SUMMARY
The mammalian CNS contains a ubiquitous population of glial progenitors known as NG2+ cells that have the ability to develop into oligodendrocytes and undergo dramatic changes in response to injury and demyelination. Although it has been reported that NG2+ cells are multipotent, their fate in health and disease remains controversial. Here, we generated PDGFαR-CreER transgenic mice and followed their fate in vivo in the developing and adult CNS. These studies revealed that NG2+ cells in the postnatal CNS generate myelinating oligodendrocytes, but not astrocytes or neurons. In regions of neurodegeneration in the spinal cord of ALS mice, NG2+ cells exhibited enhanced proliferation and accelerated differentiation into oligodendrocytes, but remained committed to the oligodendrocyte lineage. These results indicate that NG2+ cells in the normal CNS are oligodendrocyte precursors with restricted lineage potential, and that cell loss and gliosis are not sufficient to alter the lineage potential of these progenitors in ALS mice.
INTRODUCTION
The central nervous system (CNS) of mammals has a limited capacity to replace cells that have been lost as a consequence of age-related degeneration, injury, or neurological disease. Nevertheless, there are distinct groups of neural stem cells (NSCs) that retain the ability to generate neurons throughout life, indicating that significant cell replacement is possible (Zhao et al., 2008). In addition to NSCs confined to the subventricular zone of lateral ventricle and subgranular zone of the dentate gyrus, the adult brain and spinal cord contain a widely distributed, abundant class of progenitors known as NG2+ cells (also termed oligodendrocyte precursor cells, OPCs), as they express the chondroitin sulfate proteoglycan NG2 (Nishiyama et al., 2009); nearly all (99%) of these glial cells also express the alpha receptor for platelet derived growth factor (PDGFαR) (Nishiyama et al., 1996; Rivers et al., 2008). NG2+ cells are mitotically active and exhibit enhanced proliferation in response to acute CNS injury, ischemia and demyelination (Levine et al., 2001). Thus, an accurate delineation of the fate of these ubiquitous NG2+ cells is critical for determining whether they participate in cell replacement during normal CNS aging, and initiate repair following injury or disease.
Although NG2+ cells were originally thought to serve only as oligodendrocyte (OL) precursors, recent studies suggest that these progenitors may have greater lineage potential (Guo et al., 2009; Rivers et al., 2008; Zhu et al., 2008a; Zhu et al., 2008b). NG2+ cells can differentiate into OLs, astrocytes or neurons in vitro, depending on the growth conditions (Kondo and Raff, 2000), and studies in which NG2+ cells have been labeled using transgenic approaches suggest that they also have the capacity to develop into interneurons in the hippocampus and olfactory bulb (Aguirre and Gallo, 2004; Aguirre et al., 2004; Belachew et al., 2003), principal neurons in the piriform cortex (Guo et al., 2009; Rivers et al., 2008), and astrocytes in ventral areas of the brain and spinal cord in vivo (Guo et al., 2009; Zhu et al., 2008a; Zhu et al., 2008b). These findings support the hypothesis that NG2+ cells represent a widely distributed population of multipotent progenitors that can regenerate major classes of neurons and glia. However, this conclusion remains contentious, as NG2+ cells have not been observed to consistently generate these different cell types in vivo. The apparently divergent behavior of these progenitors could reflect limitations of the approaches used to define their fate, or that NG2+ cells are intrinsically heterogeneous and were incompletely sampled.
In the context of acute brain injury (Hampton et al., 2004), demyelination (Keirstead et al., 1998), and neurodegenerative disease, such as amyotropic lateral sclerosis (ALS) (Magnus et al., 2008), NG2+ cells have been shown to upregulate NG2 and dramatically increase their proliferation rate. The changes in local environment that accompany these injuries may enable NG2+ cells to exhibit greater lineage plasticity and participate in cell replacement (Magnus et al., 2008; Sellers et al., 2009), although the fate of proliferating NG2+ cells and the consequences of their change in behavior in neurodegenerative disease have not been determined.
Here, we examined the fate of NG2+ cells in the normal CNS though genetic lineage tracing in vivo using a new line of mice that express tamoxifen-inducible Cre under control of the PDGFαR promoter, and report the first analysis of NG2+ cell fate in a primary neurodegenerative disease. We show that resident NG2+ cells develop into myelinating OLs in brain and spinal cord during early postnatal and adult life; however, in contrast to previous results, these cells did not generate neurons or astrocytes in any region of brain or spinal cord. Although NG2+ cells in mature gray matter divided and differentiated less frequently than in white matter, clonal analysis indicated that individual NG2+ cells in both regions retain the capacity to proliferate and generate OLs, suggesting that NG2+ cells are not intrinsically heterogeneous with regard to their ability to divide and differentiate, as previously suggested (Bouslama-Oueghlani et al., 2005; Dimou et al., 2008; Rivers et al., 2008). Moreover, fate mapping of NG2+ cells in the spinal cord of a mouse model of ALS revealed that enhanced proliferation of these progenitors was accompanied by facilitated differentiation, but that NG2+ cells remained constrained to the OL lineage. These results suggest that NG2+ cells are not multipotent progenitors, but rather OL precursors with restricted lineage potential, which contribute to homeostatic regulation of OLs in the normal and diseased CNS.
RESULTS
Efficient in vivo induction of Cre activity in NG2+ cells
NG2+ cells in the CNS express both NG2 and PDGFαR (Nishiyama et al., 1996; Rivers et al., 2008), and both genes are rapidly downregulated upon differentiation. Although NG2 immunolabeling is most often used to identify this class of glial cells, and the NG2 promoter has been used to control transgene expression in NG2+ cells in vivo (Ziskin et al., 2007), this proteoglycan is also expressed by perivascular pericytes (Stallcup, 2002), and by some macrophage/microglial cells after CNS injury (Bu et al., 2001). In contrast, PDGFαR is not expressed by activated microglia (Bu et al., 2001), suggesting that the PDGFαR promoter may be more appropriate for restricting transgene expression to NG2+ cells in both normal and disease contexts. To determine the fate of NG2+ cells in vivo, we used the bacterial artificial chromosome (BAC) modification method (Yang et al., 1997) to generate transgenic mice in which an inducible form of Cre recombinase (CreER™) is expressed under control of mouse PDGFαR promoter (Figure S1A). After crossing to Z/EG reporter mice, three PDGFαR-CreER;Z/EG lines exhibited widespread EGFP expression in small, stellate-shaped NG2+PDGFαR+ cells in the brain and spinal cord four days after administration of 4-hydroxytamoxifen (4HT) at postnatal day 30 (P30+4). The line that exhibited the largest number of EGFP+ cells in response to 4HT was selected for further study (Figures S1B – S1H). EGFP+ cells were not observed when these PDGFαR-CreER;Z/EG mice were injected with vehicle alone, indicating that Cre activity is tightly controlled by 4HT (Figures S1B and S1C).
When PDGFαR-CreER;Z/EG mice were examined at P30+4, most EGFP+ cells in the forebrain (corpus callosum, cortex, hippocampus) and spinal cord (both gray and white matter) were PDGFαR+. Of the remaining EGFP+PDGFαR− cells, most were immunopositive for Sox10, an OL lineage-specific transcription factor in the CNS (Figures S1D and S1H), indicating that they likely represent pre-myelinating or immature OLs that developed from NG2+ cells in the period after 4HT administration. EGFP+ cells did not exhibit immunoreactivity to GFAP or NeuN in these regions (Figures S1E – S1H) indicating that Cre was not induced within astrocytes or neurons. In addition to NG2+ glial cells, EGFP also was expressed by choroid plexus ependymal cells and a subset of PDGFβR+ perivascular cells (pericytes) (Figure S2), in accordance with the pattern of PDGFαR mRNA or protein expression (Pringle et al., 1992; Su et al., 2008).
PDGFαR-CreER mice also were crossed with ROSA26-EYFP and ROSA26-mGFP (mT/mG) (Muzumdar et al., 2007) Cre reporter mice, which exhibited higher recombination efficiency; in these double transgenic mice, 83–94% of NG2+ cells in major gray matter regions became reporter-positive within a few days after 4HT administration at P30 (Figure S3 and Table S1). Thus, PDGFαR-CreER mice provide an effective means to determine the fate of NG2+ cells in vivo under both normal and pathological conditions.
