye for 1-4 months. The transplants slowly became vascularized from the host iris. The grafts developed blood vessels with laminin-immunoreactive walls and contained relatively high amounts of glial fibrillary acidic protein- and neurofilament-immunoreactivity in the neuropil after 4 months in oculo. Recordings of extracellular action potentials from the grafts revealed spontaneously active neurons with action-potential waveforms similar to those observed in immature rodents. Morphologically, the grafts showed no signs of rejection. Clusters and bands of large neurons resembling Purkinje cells and dense aggregates of smaller granule-like cells could be found in the cerebellar grafts. Large neurons were also seen in the cortex grafts. Taken together, these data suggest that the athymic rat may serve as a useful tool for studies of central nervous system tissue from otherwise immunologically incompatible species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björklund A., Stenevi U., Dunnett S. B., Gage F. H. Cross-species neural grafting in a rat model of Parkinson's disease. Nature. 1982 Aug 12;298(5875):652–654. doi: 10.1038/298652a0. [DOI] [PubMed] [Google Scholar]
- Brundin P., Nilsson O. G., Gage F. H., Björklund A. Cyclosporin A increases survival of cross-species intrastriatal grafts of embryonic dopamine-containing neurons. Exp Brain Res. 1985;60(1):204–208. doi: 10.1007/BF00237035. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Daniloff J. K., Low W. C., Bodony R. P., Wells J. Cross-species neural transplants of embryonic septal nuclei to the hippocampal formation of adult rats. Exp Brain Res. 1985;59(1):73–82. doi: 10.1007/BF00237668. [DOI] [PubMed] [Google Scholar]
- Dawson P. J., Kluskens L. F., Colston J., Fieldsteel A. H. Transplantation of human malignant tumors to the athymic rat. Cancer. 1982 Sep 15;50(6):1151–1154. doi: 10.1002/1097-0142(19820915)50:6<1151::aid-cncr2820500619>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Festing M. F., May D., Connors T. A., Lovell D., Sparrow S. An athymic nude mutation in the rat. Nature. 1978 Jul 27;274(5669):365–366. doi: 10.1038/274365a0. [DOI] [PubMed] [Google Scholar]
- Hoffer B., Seiger A., Ljungberg T., Olson L. Electrophysiological and cytological studies of brain homografts in the anterior chamber of the eye: maturation of cerebellar cortex in oculo. Brain Res. 1974 Oct 18;79(2):165–184. doi: 10.1016/0006-8993(74)90409-0. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kohsaka S., Yoshida K., Ohtani M., Toya S., Tsukada Y. Cyclosporin A enhances the survivability of mouse cerebral cortex grafted into the third ventricle of rat brain. Neurosci Lett. 1985 Feb 28;54(1):85–90. doi: 10.1016/s0304-3940(85)80122-1. [DOI] [PubMed] [Google Scholar]
- Low W. C., Lewis P. R., Terri S. T. Embryonic neural transplants across a major histocompatibility barrier: survival and specificity of innervation. Brain Res. 1983 Mar 7;262(2):328–333. doi: 10.1016/0006-8993(83)91028-4. [DOI] [PubMed] [Google Scholar]
- Maruo K., Ueyama Y., Kuwahara Y., Hioki K., Saito M., Nomura T., Tamaoki N. Human tumour xenografts in athymic rats and their age dependence. Br J Cancer. 1982 May;45(5):786–789. doi: 10.1038/bjc.1982.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M., Björklund H., Olson L., Hoffer B. Trophic effects of brain areas on the developing cerebral cortex: II. Electrophysiology of intraocular grafts. Brain Res. 1983 Jan;282(2):141–148. doi: 10.1016/0165-3806(83)90092-5. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Bygdeman M., Goldstein M., Seiger A., Olson L. Human fetal substantia nigra grafted to the dopamine-denervated striatum of immunosuppressed rats: evidence for functional reinnervation. Neurosci Lett. 1986 Nov 21;71(3):271–276. doi: 10.1016/0304-3940(86)90632-4. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Kreeftenberg J. G., Kruijt B. C., Kruizinga W., Steerenberg P. The athymic nude rat. II. Immunological characteristics. Clin Immunol Immunopathol. 1980 Feb;15(2):229–237. doi: 10.1016/0090-1229(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Zalewski A. A., Gulati A. K. Survival of nerve allografts in sensitized rats treated with cyclosporin A. J Neurosurg. 1984 Apr;60(4):828–834. doi: 10.3171/jns.1984.60.4.0828. [DOI] [PubMed] [Google Scholar]