Abstract
Traumatic brain injury (TBI) can lead to several physiologic complications including gastrointestinal dysfunction. Specifically, TBI can induce an increase in intestinal permeability, which may lead to bacterial translocation, sepsis, and eventually multi-system organ failure. However, the exact mechanism of increased intestinal permeability following TBI is unknown. We hypothesized that expression of tight junction protein ZO-1 and occludin, responsible for intestinal architectural and functional integrity, will decrease following TBI and increase intestinal permeability. BALB/c mice underwent a weight drop TBI model following anesthesia. Brain injury was confirmed by a neurologic assessment and gross brain pathology. Six hours following injury, FITC-dextran (25 mg 4.4 kDa FITC-dextran) was injected into the intact lumen of the isolated ileum. Intestinal permeability was measured in plasma 30 min following injection, by using spectrophotometry to determine plasma FITC-dextran concentrations. Whole ileum extracts were used to measure expression of tight junction proteins ZO-1 and occludin by Western blot. TBI caused a significant increase in intestinal permeability (110.0 μg/mL ±22.2) compared to sham animals (29.4 μg/mL ± 9.7) 6 h after injury (p = 0.016). Expression of ZO-1 was decreased by 49% relative to sham animals (p < 0.02), whereas expression of occludin was decreased by 73% relative to sham animals (p < 0.001). An increase in intestinal permeability corresponds with decreased expression of tight junction proteins ZO-1 and occludin following TBI. Expression of intestinal tight junction proteins may be an important factor in gastrointestinal dysfunction following brain injury.
Key words: intestinal permeability, tight junctions, traumatic brain injury
Introduction
Traumatic brain injury (TBI) continues to be a major medical problem in the United States, costing over $4 billion annually in health care costs and loss of productivity (McGarry et al., 2002). What is becoming increasingly apparent are the systemic physiological effects following TBI, including autonomic dysfunction, systemic inflammation, and organ dysfunction (Baguley et al., 2008). Specifically, gastrointestinal dysfunction is frequently observed in TBI patients, including motility abnormalities and mucosal alterations that can lead to ulceration, inflammation, and increased gut permeability (Kao et al., 1998; Hang et al., 2003). Feighery and associates have demonstrated blunting of intestinal villi and increased intestinal permeability 6 h following TBI in rat models (Feighery et al., 2008). Similarly, Hang and colleagues have also shown histopathologic changes and increased gut permeability as early as 2 h, and peaking at 24 h following TBI (Hang et al., 2003). However, the mechanism of the resultant increased gut permeability following TBI has yet to be determined.
Intestinal epithelial cells function as a physical barrier between the intestinal lumen and the underlying vasculature and lymphatics. This is largely due to the structural integrity of cellular tight junctions. Tight junctions are composed of a series of interacting transmembrane proteins, including claudins, occludin, and junctional adhesion molecules (Schneeberger and Lynch, 2004). The loss of tight junction barrier integrity has been correlated with increased intestinal permeability, and hypothetically bacterial translocation. Costantini and co-workers have demonstrated that the tight junction structural proteins zona occludens protein-1 (ZO-1) and occludin are significantly decreased following systemic burn injury in mice (Costantini et al., 2008). To our knowledge, there has been no evidence linking TBI to a loss of tight junction proteins in the intestinal epithelium as a mechanism of increased intestinal permeability. Therefore we hypothesize that TBI decreases expression of intestinal tight junction proteins ZO-1 and occludin as a mechanism for increasing intestinal permeability.
Methods
Mouse TBI model
The animal experiments, including anesthesia, TBI, and recuperation, were approved by the university institutional animal care and use committee (approval #S08110). Male BALB/c mice (body weight 20–24 g) were obtained from The Jackson Laboratory (Sacramento, CA) and placed under a 12-h light/dark cycle. A weight drop TBI model, as previously described, was used to cause cerebral contusion (Stahel et al., 2000; Habgood et al., 2007). Briefly, the animals were anesthetized with 3% inhaled isoflurane (Hospira, Lake Forest, IL) by way of a veterinary vaporizer (Ohio Medical Products, Madison, WI). The flow of isoflurane was titrated to reach appropriate anesthesia for each animal. Each animal was manually secured and its head shaved with an electric clipper. A vertical incision was made over the cranium and using a surgical drill, a burr hole 4 mm in diameter, 1 mm lateral, and 1 mm posterior to the bregma was created to expose the dura mater. A 250-g metal rod was dropped from a height of 2 cm onto the exposed dura mater. The incision was closed with vet bond and buprenorphine (100 μL) was injected subcutaneously for analgesia in both sham and TBI animals. Food and water were provided ad libitum. Sham animals underwent an identical procedure, excluding the weight drop. Six hours following TBI or sham procedure, the animals underwent an in-vivo intestinal permeability assay (see below) or were sacrificed with inhaled isoflurane and underwent removal of the terminal ileum, which was stored in formalin for histologic evaluation. Additionally, all mice brains were harvested after sacrifice and examined grossly for brain injury at 6 h following TBI.