Oligodendrocytes are generated rapidly from NG2+ cells in the early postnatal brain
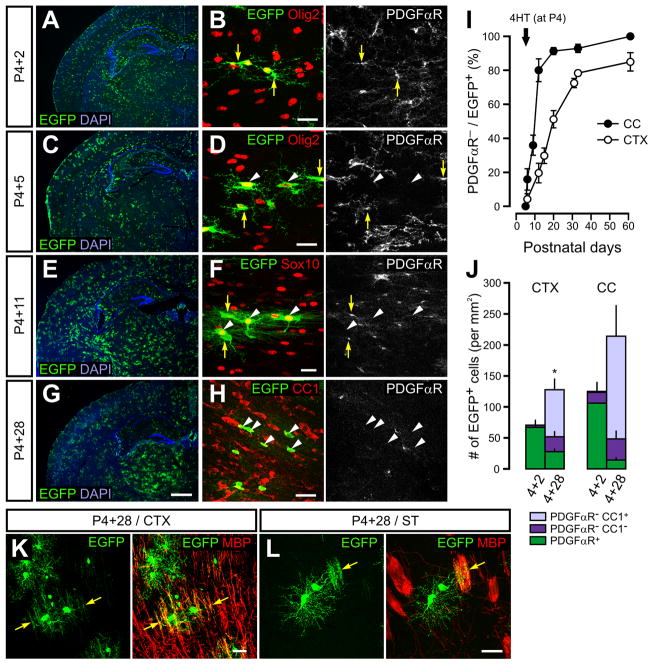
The majority of myelinating OLs appear in the mouse brain within four weeks after birth (Miller, 2002). Although NG2+ cells are regarded as OL progenitors during early postnatal life, previous studies have not directly followed the fate of NG2+ cells in vivo during this period, due to non-specific Cre expression or lack of temporal control of Cre activity in available mice lines (Dimou et al., 2008; Rivers et al., 2008; Zhu et al., 2008a). To determine the identity of the progenitors responsible for OL generation as well as the rate of OL development during this period, we administered 4HT to PDGFαR-CreER;Z/EG at P4 and followed the appearance of EGFP+ cells in the corpus callosum and dorsal cortex over the next four weeks. Two days after 4HT (P4+2), EGFP+ PDGFαR+ cells were visible throughout the brain that had stellate morphologies typical of NG2+ cells (Figures 1A and 1B). Over the next several weeks, the number of EGFP+ cells gradually increased, and many EGFP+ cells exhibited more extensive processes, particularly in the corpus callosum (Figures 1C – H). Five days after 4HT injection (P4+5), more than 35% of EGFP+ cells in the corpus callosum were PDGFαR−, although they remained within the OL lineage as they were Olig2+ and Sox10+ (Figures 1D and 1I). By P4+11, the majority of callosal EGFP+ cells were PDGFαR− and often exhibited numerous parallel-oriented processes characteristic of OLs (Figures 1F and1I), and by P4+28 most EGFP+ cells exhibited immunoreactivity to APC (CC1), a marker of mature OLs (Figures 1H and 1J). These results indicate that the vast majority of NG2+ cells residing in the corpus callosum during the first postnatal week rapidly differentiate into OLs within four weeks (Figure 1J).
Figure 1. Oligodendrocytes develop rapidly from NG2+ cells during early postnatal life.
(A, C, E, G) Images of EGFP+ cells in coronal brain sections of PDGFαR-CreER;Z/EG mice taken 2 (A), 5 (C), 11 (E) or 28 days (G) after 4HT administration at P4. Nuclei are stained by DAPI.
(B, D, F, H) Confocal images showing EGFP+ cells that were immunoreactive to PDGFαR, Olig2, Sox10, or CC1 in the corpus callosum. Yellow arrows indicate PDGFαR+ cells, and white arrowheads indicate PDGFαR−Olig2+ (D), PDGFαR−Sox10+ (F), or PDGFαR−CC1+ cells (H). Scale bars = 500μm (A, C, E, G) or 20μm (B, D, F, H).
(I) Changes in the percentage of PDGFαR−EGFP+ cells in the corpus callosum (CC, filled circles) and cortex (CTX, open circles) over time after 4HT administration at P4.
(J) Histogram showing the number of EGFP+ NG2+ cells (PDGFαR+), pre-myelinating OLs (PDGFαR−CC1−) and OLs (CC1+) in the cortex (CTX) and corpus callosum (CC) of PDGFαR-CreER;Z/EG mice 2 and 28 days after 4HT administration at P4. Error bars display mean + SEM.
(K and L) Confocal images showing EGFP and MBP immunoreactivity in the cortex (CTX) (K) and striatum (ST) (L) of PDGFαR-CreER;Z/EG mice at P4+28. Yellow arrows indicate co-localization between EGFP and MBP. Scale bars = 20μm.
Within the gray matter regions of the dorsal cortex (cingluate and motor cortex) and striatum, most NG2+ cells also developed into OLs within four weeks, as determined by the loss of PDGFαR expression (Figures 1I and 1J ) and co-localization between EGFP and CC1 or MBP at P4+28 (Figures 1J – 1L). Although the rate of OL development was slower in cortex than in corpus callosum, more than 70% of cortical EGFP+ cells were PDGFαR− after two weeks (Figure 1I), indicating that these cells had differentiated into immature or mature OLs. Together, these results suggest that the majority of NG2+ cells in white and gray matter of the developing brain rapidly mature into OLs within a few weeks after birth.
NG2+ cells continue to generate oligodendrocytes in the adult brain and spinal cord
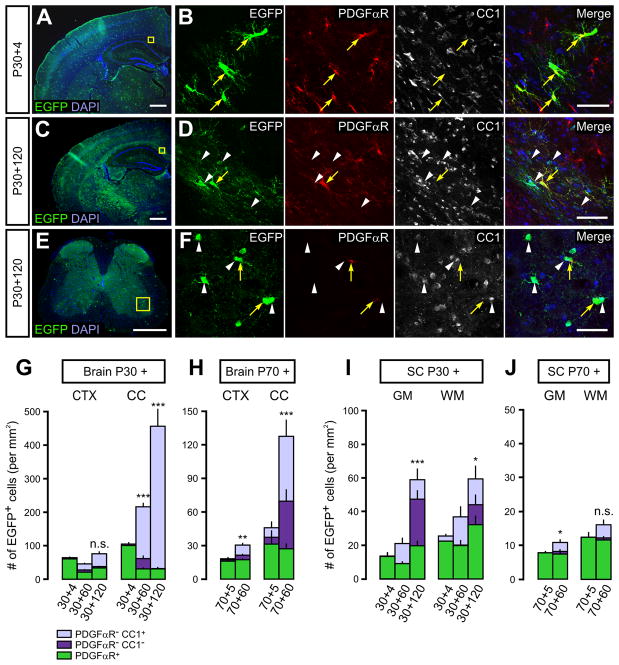
Although OLs are generated rapidly during the first postnatal month in mice, NG2+ cells remain abundant in both gray and white matter in the adult CNS, many of which retain the ability to proliferate. To determine if these resident NG2+ cells continue to develop into OLs, we followed their fate in PDGFαR-CreER;Z/EG mice over four months beginning at P30 or P70. At P30+4, EGFP+ cells were observed throughout the forebrain and almost all were PDGFαR+ (Figures 2A, 2B, 2G, S1B and S1D), and EGFP was expressed by 40–45% of all NG2+ cells. Four months after 4HT administration (P30+120), EGFP+ cells remained widely distributed (Figure 2C), but at this time point ~90% of EGFP+ cells in the corpus callosum and ~50% of EGFP+ cells in the cortex were PDGFαR−CC1+ OLs (Figures 2D and 2G). The efficiency of recombination and extent of differentiation were comparable between male and female mice, as the number of EGFP+ cells at P30+4 (107 vs. 102/mm2 in female and male mice, respectively) and the percentage of CC1+ EGFP+ OLs in the corpus callosum at P30+120 (94% vs. 88% in female and male mice, respectively) were similar; therefore, data from both genders have been pooled. EGFP+ cells that did not express PDGFαR or CC1 were likely immature or premyelinating OLs, as they were Olig2+ and Sox10+, and had highly branched processes characteristic of OL lineage cells at this stage of development (See Figure S1D, white arrowhead). The development of OLs in the corpus callosum between P34 and P150 was gradual, as intermediate numbers of CC1+EGFP+ cells were observed at P30+60 (Figure 2G). Although NG2+ cells in both white and gray matter continued to generate OLs in adult mice, the proliferation and differentiation of NG2+ cells was higher in white matter; the number of EGFP+ cells in the corpus callosum increased 4.6-fold in four months (p = 2.9×10−6), whereas they did not increase significantly in the cortex over this period (1.1-fold, p = 0.18) (Figure 2G). Moreover, the percentage of CC1+EGFP+ cells was significantly higher in the corpus callosum than in cortex at P30+120 (92 ± 2% vs. 48 ± 3%, p < 1.0×10−7).
Figure 2. NG2+ cells continue to generate OLs in the adult mouse brain and spinal cord.
(A, C and E) Images of EGFP+ cells and DAPI staining in coronal brain sections (A and C) and lumbar spinal cord (E) from PDGFαR-CreER;Z/EG mice taken 4 (A) or 120 days (C, E) after 4HT administration at P30. Yellow boxes highlight regions expanded in B, D and F. Scale bar = 500 μm.
(B, D and F) Confocal images showing EGFP, PDGFαR and CC1 immunoreactivity for the regions highlighted in A, C and E. Yellow arrows indicate EGFP+PDGFαR+ cells and white arrowheads indicate EGFP+CC1+ OLs. Scale bars = 50μm.
