Abstract
Actinic keratosis (AK), the initial lesion in a disease continuum that may progress to squamous cell carcinoma, often begins with ultraviolet B light-induced photo damage and increases in prevalence with age. Topical 5-fluorouracil (5-FU) for the treatment of widespread multiple AK lesions has cure rates of more than 90 percent. The associated skin irritation, however, may lead patients to prematurely discontinue treatment. To improve treatment efficacy and tolerability, 5-FU cream 0.5%, a novel Microsponge®-based formulation, was developed. In the elderly population, 5-FU cream 0.5% may be preferable because of its convenient (once daily) administration and its lower potential for irritation and systemic absorption, which may impact adherence to therapy and, thus, possibly increase cure rates.
Actinic keratoses (AKs), also called solar keratoses, occur more commonly in individuals with fair skin who have had extensive exposure to the sun.1,2 The prevalence of AK lesions in adults increases with age: less than 10 percent for 20- to 29-year-olds, approximately 80 percent for 60- to 69-year-olds, and greater than 80 percent for 70-year-olds and older.3,4 In the first five decades of life, the prevalence of AK lesions in men is almost twice that in women; however, after age 50, prevalence rates are similar between men and women.3,4 A study using data from the National Ambulatory Medical Care Survey from 1990 to 1999 found that 47 million visits to physicians involved an AK diagnosis, and more than 80 percent of patients with AK diagnoses were at least 50 years old.5 AK was the second most frequent diagnosis made by dermatologists.5 Because surveys and studies typically include only patients seen in clinics or by physicians, the actual number of patients with AK is probably higher.6 The incidence of AK is higher in individuals living close to the equator, but is increasing in men and women in general.7 Table 1 summarizes risk factors for AK.8
Table 1.
Risk factors for actinic keratoses8
| Fair skin, with light-colored eyes and red or blonde hair |
| Inability to tan, tendency to sunburn (Fitzpatrick I-II) |
| Chronic, cumulative exposure to ultraviolet radiation |
| Advancing age |
| Outdoor occupation or leisure activities |
| Residence in sunbelt latitudes |
| Previous nonmelanoma skin cancer (SCC and BCC)* |
| Immunosuppression |
| Genetic predisposition |
- SCC =
- squamous cell carcinoma
- BCC =
- basal cell carcinoma
Clinical Features of AK
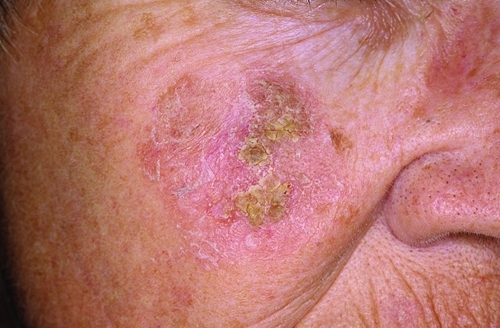
Patients with AK typically present with multiple, polymorphous, sometimes confluent lesions that appear as rough, scaly, variably erythematous, brown and/or tan, crusted papules or plaques (Figure 1). AK lesions are found on sun-exposed skin, including the face, neck, chest, ear lobes, backs of hands and arms, and bald scalp.9 Surrounding areas may show evidence of chronic sun damage, with telangiectasia, actinic collagenosis, and dyschromia.9 The lesions, which usually range from 1 to 3mm in diameter, can become considerably larger and most often can be felt before they are seen.8,9
Figure 1.
Typical presentation of a patient with actinic keratosis lesions. (Photo courtesy of Dermnet.com.)
