Abstract
Objective: This study further assessed the long-term safety and efficacy of fluorouracil cream 0.5% in patients with multiple actinic keratosis (AK) on the face/anterior scalp and other body sites. Design/setting: This 18-month, prospective, open-label, multicenter study comprised two treatment cycles separated by 12 months. Cycle 1 included treatment of AK lesions on the face, anterior scalp, posterior scalp, ears, neck, lips, arms, and/or hands. Once-daily fluorouracil cream 0.5% was applied for four weeks as tolerated, followed by four weeks of follow-up in each treatment cycle. Participants: Adults (N=277) with five or more visible and/or palpable AK lesions on the face/anterior scalp and five or more lesions on the posterior scalp, ears, neck, lips, arms, and/or hands were enrolled. Measurements: Main outcome measures included adverse events (AEs) and reduction/clearance of AK lesions on the face/anterior scalp after four weeks of treatment. Results: Results for treatment of AK lesions on the face/anterior scalp for Cycle 1 are reported. All 277 patients were treated during Cycle 1. Besides anticipated application-site reactions (67.9% and 19.1% of patients experiencing mild-to-moderate and severe events, respectively) and eye irritation, overall incidence of treatment-emergent AEs was low. No individual AE appeared in greater than four percent of patients. At the end of Cycle 1, significant reductions were noted in lesion counts on the face/anterior scalp (84.8%; P<0.0001). Clearance rate for lesions on the face and anterior scalp was 39.8 percent at eight weeks. Conclusion: Results indicate that fluorouracil cream 0.5% is safe and effective for patients with multiple AK lesions on the face/anterior scalp.
Actinic keratosis (AK) is a precancerous skin condition characterized by atypical, dysplastic keratinocytes.1 AK commonly presents as red, scaling, or hyperkeratotic plaques and usually occurs in individuals with photo-aged skin due to long-term sun exposure.2 Because AK and squamous cell carcinoma (SCC) have similar histologic and genetic mutation profiles, it has been widely proposed that these two conditions are connected and that AK is potentially “the key event in the progression to SCC.”2–4
Epidemiologic studies from the past 15 years have indicated that the risk of AK progressing to invasive SCC ranges from 6 to 12 percent per year.5 Conversely, it has been estimated that up to 60 percent of cutaneous SCCs arise from AKs.6 In a case review study of cutaneous SCC files from the University of Florida, 136 of 165 SCC lesions (82.4%) had concomitant AK lesions giving rise to and/or in close proximity to SCC.7 Of the 136 SCC lesions, 44 (32.3%) were superficial SCC appearing within an AK lesion and 92 (67.6%) had AK in close proximity (directly adjacent and up to 8mm away) to SCC.7
One of the current clinical dilemmas in the study of AK remains the identification of which AK lesions will spontaneously recede, remain as AKs, or progress to SCC. As a result, aggressive treatment of all AKs is recommended upon diagnosis, both to clear the lesions and to potentially prevent progression to SCC.2,8
Cryotherapy is the most commonly used ablative treatment of AK in patients with a small number of lesions.9 However, for patients with multiple AKs, treatment options include photodynamic therapy (with photosensitizing agents and blue light)10,11 and topical medications, such as 5-fluorouracil cream,12–18 imiquimod cream,19–22 and diclofenac sodium gel.23
Topical treatments, including 5-fluorouracil, are useful for patients who have extensive, widespread AK because they potentially treat subclinical lesions present in individuals with significant sun damage.24,25 Some patients, however, have tolerability issues with 5-fluorouracil treatment. Due to the toxic potential of fluorouracil, an ideal topical formulation would allow a substantial amount of drug to remain in the skin at the site of action, with minimal systemic exposure.26
Fluorouracil cream 0.5% (Carac®; Dermik Laboratories, Inc., Bridgewater, New Jersey), indicated for the topical treatment of multiple actinic or solar keratoses of the face and anterior scalp, uses a patented microsponge delivery system that has been shown to minimize the systemic absorption of fluorouracil, resulting in higher retention of fluorouracil in the skin. Two studies analyzed systemic absorption and skin retention of fluorouracil cream 0.5%. An in-vitro study examining the flux and percutaneous absorption of 3H-labeled 5-fluorouracil in full-thickness human cadaver skin found that higher percentages of absorbed fluorouracil were retained in the skin after 24 hours with the 0.5% formulation (86–92%) than with the 5% formulation (54%).27 An in-vivo study showed that initial mean plasma drug concentrations following administration of 5% fluorouracil cream were 10-fold higher than with fluorouracil cream 0.5%, thereby demonstrating potentially less systemic exposure with fluorouracil cream 0.5%.28
This short communication presents interim subgroup results of an 18-month, two-treatment cycle study, including safety, tolerability, and efficacy. We report on patients with AK on the face/anterior scalp who were treated with once-daily fluorouracil cream 0.5% for four weeks as tolerated during Cycle 1.