(G–J) Histograms showing the number of EGFP+ NG2+ cells (PDGFαR+), pre-myelinating OLs (PDGFαR−CC1−) and mature OLs (PDGFαR−CC1+) in the cortex (CTX) and corpus callosum (CC) of the brain (G, H), and in the gray (GM) and white matter (WM) of the spinal cord (I, J) in PDGFαR-CreER;Z/EG mice at different times after 4HT administration at P30 (G, I) or P70 (H, J). Error bars display mean + SEM.
The number of EGFP+ cells increased significantly in the corpus callosum (p = 1.3×10 −6, by one-way ANOVA) and spinal cord (p = 2.2×10 −9 for GM, and p = 0.011 for WM by one-way ANOVA) from 4 to 60 and 120 days after 4HT administration at P30. EGFP+ cell number also increased in the cortex, corpus callosum and spinal cord gray matter from 5 to 60 days after 4HT administration at P70. (* p < 0.05. ** p < 0.005, *** p < 1.0×10 −4, n.s. = not significant, when evaluated by the Student’s t-test).
When Cre activity was induced in PDGFαR-CreER;Z/EG mice at P70 and examined 5 or 60 days later, a similar increase in CC1+ EGFP+ cells was observed (Figure 2H). Although there were fewer EGFP+ cells at P70+5 compared to P30+4, reflecting less efficient Cre induction or recombination in older mice, the percentage of EGFP+ cells that were PDGFαR− after two months was similar to that observed in the corpus callosum at P30+60 (86% vs. 79%, p = 0.19). These data suggest that the rate at which NG2+ cells exit from progenitor state in this brain area is stable for several months after the peak of myelination. However, the maturation rate of these cells slowed with age, as fewer EGFP+ PDGFαR− cells became CC1+ over this two month period (83% vs. 61%, p = 0.0054) (Figures 2G and 2H). These results indicate that NG2+ cells generate OLs continuously in both the dorsal cortex and corpus callosum for at least five months after birth, and that both the rate of proliferation and the extent of differentiation of these progenitors are greater in white matter in the adult brain.
NG2+ cells also generated OLs in the spinal cord during postnatal development, as EGFP+CC1+ cells increased in both white and gray matter of the lumbar spinal cord with time after 4HT administration (Figures 2E and 2F). The rates of NG2+ cell proliferation were significantly slower in spinal than subcortical (brain) white matter from P30, as EGFP+ cells in spinal cord white matter increased by only 2.3-fold in four months. In addition, only 25% of EGFP+ cells were CC1+ at P30+120, suggesting that there also was a lower rate of NG2+ cell differentiation in the spinal cord. Moreover, there was no significant difference in the proportion of CC1+ EGFP+ cells between white and gray matter in the spinal cord (p = 0.13). The reduced proliferation and differentiation of NG2+ cells, and the smaller disparity between gray and white matter likely reflect the more advanced stage of OL development and myelination in the spinal cord at this age. Together, these results indicate that NG2+ cells that reside in the adult brain and spinal cord continue to differentiate into OLs. Differences in the behavior of these progenitors among CNS areas could arise from cell-extrinsic differences in local environment; alternatively, NG2+ cells may be heterogeneous with regard to their intrinsic capacity to proliferate and differentiate.
NG2+ cells in gray matter are not postmitotic and retain the ability to generate OLs
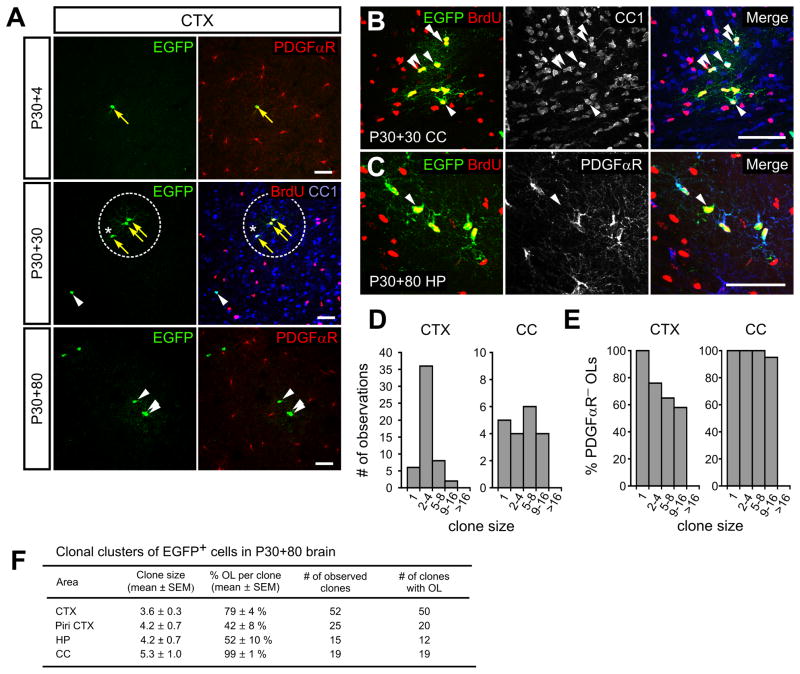
Clonal analysis offers the opportunity to study the diverse behaviors of individual progenitors in different brain regions. When PDGFαR-CreER;Z/EG mice were given a low dose of 4HT at P30 and analyzed four days later, a small number of EGFP+ PDGFαR+ cells were visible throughout the brain (Figure 3A top panels). Most were well-isolated from other EGFP+ cells, and were BrdU− (85%) after continuous delivery of BrdU from P30 to P34; the remainder consisted of closely apposed (within 10 μm) BrdU+ cell pairs. Whereas NG2+ cell somata were separated by 38 ± 2 μm at this age (n = 50), EGFP+ cell somata were separated by 230 ± 42 μm (n = 22), showing that this approach can achieve sparse labeling of NG2+ cells. After 30 days (P30+30), isolated clusters of EGFP+ cells were evident in the brains of these mice (Figure 3A middle panel), which we assumed arose from clonal expansion of individual NG2+ cells. Indeed, when BrdU was delivered continuously for up to one month, all EGFP+ cells within clusters were BrdU+ (n = 45 clusters), suggesting that each cluster arose through proliferation rather than long-distance migration (Figures 3A middle and 3B). Eighty days later (P30+80), all isolated EGFP+ cells in the cortex and corpus callosum were PDGFαR− and Olig2+ (n = 11), indicating that they had differentiated into pre-myelinating or mature OLs, while clusters of EGFP+ cells in both gray and white matter contained both PDGFαR+ and PDGFαR− cells (Figures 3A – 3C, and 3F). These findings suggest that NG2+ cells in all brain regions retain mitotic activity until they differentiate into OLs. EGFP+ cell clusters in the corpus callosum contained significantly more cells at P30+80 than in the cortex (corpus callosum: 5.3 ± 1 cells, n = 19; cortex: 3.6 ± 0.3, n = 52; p = 0.03) (Figures 3D and 3F), in accordance with the higher rate of NG2+ cell proliferation in white matter (See Figure 2G). Moreover, nearly all (98%) EGFP+ cells within clusters in the corpus callosum were PDGFαR− (Figure 3E), while the proportion of PDGFαR− cells within clusters in gray matter varied between brain regions (Figure 3F). Despite these regional differences in the propensity of NG2+ cells to differentiate, 89% of EGFP+ cell clusters in gray matter contained one or more PDGFαR− cells, suggesting that most, if not all, NG2+ cells in the mature brain are capable of becoming OLs. Although reporter-positive pericytes were occasionally observed in PDGFaR-CreER;Z/EG mice (Figure S1H), they were not observed in clones with NG2+ cells or OLs, indicating that these perivascular cells have a distinct lineage.
Figure 3. NG2+ cells in the mature brain are not postmitotic or terminally differentiated.
(A) Images of EGFP+ cells exhibiting PDGFαR, BrdU and CC1 immunoreactivity in the cortex of PDGFαR-CreER;Z/EG mice after exposure to a low dose of 4HT. Mice were examined at P30+4 (top), P30+30 (middle) and P30+80 (bottom). A single clone is highlighted by a dashed circle (middle). Yellow arrows highlight EGFP+ PDGFαR+ cells (top) and EGFP+BrdU+ cells (middle), and white arrowheads highlight an EGFP+BrdU− CC1+ cell (middle) and EGFP+ PDGFαR− cells (bottom). Asterisks indicate an EGFP+BrdU+CC1+ OLs (middle). Scale bars = 50μm.
(B, C) Confocal images of EGFP+BrdU+ cell clusters representing individual clones located in the corpus callosum (B) or in hippocampus (C) from PDGFαR-CreER;Z/EG mice at P30+30 or at P30+80, respectively. White arrowheads indicate either EGFP+CC1+ (B) or EGFP+PDGFαR− (C) OLs. Scale bar = 50μm.