Progression of AK to cancerous lesions. Visible AK lesions appear to be the first clinically evident sign of a disease continuum that begins with chronic unprotected exposure to ultraviolet (UV) light, primarily UVB, and has the potential to progress to squamous cell carcinoma (SCC).8 Unlike AK, SCC has the potential to metastasize; thus, AKs have been described as precancerous lesions. This terminology, however, is controversial, as many experts consider AK to be SCC in situ and as malignant as other neoplasias that, if left untreated, would almost certainly progress to malignancy.8,10 The inflammation that is intrinsic to photoaged skin is also a trigger for the progression from AK to SCC.8 Histopathologic analysis has demonstrated the presence of contiguous AK in more than 90 percent of SCC cases.11 In addition, AK lesions share clinical, histologic, and molecular features with SCC, including genetic and subsequent protein synthesis abnormalities.10,12 The p53 gene may act as a primary tumor suppressor gene and, when damaged, allows the growth of neoplastic cells. Mutations in the p53 gene are seen in 53 percent of AK lesions and 69 percent of SCC cases.11
Currently, no method exists to identify which AK lesions will progress to SCC. Estimates of the risk of progression of any AK lesion to SCC have ranged from 0.025 to 16.0% per year; the inclusion of younger patients (<50 years of age) in some studies may lead to underestimation.13 A database analysis by Chen et al studied more than 25,000 subjects with a mean age of 78 years, 88 percent of whom were Caucasian.14 The investigators concluded that the subjects with AK were 6.7 times more likely to develop skin cancer than those without AK (P<.01). With each year of life, the odds increased by 1.03 (P .01). The risk of AK progression to SCC may depend on characteristics similar to those that increase the risk of AK itself.8 Because no conclusive criteria exist to distinguish an AK lesion from SCC, treatment of all AK lesions is recommended to avoid possible progression to SCC.15
Treating AKs
Several treatments are available for AK, including nonpharmacologic therapies, pharmacologic therapies, and a combination of the two. Isolated AK lesions in low numbers are most commonly treated with destructive therapies, such as cryotherapy, aminolevulinic acid followed by photodynamic therapy, curettage with or without electrodissication, and excisional surgery.7,16 Dermabrasion, chemical peels, and laser resurfacing are used less frequently.7,16 Approved topical therapies for multiple, diffuse AK lesions include 5-fluorouracil (5-FU), diclofenac, and imiquimod.16 In a head-to-head study with imiquimod applied for 16 weeks and 5% 5-FU applied for 2 to 4 weeks, 5% 5-FU demonstrated greater efficacy.17 Diclofenac applied for 90 days versus 5% 5-FU applied for 30 days resulted in a greater clearance of AK lesions in the 5% 5-FU group.18 5-FU remains the most efficacious therapy for AK, with a shorter duration of therapy needed to obtain these favorable results.
Efficacy and Tolerability of Topical 5-FU
Topical 5-FU has been the cornerstone of treatment for widespread multiple AK lesions for several decades and continues to be a mainstay of therapy.17 Once inside cells, 5-FU undergoes ribosylation and phosphorylation, similar to a natural nucleotide.19 It then binds to thymidylate synthetase, which is inhibited and cannot convert deoxyuridine nucleotides to thymidine nucleotides.19 The thymidine depletion results in reduced DNA synthesis. Essentially, 5-FU acts as a “false base.” Because the dysplastic and neoplastic cells in AK lesions have a higher DNA requirement than normal cells, 5-FU acts preferentially to cause apoptosis in actinic lesions but not in healthy skin.19–21
Although cure rates of more than 90 percent have been associated with topical 5-FU treatment for AK in patients utilizing and tolerating full courses of treatment,22 adverse effects, including irritation, inflammation, erythema, and erosions, may cause patients to discontinue treatment prematurely, thereby reducing cure rates.23 Persistence in following the recommended therapy may increase efficacy, with lower levels of inflammation and discomfort. Various treatment vehicles have been used in an attempt to reduce treatment-associated irritation while maintaining efficacy.24,25
A meta-analysis of seven studies with topical 5-FU (all 2–4 weeks in duration) yielded an average AK lesion response rate of 87.8%±2.2% and an average complete patient response rate of 62.5%±12.0%.26 The analysis included concentrations of 5-FU cream 0.5%, 1%, and 5%.