Materials and Methods
Design and objectives. This 18-month, Phase 4, open-label, multicenter study consisted of two treatment/observation cycles and a final study visit. The primary objective of this paper is to report the safety of fluorouracil cream 0.5% in the treatment of AK by collecting adverse event data. Efficacy endpoints reported include the reduction in AK lesion counts of the face (including anterior scalp, if applicable) and the percentage of patients who had clearance of facial AK lesions (including anterior scalp) after Cycle 1 (Week 8).
Patients. Men and women were eligible for enrollment if they were 18 years of age or older and provided written informed consent. Eligible patients had at least five visible and/or palpable AK lesions on the face (and anterior scalp, if applicable) and at least five visible and/or palpable AK lesions on other body sites. Patients were excluded if they had a confounding skin condition on the face, scalp, ears, neck, lips, arms, or hands, including basal cell carcinoma or SCC; had excessive or prolonged exposure to sunlight; used a tanning parlor; had a known dihydropyrimidine dehydrogenase enzyme deficiency; were treated with other topical agents for AK lesions within five months of the study start or with liquid nitrogen within four weeks of the study start; were pregnant or lactating; or had a history of drug or alcohol abuse. Patients were not evaluated to determine immunocompetency, nor were any patients known to be taking immunosuppressive medications concomitantly.
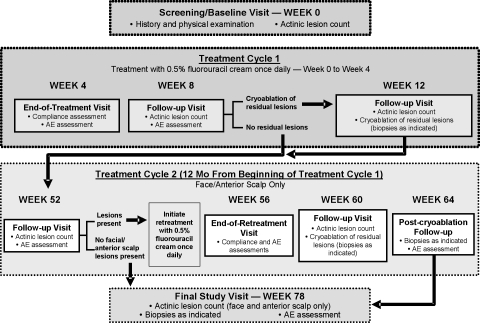
Treatment and assessments. During Cycle 1, patients were treated with fluorouracil cream 0.5% once daily for up to four weeks, as tolerated (Figure 1). A lesion count by body site was performed at the baseline visit by the evaluator; only lesions that were visible to the unaided eye and/or palpable were counted. Patients were provided with the medication and instructed on how to apply it to the designated treatment area. The first application was made to the designated treatment area under the supervision of study center personnel. A treatment diary and detailed instructions for the recording of all daily study treatment applications, as well as the recording of any other topical agents applied to their face, were also provided. Application of moisturizer or sunscreen was allowed after the study medication had been applied. If the study medication was discontinued due to intolerability at one designated body site, patients were allowed to continue treatment to other designated body sites for up to four weeks.
Figure 1.
Study design
After up to four weeks of treatment, patients returned to the study site, at which time they were observed and questioned regarding adverse events. Treatment adherence was monitored by reviewing patient diaries, direct questioning of patients regarding missed applications, and visual inspection of returned study medications. At the four-week follow-up visit (Week 8), residual AK lesions were counted as during the baseline visit, and adverse events were again recorded. At this time, any residual lesions were treated with cryotherapy. According to medical judgment, a topical corticosteroid (Hytone® hydrocortisone Cream 2.5%; Dermik Laboratories, Inc.) was provided to patients during this follow-up visit, if needed, for intolerable skin irritation. Patients who underwent cryotherapy at Week 8 returned for a postsurgical follow-up visit at Week 12, at which time residual lesions were counted and treated with cryotherapy (if needed). Any persistent suspicious lesions were biopsied, and adverse events were noted.
Efficacy assessments for the face and anterior scalp during Cycle 1 included reduction and clearance rates of AK lesions of the face at Week 8 (four weeks after the initial four-week course of fluorouracil cream 0.5% treatment). Reduction in AK lesions was defined as the percent difference in AK lesion counts at baseline compared with Week 8. Clearance rates denoted the percentage of patients without any visible/palpable AK lesions at Week 8.
Changes from baseline in AK lesions were analyzed using a paired t-test to test the statistical difference in AK lesion counts between pretreatment and post-treatment. The number and percentage of patients with complete clearance of all AK lesions or recurrence of facial AK lesions were tabulated and summarized. For efficacy analyses, statistical tests were carried out at a five-percent level of significance. Efficacy assessments for AK lesions of the face and anterior scalp are presented here.