(D) Histograms showing the frequency of EGFP+ cell clones of different sizes in the cortex (CTX) and corpus callosum (CC) at P30+80 in PDGFαR-CreER;Z/EG mice.
(E) Histograms showing the percentage of PDGFαR− OLs in EGFP+ cell clones of different sizes in the cortex (CTX) and corpus callosum (CC) for the clones shown in (D).
(F) Table showing the average size of EGFP+ cell clones in the dorso-lateral cortex (CTX), piriform cortex (Piri CTX), hippocampus (HP) and corpus callosum (CC), the percentage of PDGFαR− cells in each clone, the number of clones, and the number of clones that had at least one PDGFαR− OL (EGFP+PDGFαR− cell).
Oligodendrocytes derived from NG2+ cells in the mature brain form myelin
The continuous appearance of CC1+ EGFP+ cells after P30 and P70 suggests that NG2+ cells develop into mature OLs in the adult CNS (Figure 2). However, it was not possible to determine whether these cells extend processes to create myelin sheaths, because cytosolic EGFP is largely excluded from these thin processes (See Figures 2D and 5G). To aid in visualization of OL processes, we used PDGFαR-CreER; ROSA26-mGFP (mT/mG) mice, in which membrane anchored EGFP is expressed following Cre-mediated recombination (Muzumdar et al., 2007). Two days after 4HT administration at P30, EGFP was present in most PDGFαR+ cells in the corpus callosum (91%) and cortex (94%) (Figures S3E and S4A, Table S1). Over the next four months there was a progressive increase in EGFP fluorescence along myelinated tracts within the corpus callosum (Figure S4A) and striatum at sites where myelin basic protein (MBP) immunoreactivity was concentrated (Figure S4B). At higher magnification, EGFP+ processes of OLs were co-localized with MBP (Figures S4B – S4F). Although some EGFP+ cells were observed without 4HT exposure (Figures S4A bottom and Table S1), these OL lineage cells contributed little to the overall patterns observed (Figure S4A).
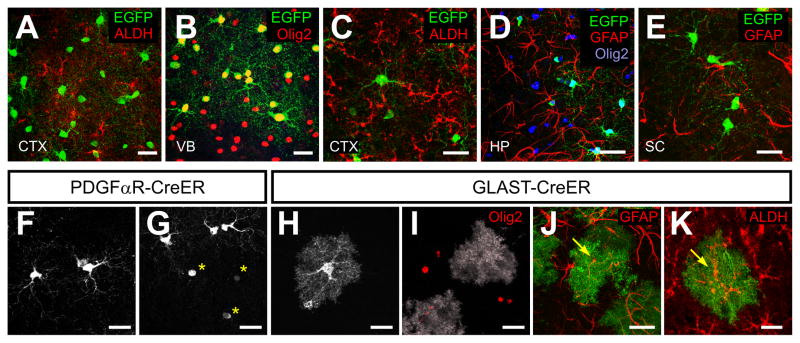
Figure 5. NG2+ cells in the postnatal brain do not generate astrocytes.
(A – E) Confocal images showing immunoreactivity to EGFP, ALDH, Olig2 and GFAP in the dorsal cortex (CTX; A, C), ventral forebrain (VB; B), hippocampus (HP; D), and spinal cord (SC; E) in PDGFαR-CreER;Z/EG mice at P4+96 (A and B) or P30+120 (C – G).
(F – K) Comparison of the morphologies of EGFP+ cells from PDGFαR-CreER;Z/EG mice at P30+120 (F, G), GLAST-CreER;Z/EG at P11+10 (H) or GLAST-CreER;ROSA26-mGFP mice at P15+21(I – K). Confocal images of EGFP+ cells from cortex (F – I), hippocampus (J) or ventral forebrain (K). Yellow asterisks highlight mature OLs, in which cytosolic EGFP is visible only within the soma (G). Yellow arrows in J and K show EGFP+ astrocytes that were GFAP+ or ALDH+. Scale bars = 20 μm.
To determine if NG2+ cell-derived OLs form myelin sheaths, we performed silver enhanced immunogold labeling of EGFP on thin sections prepared from P30+150 PDGFαR-CreER;ROSA26-mGFP mice. In both corpus callosum and cortex, silver enhanced gold particles were observed within the membranous sheets of myelin surrounding axons (Figures 4A – 4D). These particles were often most evident in the portions of the OL cytoplasm adjacent to compacted myelin (Figures 4F and 4G). Together, these results indicate that NG2+ cells generate myelinating OLs in both gray and white matter of the adult brain.
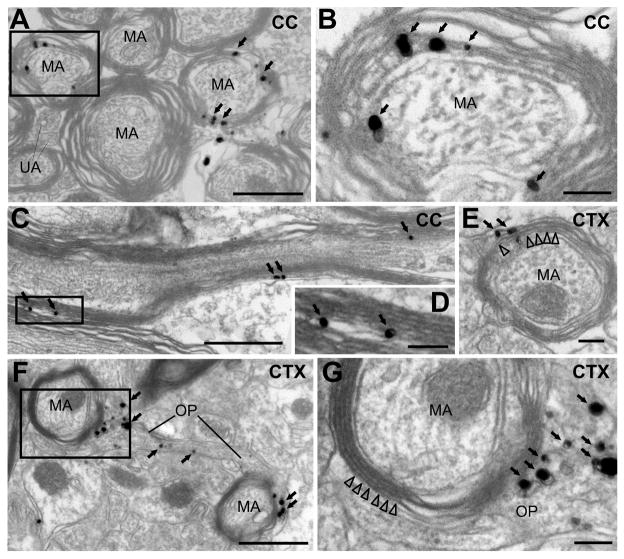
Figure 4. Oligodendrocytes generated from NG2+ cells form myelin.
Electron micrographs of the corpus callosum (A – C) and cortex (E – G) from PDGFαR-CreER;ROSA26-mGFP mice at P30+150 showing silver-enhanced immunogold labeling of EGFP. Arrows highlight silver particles located within myelin sheaths, and arrowheads in E and G indicate myelin. Black boxes in A, C and F are shown at higher magnification in B, D and G, respectively. MA, myelinated axon; OP, OL process; CC, corpus callosum; CTX, cerebral cortex. Scale bars = 100 nm (A, C, F) or 500 nm (B, D, E, G).
NG2+ cells do not develop into astrocytes in the postnatal brain, spinal cord or optic nerve
Recent studies have reported that NG2+ cells also generate astrocytes in some areas of the postnatal brain and spinal cord (Guo et al., 2009; Zhu et al., 2008a; Zhu et al., 2008b). However, none of the EGFP+ cells (0/4571 cells) in brain and spinal cord at these different ages exhibited immunoreactivity to the astrocyte-specific proteins GFAP or aldehyde dehydrogenase 1 family member L1 (ALDH) (Figure 5A– 5E). As it can be challenging to unambiguously identify astrocytes using antibodies against these proteins, we also used morphological criteria to identify astrocytes. Administration of 4HT to P10 GLAST-CreER;Z/EG or P15 GLAST-CreER;ROSA26-mGFP mice resulted in EGFP expression in many GFAP+ or ALDH+ cells that had highly ramified, complex morphologies typical of astrocytes (Bushong et al., 2004) (Figures 5H – 5K). These EGFP+ astrocytes were Olig2− (Figure 5I) and were readily distinguished from Olig2+ OL lineage cells. Notably, EGFP+ cells with the morphology of astrocytes were never observed in the brain or spinal cord of PDGFαR-CreER;Z/EG mice (Figures 5F and 5G), or in PDGFαR-CreER;ROSA26-EYFP mice at P30+120 (data not shown), where at least 80% NG2+ cells were sampled (See Table S1). Although it was previously reported that perinatal OPCs (O-2A progenitors) in the optic nerve can also become GFAP+ type 2 astrocytes (Ffrench-Constant and Raff, 1986), no EGFP+GFAP+ cells were observed in the optic nerve at various times after 4HT administration (0/477 cells) (Figure S5). Together, these results suggest that NG2+ cells are not progenitors for astrocytes in the postnatal CNS.
NG2+ cells in the postnatal brain do not develop into neurons
Recent studies also have reported that NG2+ cells serve as progenitors for GABAergic interneurons (Aguirre and Gallo, 2004; Aguirre et al., 2004) and glutamatergic principal neurons in vivo (Guo et al., 2009; Rivers et al., 2008). Although EGFP+ cells with neuronal morphologies were not observed in the dorso-medial cortex and corpus callosum, EGFP+ cells with large cell bodies and dendrite-like processes were occasionally observed in other brain regions in PDGFαR-CreER;Z/EG mice, particularly in ventral structures such as hypothalamus (Figures S6A – S6D). These atypical EGFP+ cells were PDGFαR− and Olig2−, but NeuN+ (Figures S6C and S7B), suggesting that they were neurons. These cells were rare; fewer than four EGFP+ neurons were found in each 35 μm thick coronal section, which contained >2,000 EGFP+ OL lineage cells. Labeling of these neurons was likely Cre-dependent, as they were not observed in animals treated with vehicle alone. However, EGFP+ neurons were visible within several days after 4HT administration (at P30); if they developed from NG2+ cells, these progenitors would have had to transform into neurons with unprecedented speed, as neurons derived from NSCs in the hippocampus and lateral ventricle require ~1.5 – 2 weeks to exhibit NeuN immunoreactivity (Zhao et al., 2008). If NG2+ cells serve as neuronal progenitors, the number of EGFP+ NeuN+ neurons also should increase with time after 4HT (Rivers et al., 2008), assuming that the newly generated neurons survive. However, greater numbers of EGFP+ neurons were not observed with increasing time after 4HT (Figures S6E and S6F).