Reducing Medication Exposure to Improve Adherence and Tolerability
5-FU cream 0.5% (Carac®, Dermik Laboratories, Bridgewater, New Jersey), incorporating the Microsponge® delivery system (Cardinal Health, Dublin, Ohio), is indicated for the treatment of AK on the face and anterior scalp. This technology provides a unique base for topical formulations, permitting a concentrated delivery of the active ingredient and potentially allowing less systemic absorption, which may lead to reduced systemically associated adverse effects.27–29
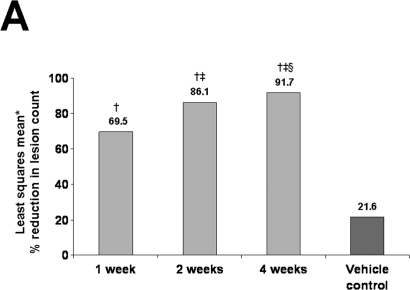
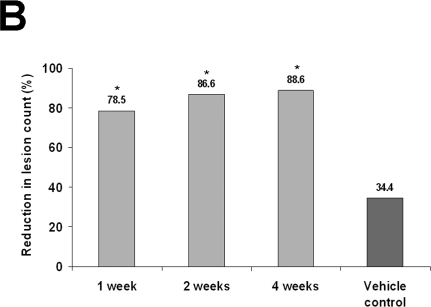
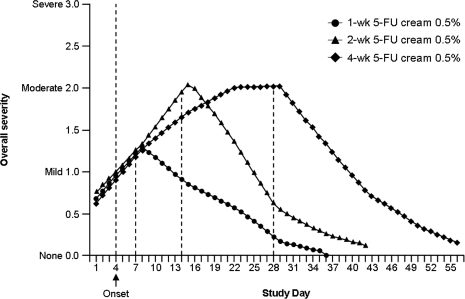
In two identically randomized, double-blind, vehicle-controlled, Phase 3 studies, 384 patients (mean age, 64 years) with AK were randomized to 5-FU cream 0.5% or vehicle once daily for 1, 2, or 4 weeks.30–32 Primary efficacy variables were the reduction in the number of AK lesions from baseline to the final visit and the percentage of patients who achieved total AK clearance at follow-up four weeks after treatment.30,31 In both studies, 5-FU cream 0.5% was significantly more effective than vehicle control (P<.001). Reductions in the number of AK lesions from baseline to Weeks 1, 2, and 4 in the first study were 69.5 percent, 86.1 percent, and 91.7 percent, respectively, for 5-FU cream 0.5% versus 21.6% for vehicle30 and in the second study, 78.5 percent, 83.6 percent, and 88.7 percent, respectively, for 5-FU cream 0.5% versus 34.4% for vehicle31 (P<.001 for both studies at all treatment weeks; Figures 2A and 2B). Total clearance of AK lesions in the first study achieved at Weeks 1, 2, and 4 was 14.9 percent, 37.0 percent, and 57.8 percent, respectively, for 5-FU cream 0.5% versus 0% for vehicle (P<.001 for all treatment weeks)30 and in the second study, 26.3 percent, 19.5 percent, and 47.5 percent, respectively, for 5-FU cream 0.5% versus 3.4% for vehicle (P<.01 for all treatment weeks).31 Patients treated for one week experienced significant improvements in the reduction of number of AK lesions and total clearance of lesions. Efficacy continued to increase after one week of therapy. Facial irritation, the most commonly occurring adverse event, was noted in approximately 95 percent of the total treatment groups and 65 percent of the vehicle groups; however, mild-to-moderate facial irritation was already present in a large percentage of patients at baseline. In both studies, facial irritation was described as mild or moderate in severity in most patients. A total of five patients from the first study and three patients from the second study withdrew because of facial irritation. Facial irritation declined quickly after treatment to be either equal to or below the average baseline facial irritation severity level. Overall, the irritation response increased during the first two weeks of treatment but demonstrated no further increase during the third and fourth weeks of treatment (Figure 3).30,31
Figures 2A and 2B.