Neurologic evaluation
The animals underwent neurological evaluation at the 6-h time point post-TBI or sham operation according to criteria adapted from Garcia and associates (Garcia et al., 1995). A total of six tests were performed on each animal, including spontaneous cage activity, symmetry of limb movements, symmetry of forelimb extension, climbing activity, reaction to touch on trunk, and reaction to touch on forepaws. Each test was assigned a score from 0–3 (with 3 being the best) and all tests were summed giving a best possible score of 18.
In-vivo intestinal permeability assay
The animals underwent an in-vivo intestinal permeability assay at 6 h following TBI, according to the method previously described by Costantini and colleagues (Costantini et al., 2008). Six hours following TBI or sham operation, the animals were anesthetized by inhaled isoflurane. A mid-line laparotomy was performed, the cecum was located, and a 5-cm segment of distal ileum was exteriorized and isolated between silk ties. Previously prepared FITC-dextran (25 mg 4.4-kDa FITC-dextran in 200 μL PBS) was injected into the lumen of the isolated ileum. The eviscerated intestine was returned to the abdominal cavity and the abdominal wall was closed. Thirty minutes following the injection blood was collected by cardiac puncture. Blood samples were placed into heparinized Eppendorf tubes and centrifuged at 10,000 g for 10 min. Plasma was removed and subsequently assayed using a SpectraMax M5 fluorescence spectrophotometer (Molecular Devices, Sunnyvale, CA) to determine the concentration of FITC-dextran. A standard curve for the assay was obtained through the serial dilution of FITC-dextran in mouse serum.
Histological evaluation
Using an automated processor, segments of distal ileum previously stored in formalin were embedded in paraffin blocks. Sections were cut, placed onto glass slides, and stained with hematoxylin-eosin (H&E; Richard Allen Scientific, Kalamazoo, MI). Images were later obtained using Q-imaging software and a light microscope at varying magnifications. A pathologist who was blinded to the groups examined each ileum specimen and scored each specimen using a modified histopathologic score by Cuzzocrea and co-workers (Cuzzocrea et al., 2002). A scale of 0–3 was used to assess intestinal damage: 0 = normal, no damage; 1 = mild, focal epithelial edema and necrosis; 2 = moderate, diffuse swelling or necrosis of the villi; and 3 = severe, diffuse necrosis of the villi with evidence of neutrophil infiltration in the submucosa or hemorrhage.
Immunoblotting
Intestinal extracts of each animal group were made by homogenizing distal ileum, dissected clean from surrounding adventitia, in commercially available protease inhibitor (Pierce Biotechnology, Rockford, IL), and phosphatase inhibitor (Pierce Biotechnology) mixed with a tissue protein extraction reagent (T-PER, Rockford, IL) in a 1:100 ratio was added. The solution was transferred to another tube, placed on ice, and centrifuged to pellet the debris. The supernatant was removed and its protein concentration determined by the bicinchoninic acid protein assay (Pierce Biotechnology). The samples were subsequently suspended in sodium dodecyl sulfate sample buffer (Invitrogen, Carlsbad, CA) and diluted with deionized water to achieve a concentration of 40 μg protein in 25 μL total volume. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (130 volts for 90 min) using 8–16% tris-glycine polyacrylamide gradient gels and then transferred onto nitrocellulose membranes (Invitrogen). The membranes were blocked with 5% BSA in TBS/Tween 20 for 1 h. A primary antibody specific to occludin (1:500 in 5% BSA; Santa Cruz Biotechnology, Santa Cruz, CA) or ZO-1 (1:500 in 5% BSA; Zymed, Carlsbad, CA) was incubated with the membranes overnight at a temperature of 4°C. The membranes were washed and incubated for 1 h at room temperature with the secondary antibody horseradish peroxidase-linked anti-rabbit IgG (1:2000; Cell Signaling, Danvers, MA) prepared in blocking solution. After thorough washing, the Pierce Supersignal West Pico Chemiluminescent Kit was applied for antibody detection with the Xenogen IVIS Lumina Imaging System (Caliper Life Sciences, Hopkinton, MA). Mean pixel density was estimated using UN-SCAN-IT Gel Digitizing software (Silk Scientific, Orem, UT). Relative band density was calculated by dividing the pixel density of each sample by the mean pixel density of sham samples.