NG2+ cells have been reported to generate neurons in the dentate gyrus (Aguirre et al., 2004), olfactory bulb (Aguirre and Gallo, 2004), and piriform cortex (Guo et al., 2009; Rivers et al., 2008). Although no EGFP+ neurons were observed in these regions in PDGFαR-CreER;Z/EG mice (Figure S6D for piriform cortex), it is possible that the subset of NG2+ cells with neurogenic potential were not sampled, as only 25–45% of NG2+ cells became EGFP+ in these mice (Table S1). However, in PDGFαR-CreER;ROSA26-mGFP mice, where more than 90% of NG2+ cells were labeled (Table S1), EGFP+ NeuN+ neurons also were not observed in the piriform cortex, dentate gyrus or olfactory bulb, either 4 days (P30+4) or 150 days (P30+150) after 4HT administration (Figures 6A – 6I). All EGFP+ cells in these mice at P30+150 were Olig2+ (Figure 6J – 6L).
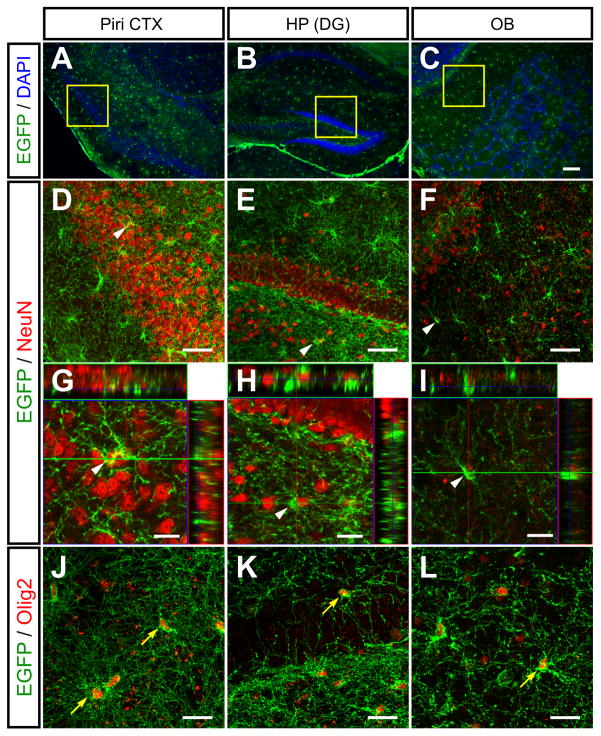
Figure 6. NG2+ cells do not give rise to neurons in the postnatal CNS.
(A – C) Images of EGFP+ cells the piriform cortex (Piri CTX; A) dentate gyrus (DG; B) and olfactory bulb (OB; C) in PDGFαR-CreER;ROSA26-mGFP mice at P30+150. Nuclei are labeled with DAPI.
(D – F) Confocal images showing EGFP and NeuN immunoreactivity in the regions highlighted by the yellow boxes in A – C. Orthogonal views are shown in (G – H) to indicate the spatial relationship between the NeuN+ neurons and EGFP+ cells indicated by the white arrowheads.
(J – L) Confocal images of EGFP+ cells exhibiting Olig2+ immunoreactivity in these brain regions. Yellow arrows highlight several EGFP+Olig2+ cells. Scale bars = 100μm (A – C), 50μm (D – F) and 20 μm (G – I).
Neurogenesis in the subventricular and subgranular zones occurs through asymmetric division of NSCs (Gould, 2007; Zhao et al., 2008). Thus, if EGFP+ neurons in PDGFαR-CreER mice are derived from NG2+ cells, it is expected that they would incorporate BrdU after 4HT administration. To increase the probability of detecting dividing neuronal progenitors, ROSA26-EYFP reporter mice were used to maximize NG2+ cell sampling (Figure S3A – S3D, and Table S1) and NG2+ cells were pre-labeled by continuously delivering BrdU to PDGFαR-CreER;ROSA26-EYFP mice for 11 or 21 days beginning at P23 (Figure S7A). Although 83% of NG2+ cells in the hypothalamus were BrdU+ at P30+14, none of the EYFP+NeuN+ cells observed were BrdU+ (n = 53) (Figure S7B). Together, these results suggest that NG2+ cells do not serve as progenitors for neurons in the postnatal brain, and that the infrequent appearance of reporter-positive neurons arose from direct Cre expression in these cells.
Proliferating NG2+ cells in ALS spinal cord differentiate into oligodendrocytes, but not astrocytes or neurons
NG2+ cells can develop into most major CNS cell types in vitro when exposed to different growth factors (Kondo and Raff, 2000), suggesting that environmental cues can influence the fate of these progenitors. Although our studies indicate that the fate of NG2+ cells was confined to the OL lineage in the normal CNS, NG2+ cells may adopt diverse fates following injury or during disease, when cytokine and growth factor levels change. It has been proposed that NG2+ progenitors in the spinal cord may have the ability to differentiate into astrocytes in ALS mice (Magnus et al., 2008), astrocytes and microglia after contusion injury (Sellers et al., 2009), and astrocytes and Schwann cells following focal injection of the demyelinating agent lysolecithin (Zawadzka et al., 2010). To determine whether NG2+ cells exhibit lineage plasticity in the context of a primary neurodegenerative disease, we followed the fate of these progenitors in the spinal cord of a mouse model of ALS (SOD1, G93A) (Gurney et al., 1994), in which progressive neurodegeneration and gliosis occur in a highly reproducible manner without external manipulation. Previous studies have shown that both neurons (Gurney et al., 1994) and astrocytes (Rossi et al., 2008) degenerate in the spinal cord of these mice, and astrocytes have been shown to incorporate BrdU (Guan et al., 2007; Magnus et al., 2008), suggesting that some astrocytes are replaced. Although the fate of OLs has not been determined in this model, it is also possible that they degenerate following the loss of motor neurons. Moreover, disease progression is accompanied by microglial activation, inflammation (Papadimitriou et al., 2010), disruption of the blood-spinal cord barrier, and a reduction in microcirculation (Zhong et al., 2008). Thus, ALS mice provide the opportunity to study the behavior of NG2+ cells in vivo in the context of extensive reactive changes that accompany cellular degeneration.
To examine how NG2+ cells in the spinal cord respond to changes in their local environment in ALS mice, BrdU was administered to SOD1 (G93A) mice for two days prior to analysis at P75 (pre-symptomatic), P90 (symptomatic) or ~P120 (end stage). The number of BrdU+ NG2+ cells (Olig2+) increased 4-fold (p = 0.02) in the lumbar spinal cord by the symptomatic stage, and 20-fold by end stage (p = 3.0×10−6 vs. control) (Figure 7), indicating that NG2+ cells contribute to the extensive gliosis observed in this model of ALS. To determine the fate of NG2+ cells in the spinal cord of these mice, we crossed PDGFαR-CreER;Z/EG mice to SOD1 (G93A) mice, administered 4HT at P60 and examined EGFP+ cells at end stage (~P120). Consistent with the increase in BrdU incorporation by NG2+ cells, there was a significant increase in EGFP+ cells at end stage as compared to control (p <1.0× 10−4) (Figures 8A, 8B and 8E). Moreover, a greater proportion of these EGFP+ cells were PDGFαR− and CC1+ in SOD1 (G93A) mice, indicating that the NG2+ cell differentiation was also enhanced (Figures 8C – 8E). Notably, none of the EGFP+ cells in SOD1 (G93A) mice exhibited immunoreactivity to GFAP+ (0/1,020 cells), Iba1+ (0/661 cells) or NeuN+ (0/1,511 cells) (Figures 8F – 8I), indicating that NG2+ cells in the spinal cord remain restricted to the OL lineage, despite the extensive neurodegeneration and gliosis that occur at later stages of this disease.