Shown are (A) the least squares mean30 and (B) the mean percentage reductions in actinic keratosis lesions for 5-FU cream 0.5% and vehicle control.31
Footnotes: (A) *Least squares mean is a method of predicting the difference between observed and predicted response for a variable that is a function of ≥1 independent variables. †P<.001 versus vehicle control. ‡P<.001 versus 5-FU cream 0.5% for one week. §P=.016 versus 5-FU cream 0.5% for two weeks. (B) *P<.001 versus vehicle control. (Figure 2A Reprinted with permission from Cutis. 2002;70:335–339. ©2002 Quadrant HealthCom.30)
Figure 3.
Overall severity and time course of facial irritation for 5-FU cream 0.5% during 1, 2, and 4 weeks of treatment. Severity ratings are based on the following scale: 0 = none, 1 = mild, 2 = moderate, 3 = severe. (Reprinted with permission from Cutis. 2002;70:335–339. ©2002 Quadrant HealthCom.30)
Comparison of 0.5% and 5% Preparations
The efficacy and tolerability of the Microsponge®-based 5-FU cream 0.5% and a 5% 5-FU cream have been compared. 5-FU cream 0.5% was as effective as the 5% 5-FU cream in reducing the number of AK lesions and in the percentage of patients achieving total clearance of lesions.33–35 Facial irritation and patient-rated treatment tolerance favored 5-FU cream 0.5% over the 5% cream, suggesting that irritation may not be related to efficacy.
A split-face study involving 21 patients (mean age ± SD, 70.4±8.5 years) with a mean of 10.85 AK lesions on each side of the face compared the efficacy and tolerability of once-daily 5-FU cream 0.5% and twice-daily 5% 5-FU cream.33 Both creams were effective in reducing AK lesions and achieving total AK clearance; however, the 0.5% formulation was significantly more effective than the 5% formulation in reducing the absolute number of AK lesions from baseline: 8.8 versus 6.1 lesions, respectively (P=.04). Although irritation associated with the treatments was similar, there was less irritation with 5-FU cream 0.5%, which was preferred by 85 percent of the patients (P=.003).33 Reasons contributing to the preference score were less irritation, preferred application schedule, ease of application, skin appearance, and esthetics of the product on the skin. The study was well-controlled and evaluator-blinded; however, the author cautioned that the small population and non–patient-blinded nature of the study warranted further clinical studies to confirm these results.
In a study using human cadaver skin, 86% to 92% of the absorbed 5-FU cream 0.5% was retained in the skin versus 54 percent of the 5% 5-FU cream 24 hours after application,27 demonstrating a localized effect for the 0.5% formulation. In a pharmacokinetic comparison study, Levy et al, measuring plasma and urine concentrations in patients (mean age, 64 years), estimated systemic absorption rates of 0.55 percent and 2.4 percent for the 0.5% and 5% formulations, respectively.28 The results of both of these studies suggest that the degree of systemic absorption is dose dependent, with greater systemic absorption achieved with higher concentration formulations.34 Further studies are needed to confirm the potential benefits of a targeted delivery system applied to the skin.