Safety was assessed by summarizing the incidence of adverse events, including all application-site reactions and eye irritations. Application-site reactions were recorded as erythema, edema, dryness, pain, erosion, burning, and pruritus; eye irritation was defined as burning, sensitivity, itching, stinging, and watering.
Results
All 277 patients who were screened were enrolled in the study. All were Caucasian with a mean age of 67.4 years (range, 36–92 years) and the majority were male (81.2%) with a fair complexion (73.6%). A history of facial irritation (dryness, erythema, burning, erosion, pruritus, edema, or pain) was reported by 67 patients (24%), and irritation of the face was ongoing at entry into the study in approximately 65 (97%) of these patients. A total of 39 patients (14%) reported a history of eye irritation (watering, burning, itching, sensitivity, or stinging).
The mean number of total AK lesions among these patients was 39.8±28.09; the mean number of AK lesions on the face/anterior scalp was 15.7±10.95. All 277 patients enrolled in the study (100%) received at least one application of study medication during Cycle 1, and 203 (73.3%) completed treatment. During Cycle 1, 229 (82.7%) of the 277 patients enrolled in the study were judged to be compliant—defined as applying 80 percent or more of 28 protocol-specified doses.
With the exception of the anticipated application-site reactions, the overall incidence of treatment-emergent adverse events was low, with no individual adverse event appearing in greater than four percent of patients. No unexpected adverse events were reported. The most common treatment-emergent adverse events during Cycle 1 were related to application-site reactions (i.e., erythema, burning, pruritus, edema, and pain) and symptoms of eye irritation (i.e., watering, burning, itching, sensitivity, and stinging). Most adverse events were mild or moderate in intensity. A total of 245 patients (88.4%) reported at least one treatment-related adverse event during Weeks 1 to 8 (Table 1), and these events were rated as severe in 54 patients (19.5%). Erythema and/or burning of the face were the most common treatment-related application-site adverse events, whereas conjunctivitis (watering, burning) was the most common adverse event of the special senses (Table 1). One patient (0.4%) was discontinued from the study due to a treatment-related adverse event (moderate conjunctivitis during Week 2).
Table 1.
Treatment-related adverse events experienced by ≥2% of patients (N=277)
| SYSTEM ORGAN CLASS/PREFERRED TERM | MILD n (%) | MODERATE n (%) | SEVERE n (%) | TOTAL n (%) |
|---|---|---|---|---|
| Patients with at least one event | 142 (51.3) | 126 (45.5) | 54 (19.5) | 245 (88.4) |
| Skin and appendages | 87 (31.4) | 101 (36.5) | 53 (19.1) | 241 (87.0) |
| Application-site reactions | 4 (1.4) | 9 (3.2) | 3 (1.1) | 16 (5.8) |
| Arms (erythema) | 6 (2.2) | 2 (0.7) | 0 | 8 (2.9) |
| Hand (erythema) | 5 (1.8) | 1 (0.4) | 2 (0.7) | 8 (2.9) |
| Face (burning) | 8 (2.9) | 2 (0.7) | 0 | 10 (3.6) |
| Face (burning, other) | 3 (1.1) | 3 (1.1) | 0 | 6 (2.2) |
| Face (dryness, erythema) | 2 (0.7) | 4 (1.4) | 1 (0.4) | 7 (2.5) |
| Face (edema, dryness, pain, erythema, burning, pruritus, other) | 0 | 1 (0.4) | 5 (1.8) | 6 (2.2) |
| Face (erythema) | 9 (3.2) | 7 (2.5) | 0 | 16 (5.8) |
| Face (erythema, burning, other) | 3 (1.1) | 2 (0.7) | 1 (0.4) | 6 (2.2) |
| Face (erythema, other) | 5 (1.8) | 1 (0.4) | 1 (0.4) | 7 (2.5) |
| Face (erythema, pruritus) | 3 (1.1) | 2 (0.7) | 1 (0.4) | 6 (2.2) |
| Face (pain, erythema) | 4 (1.4) | 3 (1.1) | 0 | 7 (2.5) |
| Face (pain, erythema, other) | 3 (1.1) | 3 (1.1) | 0 | 6 (2.2) |
| Face (other) | 5 (1.8) | 4 (1.4) | 0 | 9 (3.2) |
| Special senses | 45 (16.2) | 20 (7.2) | 3 (1.1) | 68 (24.5) |
| Conjunctivitis—eye (burning) | 9 (3.2) | 1 (0.4) | 0 | 10 (3.6) |
| Conjunctivitis—eye (other) | 5 (1.8) | 2 (0.7) | 0 | 7 (2.5) |
| Conjunctivitis—eye (watering) | 10 (3.6) | 10 (3.6) | 1 (0.4) | 21 (7.6) |
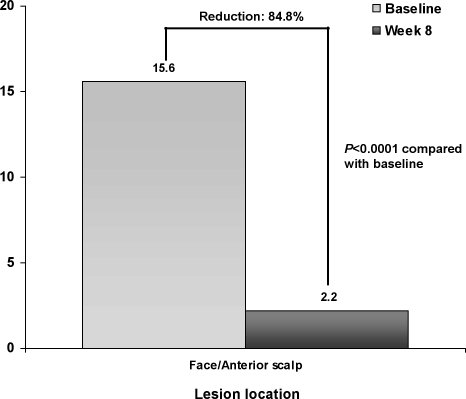
During Cycle 1, a statistically significant (P<0.0001) decrease in the number of AK lesions was observed from baseline to Week 8 on the face and anterior scalp (Figure 2). Mean percent reduction in AK lesions from baseline to Week 8 on the face and anterior scalp was 84.8 percent (P<0.0001). At Week 8, the clearance rate for AK lesions on the face and anterior scalp was 39.8 percent.