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). The statistical significance among groups was determined by a two-tailed t-test, and a p value < 0.05 was considered statistically significant. Comparison of intestinal histopathologic scores was determined by a Mann-Whitney U test.
Results
Brain findings following TBI
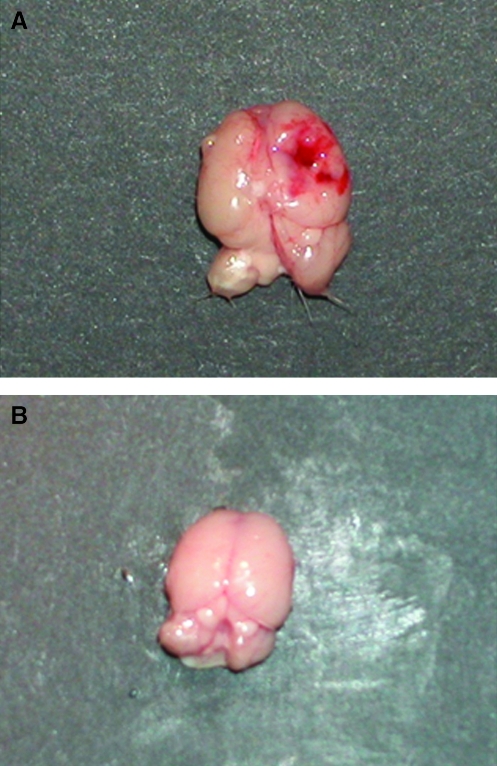
The weight drop TBI model caused a contusion to the right parietal cortex resulting in multiple areas of hemorrhage as well as diffuse edema confirmed by brain extraction and gross examination. Figure 1 shows a representative post-TBI brain specimen.
FIG. 1.
Representative brain tissue from mice following the weight drop TBI model (A) and sham (B). The TBI brain shows a focal hemorrhagic contusion.
Neurologic assessment
All animals underwent neurological assessment and scoring 6 h following either sham operation or TBI. Mice undergoing TBI had a decreased neurological score (10.5 ± 1) compared to sham animals (18.0 ± 0) at 6 h, which confirmed the neurologic injury (p < 0.01).
Intestinal permeability
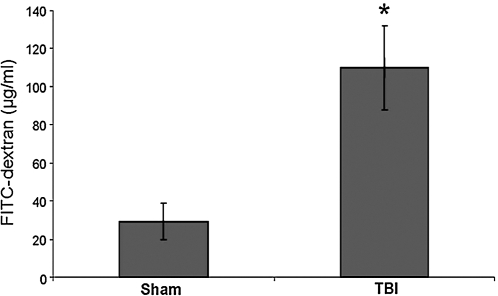
In-vivo intestinal permeability was determined 6 h following either sham or TBI by spectrophotometric measurement of plasma following intraluminal injection of 4.4-kDa FITC-dextran. TBI caused a significant increase in intestinal permeability (110.0 ± 22.2 μg/mL) compared to sham (29.4 ± 9.7 μg/mL) after 6 h (p = 0.016) (Fig. 2).
FIG. 2.
Intestinal permeability as determined by serum FITC-dextran (25 mg 4.4-kDa FITC-dextran in 200 μL PBS) injected into the lumen of the isolated ileum in both sham (n = 4) and TBI mice (n = 4). TBI caused a significant increase in intestinal permeability (110.0 ± 22.2 μg/mL) compared to sham (29.4 ± 9.7 μg/mL) after 6 h (p = 0.016).