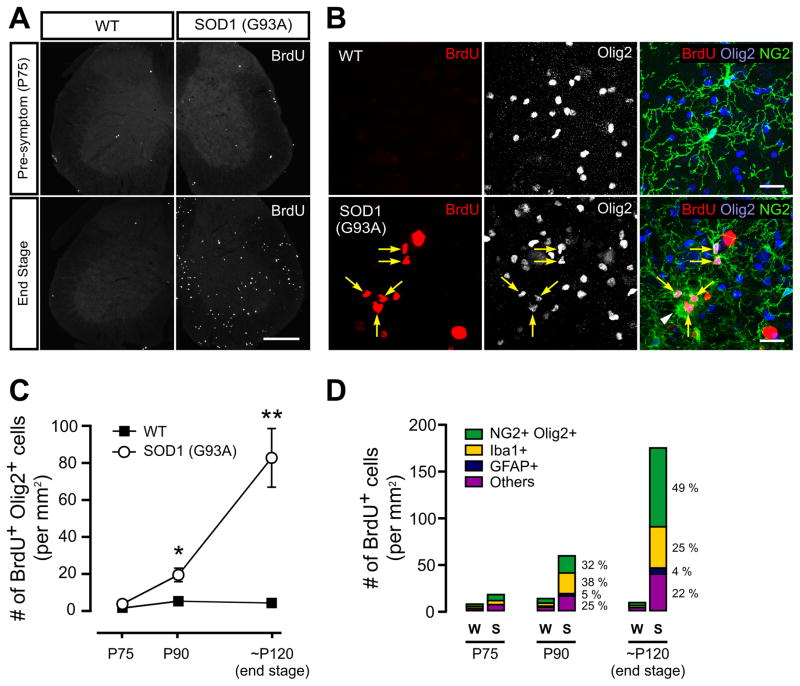
Figure 7. Enhanced proliferation of NG2+ cells in the spinal cord of ALS mice.
(A) Sections of lumbar spinal cord from wild type (WT) and SOD1 mutant (G93A) mice showing BrdU+ cells at the presymptomatic (upper panels) and end stages (lower panels). Scale bars = 500 μm.
(B) Confocal images showing BrdU, Olig2 and NG2 immunoreactivity in the lumbar spinal cord gray matter from WT and SOD1 (G93A) mice at end stage. Yellow arrows indicate BrdU+NG2+ cells, and the white arrowhead represents an NG2-expressing macrophage/microglia-like cell (Iba1+Olig2−). Scale bars = 20μm.
(C) Plot of the number of BrdU+ Olig2+ cells in the lumbar spinal cord of wild type and SOD1 (G93A) mice during disease progression (from P75 to end stage). The number of BrdU+ Olig2+ cells was significantly greater at P90 and end stage in SOD1 (G93A) mice than in age matched wild type littermates. * p <0.05. ** p < 0.0001. Statistical comparisons were made by Student’s t-test.
(D) Histograms showing the number of NG2+ cells (NG2+Olig2+), microglial cells (Iba1+), astrocytes (GFAP+) and other (undefined) cells that were BrdU+ in the lumbar spinal cord of wildtype and SOD1 (G93A) mice at different ages.
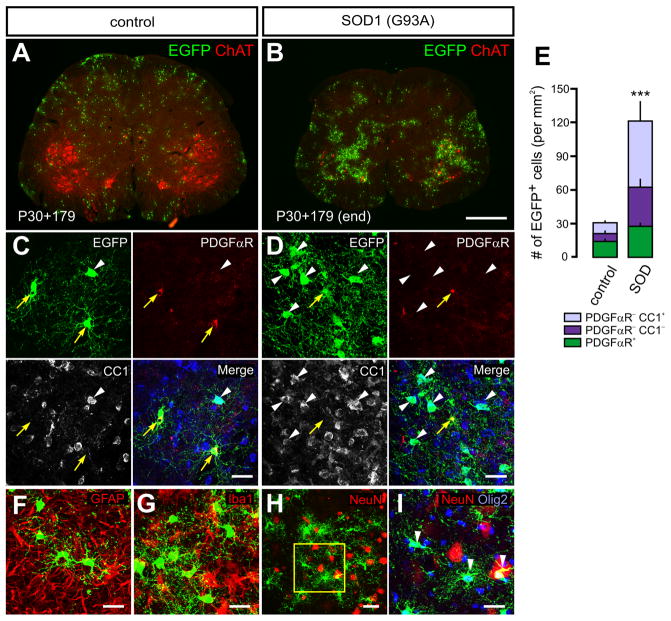
Figure 8. Enhanced differentiation of NG2+ cells into oligodendrocytes in ALS mice.
(A and B) Images of EGFP and choline acetyl transferase (ChAT) immunoreactivity in sections of lumbar spinal cord from aged-matched, littermate PDGFαR-CreER;Z/EG (A) and PDGFαR-CreER;Z/EG;SOD1(G93A) (B) mice at P30+179. Scale bars = 500μm.
(C and D) Confocal images of EGFP, PDGFαR and CC1 immunoreactivity in the lumbar spinal cord of PDGFαR-CreER;Z/EG (C) and PDGFαR-CreER;Z/EG;SOD1(G93A) (D) mice at P60+60. Yellow arrows and white arrowheads indicate EGFP+PDGFαR+ cells and EGFP+CC1+ cells, respectively. Scale bars = 20μm.
(E) Histograms of the number of EGFP+NG2+ cells (PDGFαR+), pre-myelinating OLs (PDGFαR−CC1−) and OLs (PDGFαR−CC1+) in the lumbar spinal cord of PDGFαR-CreER;Z/EG (control) and PDGFαR-CreER;Z/EG;SOD1(G93A) mice (SOD) that were administered 4HT at P60 and examined at end stage (~P120). *** p < 5.5×10−5 for comparisons of the number of EGFP+ cells between WT and SOD1(G93A) PDGFαR-CreER;Z/EG mice.
(F – I) Images of EGFP+ cells in PDGFαR-CreER;Z/EG;SOD1(G93A) mice at P60+60 (end stage) showing that these cells were not GFAP+ astrocytes (F), Iba1+ microglia (G) or NeuN+ neurons (H and I). (I) Higher magnification image of boxed area in H showing that all of EGFP+ cells were Olig2+. Scale bars = 20μm.
DISCUSSION
NG2+ cells constitute an abundant, widely distributed population of glial cells in the mammalian CNS that retain the ability to divide during adult life. As they can develop into both glial cells and neurons in vitro, NG2+ cells may serve as multipotent progenitors in vivo with the capacity to promote extensive cell replacement. The fate of NG2+ cells in the mouse brain has recently been evaluated in vivo using Cre-lox fate mapping in transgenic mouse lines (Dimou et al., 2008; Guo et al., 2009; Rivers et al., 2008; Zhu et al., 2008a). Although these studies observed that NG2+ cells are capable of generating OLs, they also reported that NG2+ cells can develop into astrocytes and neurons. However, conclusions about the alternative fate of NG2+ cells remain controversial, as NG2+ cells in some cases generated astrocytes, but not neurons (Zhu et al., 2008a; Zhu et al., 2008b), while in others they gave rise to neurons, but not astrocytes (Rivers et al., 2008), or both astrocytes and neurons (Guo et al., 2009). These contrasting results were attributed to differences in the developmental stages examined or to incomplete sampling of the population, implying that NG2+ cells are comprised of distinct groups with different lineage potential (Guo et al., 2009; Rivers et al., 2008).
In this study, we developed a new line of PDGFαR-CreER mice that allows sampling of up to 90% of NG2+ cells in the CNS during both early postnatal and adult life. Inducible labeling of these progenitors during early postnatal development revealed that NG2+ cells gave rise to myelinating OLs in both gray and white matter, in accordance with previous studies in rat using OL lineage markers (Levine et al., 1993), BrdU (Horner et al., 2000), and retrovirus (Levison et al., 1999). Moreover, NG2+ cells retained this capacity into adulthood, as shown recently using a different line of PDGFαR-CreER mice (Rivers et al., 2008). However, in contradiction to previous reports, our studies indicate that NG2+ cells do not develop into astrocytes or neurons during postnatal life, even in the context of gliosis and extensive neurodegeneration in the spinal cord of ALS mice. These findings suggest that NG2+ cells are not multipotent progenitors retained to generate diverse cell types in normal or degenerating states, but rather lineage restricted precursors that allow new OLs to be generated throughout life.
Sources of heterogeneity in NG2+ cell behavior
By labeling a cohort of NG2+ cells in different gray and white matter regions, it was possible to compare their rates of proliferation and differentiation at different times during development across many CNS regions. These studies show that the proliferation, differentiation, and eventual maturation of postnatal NG2+ cells peaked in the mouse brain during early postnatal life and slowed thereafter. However, NG2+ cell behaviors were not homogeneous, as these developmental changes occurred more rapidly in gray matter regions such as the cortex, than in white matter regions such as the corpus callosum, consistent with previous observations (Dimou et al., 2008; Rivers et al., 2008). By contrast, this difference in NG2+ cell behavior between white and gray matter was not apparent in spinal cord, where the overall rate of oligodendrogenesis is slower than in brain during the same period (between P30 and P120) (Figure 2). Previous studies have shown that manipulation of EGF or PDGFαR signaling in NG2+ cells has dramatic effects on their proliferation and differentiation in vivo (Aguirre and Gallo, 2007; Calver et al., 1998), raising the possibility that these regional differences in NG2+ cell behavior reflect differential availability of growth factors. An alternative, but not mutually exclusive possibility is that NG2+ cells are comprised of physiologically distinct subgroups destined for different fates (Dimou et al., 2008; Karadottir et al., 2008; Rivers et al., 2008). Consistent with the latter hypothesis, more than half of the NG2+ cells in the adult brain failed to incorporate BrdU when it was delivered continuously for several weeks to months (Dimou et al., 2008; Psachoulia et al., 2009; Rivers et al., 2008), and in the mature cortex most did not differentiate into OLs, suggesting that there may be a population of quiescent or postmitotic NG2+ cells (Dimou et al., 2008; Rivers et al., 2008).