Combining 5-FU and Cryotherapy to Reduce Therapy Duration
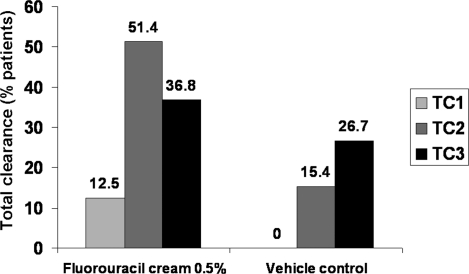
A seven-day treatment with 5-FU cream 0.5% also has been shown to be effective in combination with cryotherapy. In a double-blind study by Jorizzo et al, 144 patients (mean age, 62.6 years) were randomized to 5-FU cream 0.5% or vehicle daily for seven days.36 At the four-week follow-up, any remaining AK lesions were treated with cryotherapy. Patients were evaluated six months later, and the cycle was repeated, if necessary. Final assessments were performed at the third six-month follow-up. Patients experiencing clearance of AK lesions in all treatment areas favored 5-FU cream 0.5% plus cryotherapy over vehicle (36.8% vs. 26.7%) at the end of the study (P=.028; Figure 4).
Figure 4.
Percentage of patients with clearance of AK lesions in all treatment areas.36 TC1 = Treatment Cycle 1; TC2 = Treatment Cycle 2; TC3 = Treatment Cycle 3. P=.002 at TC1, P<.001 at TC2, and P=.028 at TC3 versus the vehicle control group.
Implications for Treatment in the Elderly Population
Optimal treatment of AK lesions in the elderly depends on several factors, including adherence to treatment regimens and modification of regimens to adjust to pharmacokinetic and pharmacodynamic changes that occur. Treatment adherence may be influenced by the number of medication doses required each day, duration of therapy, and adverse events that affect tolerability.37–39 Because of declining organ function, metabolic function also may impact adverse events as well as tolerability.40
Studies estimate nonadherence to long-term treatment regimens from 40 percent to as high as 75 percent among elderly patients.39 These patients, who often have several chronic diseases, have a higher incidence of impaired cognitive function, including memory and ability to understand directions, which may affect adherence.37 Low medication adherence among elderly patients also may be the result of complex drug regimens as well as the number of medications and daily doses prescribed.37,39 Studies have found that persistence decreases with increasing duration of therapy.37,38 The clearance rates of many medications are reduced with increased age.40 Therefore, decreased systemic absorption may be particularly important in this age group.
Potential Benefits of 5-FU Cream 0.5% in the Elderly Population
Microsponge®-based 5-FU cream 0.5% may improve adherence to treatment among elderly patients who have been prescribed topical 5-FU, particularly since it is applied once daily, unlike the twice-daily application of other topical formulations, and causes less irritation. Once-daily application of 5-FU cream 0.5% has been shown to be as equally effective as twice-daily application of a 5% 5-FU cream in reducing AK lesions and in achieving total AK clearance.33,34 The impact of adverse events may be of particular importance in this patient population, among whom adherence to treatment regimens is generally low.39 Furthermore, once-daily 5-FU cream 0.5% results in lower systemic 5-FU exposure than the 5% cream,28 potentially decreasing adverse events that also may impact adherence to treatment. The combination of 5-FU cream 0.5% and cryotherapy reduces the duration of therapy and treats subclinical lesions, another advantage in improving treatment adherence in the elderly population.
Conclusion
AK primarily affects elderly patients after chronic UV exposure. Left untreated, up to 16 percent of AK lesions may progress to skin cancer. Topical 5-FU is an effective treatment for widespread multiple AK lesions; however, treatment efficacy does not necessarily depend on high concentrations or long durations of therapy. Lesion reduction is usually observed after one week of treatment, with a greater reduction in lesion count observed with therapy for four weeks. Microsponge®-based 5-FU cream 0.5% applied once daily has been shown to be a safe and effective therapy. Use of the 0.5% formulation for as little as one week when combined with cryotherapy improved outcomes versus cryotherapy alone.
Because of its once-daily application schedule, duration of therapy from 1 to 4 weeks as tolerated, and potentially minimized systemic absorption, 5-FU cream 0.5% may be an alternative for elderly patients with AK. These attributes may improve adherence to therapy as well as potentially improve cure rates.