Histopathologic evaluation
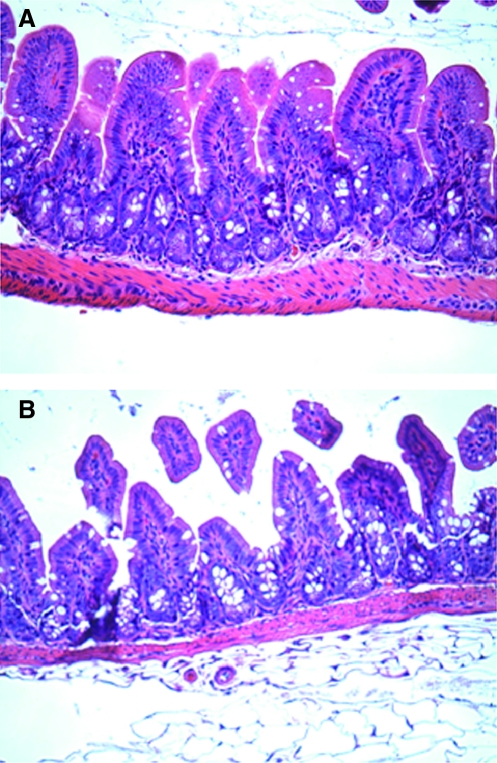
The terminal ileum was harvested 6 h following both TBI and sham operation for histologic analysis using H&E staining. Sham animals had normal-appearing villi with consistent villous height. The histologic appearance of intestinal specimens from TBI animals was notable for marked blunting of intestinal villi with architectural deformity and intestinal villi necrosis (Fig. 3). The degree of mean intestinal injury was markedly increased in the TBI group (2.4 ± 0.5) compared to the sham animals (1.0 ± 0; p < 00.03).
FIG. 3.
Representative H&E staining and microscopy (60×) from the terminal ileum harvested 6 h following both TBI and sham mice. Sham animals (A) had normal-appearing villi with consistent villous height. The histologic appearance of intestinal specimens from TBI animals (B) were notable for marked blunting of intestinal villi.
ZO-1 expression
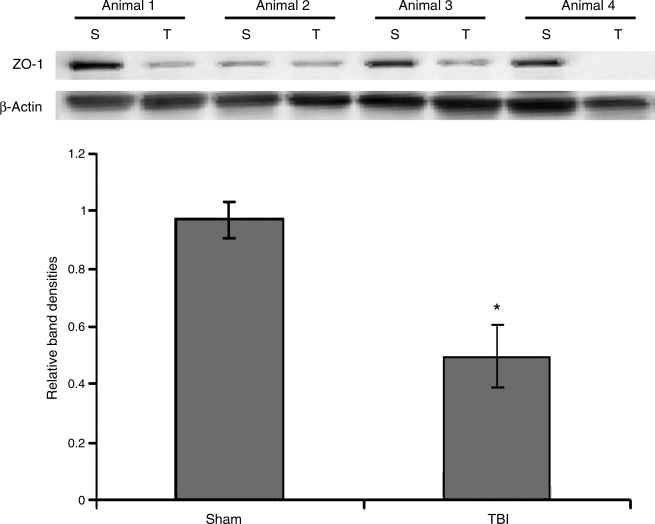
The tight junction protein ZO-1 was measured at 6 h following TBI or sham procedure by Western immunoblotting. At 6 h, a 49% decrease in intestinal ZO-1 was observed in TBI animals compared to sham counterparts (p < 0.02). A graphical representation of relative band densities and representative Western blots are shown in Figure 4.
FIG. 4.
Expression of ZO-1 was measured in intestinal protein extracts 6 h following TBI or sham injury by Western immunoblotting (n = 4 animals in each group). Intestinal ZO-1 expression was decreased by 49% in TBI animals compared to sham animals (p < 0.02). The Western blots of all four animals from each group are shown with β-actin (S, sham; T, TBI).
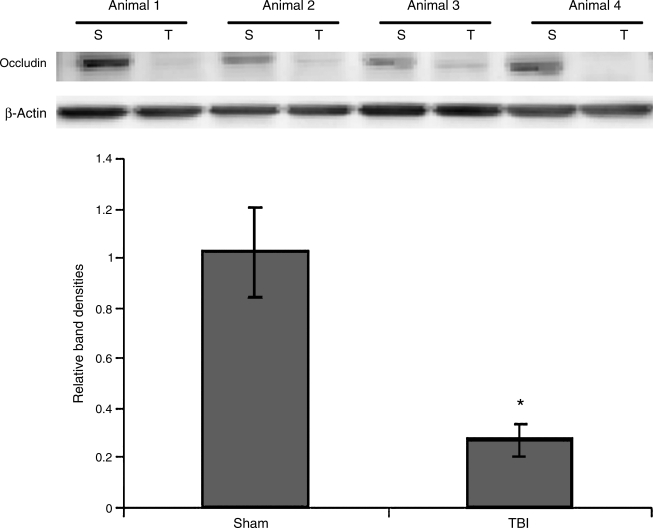
Occludin expression
Intestinal occludin was measured 6 h following TBI by Western immunoblotting. At 6 h following TBI, intestinal occludin was decreased 73% compared to sham animals (p < 0.001). A graphical representation of relative band densities and representative Western blots are shown in Figure 5.