To determine whether NG2+ cells are intrinsically heterogeneous in their ability to proliferate and differentiate, we performed a clonal analysis in vivo (Figure 3), by inducing Cre-mediated recombination in isolated NG2+ cells. Analysis of discrete EGFP+ cell clusters several months after administration of 4HT at P30 revealed that clones in white matter had more cells and contained a greater proportion of OLs than in gray matter, as expected from the higher rates of proliferation and differentiation of NG2+ cells in white matter; however, 96% of clones within the cortex also contained OLs, indicating that NG2+ cells within gray matter retained the ability to differentiate. If a significant proportion of NG2+ cells in brain are postmitotic, many isolated EGFP+NG2+ cells should have been visible at the end of the sample period (P30+80). However, all EGFP+NG2+ cells at this age occurred in multi-cellular clusters, while all isolated EGFP+ cells were PDGFαR−, indicating that they had undergone differentiation (Figure 3). These results suggest that all NG2+ cells in the mature brain, regardless of region, have the ability to divide and differentiate into OLs. These data do not support the conclusion that there is a distinct subpopulation of NG2+ cells which is postmitotic (Dimou et al., 2008; Rivers et al., 2008) or destined for a different cell fate (Guo et al., 2009; Rivers et al., 2008). The persistent differentiation of NG2+ cells into OLs in the adult CNS, raises the possibility that OLs in both the brain and spinal cord periodically degenerate, or that previously unmyelinated axons become myelinated. The decline in proliferation of NG2+ cells with age (Psachoulia et al., 2009; Rivers et al., 2008), which occurs most prominently in gray matter areas, may reflect local decreases in need for new OLs or limited access to growth factors, rather than transition to a postmitotic state.
NG2+ cells in the postnatal CNS do not develop into astrocytes
Despite extensive sampling of resident NG2+ cells, EGFP+ (or EYFP+) astrocytes were not observed in either brain or spinal cord, regardless of postnatal developmental stage or period of fate tracing (Figure 5). These findings are in agreement with observations made by Rivers et al. (2008) using a different line of PDGFαR-CreER mice, but contrast with studies using other lines of mice (Guo et al., 2009; Zhu et al., 2008a; Zhu et al., 2008b). What accounts for the discrepancy among studies that have studied the fate of NG2+ progenitors? In NG2-Cre mice (Zhu et al., 2008a; Zhu et al., 2008b), Cre is constitutively expressed under control of the NG2 promoter. If this promoter is active in astrocytes or astrocyte progenitors at any time during embryonic development, it would result in the presence of reporter-positive astrocytes in the postnatal CNS. Studies using PLP-CreER mice, in which Cre-mediated reporter expression was induced within a subpopulation of NG2+ cells, as well as mature OLs (Guo et al., 2009), also found that astrocytes became reporter-positive eight days following 4HT administration at P7. However, whereas more than 100kb of upstream and downstream regulatory sequence was used to construct the PDGFαR-CreER BAC transgene, Cre expression in PLP-CreER mice is controlled by a 2.4kb fragment of the PLP promoter (Doerflinger et al., 2003). It is possible that this promoter sequence does not contain sufficient regulatory elements to prevent Cre expression in cells outside the OL lineage. We tested the specificity of promoter activity in the same PLP-CreER;ROSA26-EYFP mice (Guo et al., 2009), and observed many EYFP+ astrocytes and neurons less than two days after 4HT administration to P7 mice (P7+1.5) (Figure S8). These observations indicate that expression of Cre is not limited to OL lineage cells in these mice, confounding clear interpretation of these fate tracing experiments.
Genetic fate mapping studies also have been performed using Olig2-CreER mice in which a CreER-encoding sequence was placed within the Olig2 locus (Dimou et al., 2008). Although Olig2 is a transcription factor that specifies OL fate (Ligon et al., 2006), some reporter-positive astrocytes were visible in Olig2-CreER mice when tamoxifen was administered to adult mice (Dimou et al., 2008). However, as discussed by Dimou et al. (2008), it is likely that Olig2 levels are significantly lower in these heterozygous mice due to disruption of one Olig2 allele by the CreER sequence (Takebayashi et al., 2002). Thus, it is possible that reporter-positive astrocytes arise in these mice because Olig2-dependent OL specification is weakened in NG2+ cells or their progenitors (Zhou and Anderson, 2002).
NG2+ cells do not develop into neurons in the postnatal brain
Among the most provocative questions concerning NG2+ cells is whether they serve as progenitors for neurons in the postnatal CNS. In support of this hypothesis, EGFP+ interneurons have been observed in the dentate gyrus of CNP-EGFP mice (Belachew et al., 2003), and some EGFP+NG2+ cells isolated from the subventricular zone of perinatal CNP-EGFP mice developed into interneurons after they were transplanted into neurogenic regions of early postnatal brains (Aguirre and Gallo, 2004; Aguirre et al., 2004). In addition, reporter-positive projection neurons were observed in recent Cre-lox fate mapping studies using both PLP-CreER mice (Guo et al., 2009) and a different line of PDGFαR-CreER mice (Rivers et al., 2008). A small number of EGFP+ neurons were present in our PDGFαR-CreER mice after postnatal 4HT administration, particularly in ventral brain regions (Figures S6 and S7). However, several observations suggest that these reporter-positive neurons arose through direct expression of CreER by neurons, rather than through differentiation of NG2+ cells. First, EGFP+ neurons were visible within a few days after 4HT exposure. By comparison, generation of morphologically distinct NeuN+ neurons in the dentate gyrus requires several weeks (Zhao et al., 2008), suggesting that it is unlikely that NG2+ cells could undergo such a rapid morphological transformation, given that time is also required for translocation of Cre, recombination and EGFP expression. Second, in contrast to Rivers et al. (2008), EGFP+ neurons did not accumulate over time in our mice, as would be expected if NG2+ cells serve continuously as neuronal progenitors. Third, although all reported forms of neurogenesis in the CNS occur through asymmetric division of progenitors (Gould, 2007; Zhao et al., 2008), BrdU+ EGFP+ neurons were never observed, despite near-saturation of NG2+ cells with BrdU, as also reported by Rivers et al. (2008). Thus, if these neurons developed from NG2+ cells, they must have been formed through direct differentiation without cell division. In this scenario, postmitotic NG2+ cells would be expected, but were not observed in our studies. And fourth, we did not observe any EGFP+ neurons in the pyriform cortex, dentate gyrus or olfactory bulb, regions where NG2+ cell-derived neurons have been previously reported (Aguirre and Gallo, 2004; Aguirre et al., 2004; Guo et al., 2009; Rivers et al., 2008), despite sampling of a larger proportion of the NG2+ cell population (e.g. > 90% of NG2+ cells in the piriform cortex). Thus, the appearance of reporter-positive neurons in this study likely resulted from direct expression of Cre by neurons, similar to that reported by Dimou et al. in Olig2-CreER mice (2008). Collectively, our results suggest that NG2+ cells are not multipotent in the normal brain and spinal cord.
NG2+ cells remain lineage restricted in the spinal cord of ALS mice
NG2+ cells in the adult CNS increase their rate of proliferation and often undergo dramatic reactive changes in response to acute injury or demyelination (Levine et al., 2001). Although our studies indicate that NG2+ cells are constrained to the OL lineage under normal conditions, alterations in growth factor availability and exposure to cytokines in degenerating regions could force greater lineage diversity. A demonstration of such lineage plasticity in vivo would indicate that NG2+ cells in the mature CNS are intrinsically multipotent, and present new opportunities for initiating cell replacement through manipulation of these endogenous progenitors. Moreover, because NG2+ cells are widely distributed throughout the CNS, stimulation of these progenitors could allow more extensive repair than is likely to be achieved through cell transplantation or viral-mediated cell transformation. Indeed, recent studies have reported that NG2+ cells in the spinal cord develop into astrocytes, microglia and pericytes after spinal cord hemisection (Sellers et al., 2009), and both astrocytes and Schwann cells after lysolecithin injection (Zawadzka et al., 2010).