References
- 1. de Berker D, McGregor JM, Hughes BR. on behalf of the British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for the management of actinic keratoses. Br J Dermatol. 2007;156:222–230. doi: 10.1111/j.1365-2133.2006.07692.x. [DOI] [PubMed] [Google Scholar]
- 2. Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995;32:95–98. doi: 10.1016/0190-9622(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 3. Holman CD, Armstrong BK, Evans PR, et al. Relationship of solar keratosis and history of skin cancer to objective measures of actinic skin damage. Br J Dermatol. 1984;110:129–138. doi: 10.1111/j.1365-2133.1984.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 4. Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):S4–S7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 5. Gupta AK, Cooper EA, Feldman SR, Fleischer AB JR. A survey of office visits for actinic keratosis as reported by NAMCS, 1990-1999. National Ambulatory Medical Care Survey. Cutis. 2002;70(2 Suppl):8–13. [PubMed] [Google Scholar]
- 6. Frost CA, Green AC. Epidemiology of solar keratoses. Br J Dermatol. 1994;131:455–464. doi: 10.1111/j.1365-2133.1994.tb08544.x. [DOI] [PubMed] [Google Scholar]
- 7. Berman B, Bienstock L, Kuritzky L, et al. Actinic keratoses: sequelae and treatments. Recommendations from a consensus panel. J Fam Pract. 2006;55(Suppl):1–8. [PubMed] [Google Scholar]
- 8. Oppel T, Korting HC. Actinic keratosis: the key event in the evolution from photoaged skin to squamous cell carcinoma. Therapy based on pathogenetic and clinical aspects. Skin Pharmacol Physiol. 2004;17:67–76. doi: 10.1159/000076016. [DOI] [PubMed] [Google Scholar]
- 9. Moy RL. Clinical presentation of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):8–10. doi: 10.1067/mjd.2000.103343. [DOI] [PubMed] [Google Scholar]
- 10. Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st-century perspective. Arch Dermatol. 2003;139:66–70. doi: 10.1001/archderm.139.1.66. [DOI] [PubMed] [Google Scholar]
- 11. Cockerell CJ. Should we rename actinic keratosis? A leading dermatologist debates a growing dispute in the medical community—the renaming of this precancerous lesion. Skin & Aging. 2002;10:31–32. [Google Scholar]
- 12. Ashton KJ, Weinstein SR, Maguire DJ, Griffiths LR. Chromosomal aberrations in squamous cell carcinoma and solar keratoses revealed by comparative genomic hybridization. Arch Dermatol. 2003;139:876–882. doi: 10.1001/archderm.139.7.876. [DOI] [PubMed] [Google Scholar]
- 13. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):23–24. doi: 10.1067/mjd.2000.103339. [DOI] [PubMed] [Google Scholar]
- 14. Chen GJ, Feldman SR, Williford PM, et al. Clinical diagnosis of actinic keratosis identifies an elderly population at high risk of developing skin cancer. Dermatol Surg. 2005;31:43–47. doi: 10.1111/j.1524-4725.2005.31009. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 16. Gold MH, Nestor MS. Current treatments of actinic keratosis. J Drugs Dermatol. 2006;5(2 Suppl):17–25. [PubMed] [Google Scholar]
- 17. Tanghetti E, Werschler P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J Drugs Dermatol. 2007;6:144–147. [PubMed] [Google Scholar]
- 18. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156–159. [PubMed] [Google Scholar]
- 19. Newman MD, Weinberg JM. Topical therapy in the treatment of actinic keratosis and basal cell carcinoma. Cutis. 2007;79(4 Suppl):18–28. [PubMed] [Google Scholar]