Figure 2.
Change from baseline to Week 8 in mean (SD) AK lesion counts on face and anterior scalp during Treatment Cycle 1
Discussion
The results for treatment of AK lesions on the face and anterior scalp for this 18-month, Phase 4, open-label study indicate that fluorouracil cream 0.5%, formulated using a patented microsponge delivery system, was safe and effective. The safety and tolerability results during one treatment cycle in this study are consistent with and corroborate the results of earlier studies with fluorouracil cream 0.5% for the treatment of AK.15,16 For example, based on pooled data from two vehicle-controlled pivotal trials (N=384), 243 patients (94.6%) treated with fluorouracil cream 0.5% experienced application-site reactions and 14 (5.4%) reported eye irritation.29 However, only 31 patients (12%) in these studies discontinued due to facial irritation.29 In the current study, although adverse events related to the skin and appendages were rated as severe in 54 patients (19.1%) and eye irritation was judged as severe in three patients (1.1%), only one patient discontinued from the study due to a treatment-related adverse event (i.e., moderate conjunctivitis).
The efficacy results after one treatment cycle in this study are consistent with the results from previous studies with this treatment.15–17 The reduction rates for facial/anterior scalp lesions ranged from 67 to 92 percent in the two large, vehicle-controlled, pivotal trials15,16; in the current study, the reduction rate for facial/anterior scalp lesions was 84.8 percent.
Conclusion
The interim results of this study provide additional evidence that one four-week cycle of once-daily treatment with fluorouracil cream 0.5%, formulated using a patented microsponge delivery system, is a safe and effective treatment for AK lesions on the face and anterior scalp. Additional data, including safety results up to Week 78, lesion counts on the face/anterior scalp and other body sites up to Week 78, and facial lesion clearance rates at Week 60 will be reported in a future publication.
Acknowledgments
Editorial support for this article was provided by Dermik Laboratories, a business of sanofi-aventis U.S. LLC. The opinions expressed in the current article are those of the authors. The authors received no honoraria or other form of financial support related to the development of this manuscript.