In the SOD1(G93A) mouse model of ALS, motor neurons in the spinal cord degenerate, leading to progressive paralysis and premature death (Gurney et al., 1994). This degeneration is accompanied by extensive gliosis, involving proliferation, activation, and invasion of microglia/macrophages, as well as reactive changes in astrocytes (Hall et al., 1998) and astrocyte degeneration (Rossi et al., 2008). Our studies, and those reported previously (Magnus et al., 2008), indicate that NG2+ cells are prominent contributors to this gliosis; by end stage, they were the most actively proliferating cells, with proliferation rates 20-fold higher than normal (Figure 7). Our fate tracing results revealed that a greater proportion of EGFP+ cells became CC1+ (or PDGFαR−CC1−) by end stage, indicating there is also enhanced differentiation of these progenitors. However, no EGFP+ neurons or astrocytes were observed, indicating that NG2+ cells remained restricted to the OL lineage despite the dramatic changes that occur in the spinal cord in this model of ALS. Although the changes that occur in the spinal cord during the course of disease in ALS mice, such as cell death, inflammation, and gliosis, also occur in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease (Ilieva et al., 2009), and following stroke and acute CNS trauma, it is possible that NG2+ cells exhibit greater lineage plasticity in response to different disease and injury conditions (Sellers et al., 2009; Zawadzka et al., 2010 ). Analysis of the behavior of NG2+ cells in these different contexts may yield further insight into the factors that regulate their behavior, and the means to stimulate cell replacement by manipulating the proliferation and fate of these ubiquitous progenitors.
Why do NG2+ cells increase their rates of proliferation and differentiation during the course of disease in ALS mice? If NG2+ cells continue to serve as progenitors for OLs throughout life, their behavior may be a response to degeneration of OLs following motor neuron loss. It is possible that newly generated OLs exist in a differentiated, but non-myelinating state in these mice, perhaps due to the lack of appropriate targets, and eventually undergo cell death. Such a continuous cycle of proliferation, differentiation, and death of OL lineage cells during the course of this disease may consume resources and hasten the demise of remaining neurons already under stress. Moreover, as OLs are crucial for metabolic support of axons (Nave, 2010), a loss of OLs may further accelerate neuronal degeneration. The ability to manipulate gene expression within NG2+ cells in vivo using PDGFαR-CreER mice will help define the mechanisms that underlie these changes in NG2+ cell behavior, as well as the consequences of these changes for disease progression in different neurodegenerative disorders and in response to acute injury to the CNS.
EXPERIMENTAL PROCEDURES
Generation of PDGFαR-CreER transgenic mice
A bacterial artificial chromosome (BAC) containing the mouse PDGFαR gene was modified by homologous recombination method (Yang et al., 1997) to generate PDGFaR-CreER mice (See supplemental information). The PDGFαR-CreER mouse line that exhibited the highest Cre recombination efficiency (line 467) was selected for this study and maintained as hemizygous state with various reporter mice. Z/EG, ROSA26-EYFP and ROSA26-mGFP (mT/mG) mice were purchased from the Jackson Laboratory. All reporter mice were used as hemizygotes or heterozygotes. GLAST-CreER™ BAC mice were kindly provided by Drs. A. Rattner and J. Nathans, Johns Hopkins University. SOD1 (G93A) mutant mice were obtained from the Jackson Laboratory and crossed with PDGFαR-CreER;Z/EG mice. All experiments were carried out in strict accordance with protocols approved by the Animal Care and Use Committee at Johns Hopkins University.
Induction of Cre activity and BrdU administration
4-hydroxytamoxifen (4HT, Sigma H7904) was prepared as described previously (Badea et al., 2003), and 1mg or less was used per injection, unless stated otherwise. Each mouse received up to two injections per day, at least 8 hours apart. A total 0.1 mg, 4 mg or 5 mg of 4HT was used for the fate tracing from P4, P30 or P70, respectively. A single dose of 0.5 mg was injected at P30 for clonal analysis. For continuous exposure to BrdU (Sigma), mice were provided with BrdU-containing drinking water (1mg/ml supplemented with 1% sucrose), and received daily injections of BrdU (BrdU in 0.9% NaCl, 50mg/kg b.w., i.p.) additionally. For cell proliferation analysis in SOD1 (G93A) mice, BrdU (50mg/kg) was injected twice per day, at least 8 hrs apart, for two days before analysis.
Immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg b.w.) and perfused trancardially with 4% paraformaldehyde (PFA in 0.1M phosphate buffer, pH 7.4). Brains and spinal cords were postfixed in 4% PFA overnight at 4 °C, transferred to 30% sucrose solution (in PBS, pH 7.4), and stored at 4 °C for more than 36hrs. Tissue was sectioned (35 μm thick, unless stated otherwise) with a cryostat and immunofluorescence was performed on free-floating sections. Brain (coronal, bregma 0.5 to −1.9 mm) and spinal cord sections (from lumbar cord) were preincubated in blocking solution (5% normal donkey serum, 0.3% Triton X-100 in PBS pH 7.4) for 1 or 2 hrs at room temperature, then incubated overnight at 4 °C in primary antibodies. The primary and secondary antibodies used in this study are described in Tables S2 and S3. For Olig2, ALDH or APC(CC1) immunostaining, tissue sections were incubated for 10 minutes in LAB solution (Polysciences) before blocking. For BrdU immunostaining, sections were preincubated in 2N HCl at 37 °C for 30min, followed by neutralization with 0.1M sodium borate buffer (pH 8.5) prior to immunolabeling. For combined immunostaining of BrdU and EYFP, EYFP signal was enhanced using the ABC kit (Vector Lab) and TSA amplification system (Perkin Elmer) before acid denaturation. Secondary antibody incubation was performed at room temperature for 2 hrs. Sections were mounted on slides with ProLong antifade reagent (Invitrogen). Confocal images represent projected stacks of 15 – 45 images collected at 0.5 – 1.5μm steps.
Cell counting and Analysis
Mounted slides were imaged using an epifluorescence microscope (Zeiss Axio-imager M1), and Axiovision software (Zeiss), or a confocal laser scanning microscope (Zeiss LSM 510 Meta) using appropriate excitation and emission filters. A total of 3 – 12 sections were examined per mouse, and 3 – 5 mice were analyzed per group. For all studies except the clonal analysis, areas were chosen randomly within the indicated regions of brain or spinal cord. Significance was measured using the Student’s t-test or one-way ANOVA, and all data are reported as mean ± standard error of the mean (SEM).
Electron Microscopy
For pre-embedding immunoelectron microscopy, PDGFαR-CreER;mGFP mice (P30+150) were perfused transcardially with 4% paraformaldehyde/0.1% glutaraldehyde in 0.1 M PB under deep pentobarbital anesthesia. After blocking with 5% normal donkey serum in PBS, coronal sections (50 μm in thickness) including the corpus callosum were incubated overnight with rabbit anti-EGFP IgG and then with anti-rabbit IgG conjugated to 1.4 nm gold particles (Nanoprobes). Following silver enhancement (HQ silver, Nanoprobes), sections were osmificated, dehydrated and embedded in Epon 812 resin. Ultrathin sections were prepared with an ultramicrotome (Leica Ultracut UCT) and stained with 2% uranyl acetate. Electron micrographs were taken with an H-7600 electron microscope (Hitachi, Tokyo, Japan).
Highlights.
NG2+ cells generate myelinating oligodendrocytes in the developing and adult CNS
Gray matter NG2+ cells are not postmitotic and retain the ability to form OLs
NG2+ cells are not progenitors of astrocytes or neurons in the postnatal CNS
Proliferating NG2+ cells in ALS mice do not exhibit lineage plasticity
Supplementary Material
Acknowledgments
We thank N. Ye and N. Nishiyama for mouse colony maintenance and genotyping, I. Srivastava for technical assistance in histological procedures, Dr. R. Huganir (Johns Hopkins University, Baltimore) for antibodies to GFP, Dr. B. Novitch (University of California, Los Angeles) for antibodies to Olig2, Dr. W. Stallcup (Burnham Institute, San Diego) for antibodies to NG2 and PDGFαR, Dr. Watanabe (Hokkaido University, Japan) for antibodies to GFP and GLUT1, and Dr. M. Wegner (University of Erlangen) for antibodies against Sox10. GLAST-CreER mice were generously provided by Drs. A. Ratner and J. Nathans (Johns Hopkins University). This work was supported by the US National Institutes of Health grants NS051509, NS050274 and MH084020, the Muscular Dystrophy Association, the Packard Center for ALS Research at Johns Hopkins, and P2ALS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3:209–220. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouslama-Oueghlani L, Wehrle R, Sotelo C, Dusart I. Heterogeneity of NG2-expressing cells in the newborn mouse cerebellum. Dev Biol. 2005;285:409–421. doi: 10.1016/j.ydbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. Nature. 1986;323:335–338. doi: 10.1038/323335a0. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Guan YJ, Wang X, Wang HY, Kawagishi K, Ryu H, Huo CF, Shimony EM, Kristal BS, Kuhn HG, Friedlander RM. Increased stem cell proliferation in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. J Neurochem. 2007;102:1125–1138. doi: 10.1111/j.1471-4159.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56:200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiol Dis. 2010;37:493–502. doi: 10.1016/j.nbd.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29:6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.