- 20. Eaglstein WH, Weinstein GD, Frost P. Fluorouracil: mechanism of action in human skin and actinic keratoses. I. Effect on DNA synthesis in vivo. Arch Dermatol. 1970;101:132–139. [PubMed] [Google Scholar]
- 21. Lawrence N. New and emerging treatments for photoaging. Dermatol Clin. 2000;18:99–112. doi: 10.1016/s0733-8635(05)70151-0. [DOI] [PubMed] [Google Scholar]
- 22.Efudex [package insert] Costa Mesa, CA: Valeant Pharmaceuticals North America; 2005. [Google Scholar]
- 23. Dinehart SM. The treatment of actinic keratoses. J Am Acad Dermatol. 2000;42(1 Pt 2):25–28. doi: 10.1067/mjd.2000.103338. [DOI] [PubMed] [Google Scholar]
- 24. Jury CS, Ramraka-Jones VS, Gudi V, Herd RM. A randomized trial of topical 5% 5-fluorouracil (Efudix cream) in the treatment of actinic keratoses comparing daily with weekly treatment. Br J Dermatol. 2005;153:808–810. doi: 10.1111/j.1365-2133.2005.06858.x. [DOI] [PubMed] [Google Scholar]
- 25. Epstein E. Does intermittent “pulse” topical 5-fluorouracil therapy allow destruction of actinic keratoses without significant inflammation? J Am Acad Dermatol. 1998;38:77–80. doi: 10.1016/s0190-9622(98)70542-0. [DOI] [PubMed] [Google Scholar]
- 26. Gupta AK. The management of actinic keratoses in the United States with topical fluorouracil: a pharmacoeconomic evaluation. Cutis. 2002;70(2 Suppl):30–36. [PubMed] [Google Scholar]
- 27. Levy S, Furst K, Chern W. A comparison of the skin permeation of three topical 0.5% fluorouracil formulations with that of a 5% formulation. Clin Ther. 2001;23:901–907. doi: 10.1016/s0149-2918(01)80077-1. [DOI] [PubMed] [Google Scholar]
- 28. Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23:908–920. doi: 10.1016/s0149-2918(01)80078-3. [DOI] [PubMed] [Google Scholar]
- 29.Wide-ranging benefits. Dublin, OH: Cardinal Health; 2004. Precision dosing. [Google Scholar]
- 30. Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70:335–339. [PubMed] [Google Scholar]
- 31. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 Suppl):22–29. [PubMed] [Google Scholar]
- 32. Gupta AK, Weiss JS, Jorizzo JL. 5-fluorouracil 0.5% cream for multiple actinic or solar keratoses of the face and anterior scalp. Skin Therapy Lett. 2001;6:1–4. [PubMed] [Google Scholar]
- 33. Loven K, Stein L, Furst K, Levy S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther. 2002;24:990–1000. doi: 10.1016/s0149-2918(02)80012-1. [DOI] [PubMed] [Google Scholar]
- 34. Jorizzo JL, Carney PS, Ko WT, et al. Fluorouracil 5% and 0.5% creams for the treatment of actinic keratosis: equivalent efficacy with a lower concentration and more convenient dosing schedule. Cutis. 2004;74(6 Suppl):18–23. [PubMed] [Google Scholar]
- 35. Levy S, Furst K, Chern W. A novel 0.5% fluorouracil cream is minimally absorbed into the systemic circulation yet is as effective as 5% fluorouracil cream. Cutis. 2002;70(2 Suppl):14–21. [PubMed] [Google Scholar]
- 36. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133–139. [PubMed] [Google Scholar]
- 37. Claesson S, Morrison A, Wertheimer AI, Berger ML. Compliance with prescribed drugs: challenges for the elderly population. Pharm World Sci. 1999;21:256–259. doi: 10.1023/a:1008786004974. [DOI] [PubMed] [Google Scholar]
- 38. Gottlieb H. Medication nonadherence: finding solutions to a costly medical problem. Drug Benefit Trends. 2000;12:57–62. [Google Scholar]
- 39. Salzman C. Medication compliance in the elderly. J Clin Psychiatry. 1995;56(Suppl 1):18–22. [PubMed] [Google Scholar]
- 40. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]