References
- 1. Cockerell CJ, Wharton JR. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma) J Drugs Dermatol. 2005;4(4):462–467. [PubMed] [Google Scholar]
- 2. Oppel T, Korting HC. Actinic keratosis: the key event in the evolution from photoaged skin to squamous cell carcinoma. Therapy based on pathogenetic and clinical aspects. Skin Pharmacol Physiol. 2004;17(2):67–76. doi: 10.1159/000076016. [DOI] [PubMed] [Google Scholar]
- 3. Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”) J Am Acad Dermatol. 2000;42(1 Pt 2):11–17. doi: 10.1067/mjd.2000.103344. [DOI] [PubMed] [Google Scholar]
- 4. Cockerell CJ. Should we rename actinic keratosis? A leading dermatologist debates a growing dispute in the medical community—the renaming of this precancerous lesion. Skin & Aging. 2002;10(9):31–32. [Google Scholar]
- 5. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):S23–S24. doi: 10.1067/mjd.2000.103339. [DOI] [PubMed] [Google Scholar]
- 6. Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 7. Mittelbronn MA, Mullins DL, Ramos-Caro FA, Flowers FP. Frequency of pre-existing actinic keratosis in cutaneous squamous cell carcinoma. Int J Dermatol. 1998;37(9):677–681. doi: 10.1046/j.1365-4362.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 8. Moy RL. Clinical presentation of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):8–10. doi: 10.1067/mjd.2000.103343. [DOI] [PubMed] [Google Scholar]
- 9. Lober BA, Fenske NA. Optimum treatment strategies for actinic keratosis (intraepidermal squamous cell carcinoma) Am J Clin Dermatol. 2004;5(6):395–401. doi: 10.2165/00128071-200405060-00004. [DOI] [PubMed] [Google Scholar]
- 10. Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140(1):41–46. doi: 10.1001/archderm.140.1.41. [DOI] [PubMed] [Google Scholar]
- 11. Touma D, Yaar M, Whitehead S, et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140(1):33–40. doi: 10.1001/archderm.140.1.33. [DOI] [PubMed] [Google Scholar]
- 12. Jury CS, Ramraka-Jones VS, Gudi V, Herd RM. A randomized trial of topical 5% 5-fluorouracil (Efudix cream) in the treatment of actinic keratoses comparing daily with weekly treatment. Br J Dermatol. 2005;153(4):808–810. doi: 10.1111/j.1365-2133.2005.06858.x. [DOI] [PubMed] [Google Scholar]
- 13. Labandeira J, Pereiro M, Jr, Valdes F, Toribio J. Intermittent topical 5-fluorouracil is effective without significant irritation in the treatment of actinic keratoses but prolongs treatment duration. Dermatol Surg. 2004;30(4 Pt 1):517–520. doi: 10.1111/j.1524-4725.2004.30167.x. [DOI] [PubMed] [Google Scholar]
- 14. Jorizzo J, Weiss J, Furst K, et al. Effect of a 1-week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: a randomized, vehicle-controlled clinical trial. Arch Dermatol. 2004;140(7):813–816. doi: 10.1001/archderm.140.7.813. [DOI] [PubMed] [Google Scholar]
- 15. Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis. 2002;70(6):335–339. [PubMed] [Google Scholar]
- 16. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 Suppl):22–29. [PubMed] [Google Scholar]
- 17. Loven K, Stein L, Furst K, Levy S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther. 2002;24(6):990–1000. doi: 10.1016/s0149-2918(02)80012-1. [DOI] [PubMed] [Google Scholar]
- 18. Jorizzo JL, Carney PS, Ko WT, et al. Fluorouracil 5% and 0.5% creams for the treatment of actinic keratosis: equivalent efficacy with a lower concentration and more convenient dosing schedule. Cutis. 2004;74(6 Suppl):18–23. [PubMed] [Google Scholar]
- 19. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57(2):265–268. doi: 10.1016/j.jaad.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 20. Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126(6):1251–1255. doi: 10.1038/sj.jid.5700264. [DOI] [PubMed] [Google Scholar]
- 21. Korman N, Moy R, Ling M, et al. Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch Dermatol. 2005;141(4):467–473. doi: 10.1001/archderm.141.4.467. [DOI] [PubMed] [Google Scholar]
- 22. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157(1):133–141. doi: 10.1111/j.1365-2133.2007.07942.x. [DOI] [PubMed] [Google Scholar]
- 23. Pirard D, Vereecken P, Melot C, Heenen M. Three percent diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses: a meta-analysis of the recent studies. Arch Dermatol Res. 2005;297(5):185–189. doi: 10.1007/s00403-005-0601-9. [DOI] [PubMed] [Google Scholar]
- 24. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5(2):156–159. [PubMed] [Google Scholar]
- 25. Tanghetti E, Werschler P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J Drugs Dermatol. 2007;6(2):144–147. [PubMed] [Google Scholar]
- 26. Levy S, Furst K, Chern W. A novel 0.5% fluorouracil cream is minimally absorbed into the systemic circulation yet is as effective as 5% fluorouracil cream. Cutis. 2002;70(2 Suppl):14–21. [PubMed] [Google Scholar]
- 27. Levy S, Furst K, Chern W. A comparison of the skin permeation of three topical 0.5% fluorouracil formulations with that of a 5% formulation. Clin Ther. 2001;23(6):901–907. doi: 10.1016/s0149-2918(01)80077-1. [DOI] [PubMed] [Google Scholar]
- 28. Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther. 2001;23(6):908–920. doi: 10.1016/s0149-2918(01)80078-3. [DOI] [PubMed] [Google Scholar]
- 29.Carac [package insert] Bridgewater, NJ: Dermik Laboratories; 2006. [Google Scholar]