FIG. 5.
Expression of occludin was measured in intestinal protein extracts 6 h following TBI or sham injury by Western immunoblotting (n = 4 animals in each group). Intestinal occludin expression was decreased by 73% in TBI animals compared to sham animals (p < 0.001). The Western blots of all four animals from each group are shown with β-actin (S, sham; T, TBI).
Discussion
Isolated TBI has systemic consequences for several organs, leading to important clinical sequelae, including pneumonia, cardiovascular disorders, autonomic abnormalities, intestinal dysfunction, and multi-system organ failure (Osborn et al., 2004; Kemp et al., 2008). Several areas of intestinal dysfunction following TBI have been described, including stomach ulceration and gastritis (Cushing's ulcer), prolonged ileus and other motility problems, and most importantly impairment of gut barrier function (Kao et al., 1998; Feighery et al., 2008). However, the precise mechanism through which TBI leads to these gastrointestinal events is unknown. Normally-functioning organs with intact epithelial lining rely on the establishment and maintenance of compartments by cell-to-cell integrity that is regulated in part by tight junction proteins (Anderson and Van Itallie, 1995). This barrier, most notably in the gut and lung, plays a critical role in preventing systemic contamination by microbes and toxins present in the external environment (Anderson and Van Itallie, 1995; Han et al., 2004).
Previously, using a mouse model we have shown a decrease in the protein expression of the tight junction proteins ZO-1 and occludin occurs following a 30% body surface area burn. Decreased expression of tight junction proteins was correlated with an increase in intestinal TNF-α (Costantini et al., 2008). Several investigators have reported a similar increase in inflammatory cytokines following TBI, thus providing a possible explanation for the observed increased intestinal permeability. Studies analyzing post-TBI intestines and serum have shown the importance of a local inflammatory response, mediated in part by an increase in nuclear factor-κB activation, and intestinal concentrations of pro-inflammatory cytokines such as TNF-α, and IL-6 (Hang et al., 2005a; Hang et al., 2005b). Costantini and associates have shown that immunomodulation by pentoxifyline, a potent anti-inflammatory agent, protected against intestinal permeability following burn injury (Costantini et al., 2008). Likewise, several other investigators have demonstrated the effects of other mediators following TBI, including glutamine and progesterone, which decrease IL-1, TNF-α, and IL-6 levels in intestinal and brain tissue, respectively (Chen et al., 2008b; Chen et al., 2008a). Jin and colleagues, using transgenic nuclear factor-erythroid 2–related factor 2 knockout mice (a transcriptional factor having cytoprotective properties against inflammation), showed that increased levels of TNF-α, IL-1β, and IL-6 production correlated with higher intestinal permeability following TBI (Jin et al., 2008). Despite this evidence, the molecular mechanism behind increasing gut permeability following TBI or other traumatic stress is still unknown.
Modulation of tight junction protein expression may explain, at least in part, the increase in intestinal permeability seen following brain injury. Mazzon and Cuzzocrea demonstrated that the structure and function of tight junction proteins on the intestinal epithelia depends on the regulation and production of TNF-α (Mazzon and Cuzzocrea, 2006). Another possible mechanism to explain the attenuation of tight junction protein expression following TBI may involve intestinal epithelial cell apoptosis, since apoptotic cells in the mucosal epithelium have been described in rat TBI experiments (Hang et al., 2007). Overexpression of Bcl-2, a known anti-apoptotic factor, can also significantly reduce intestinal damage, preventing tight junction breakdown (Hang et al., 2007; Mazzon and Cuzzocrea, 2008). However, the relationship of apoptosis and its potential effects on tight junction proteins has not been well characterized (Zeissig et al., 2004).
In this study, we have shown that TBI significantly decreased the expression of the intestinal tight junction proteins ZO-1 and occludin, which correlates to increased intestinal permeability and distinct changes in intestinal histology. The role of ZO-1 and occludin in maintaining intestinal tight junctions has been investigated in the clinical arena. In an analysis of colons from patients with active Crohn's disease, Zeissig and co-workers showed a reduced number of intestinal tight junction strands by electron microscopy (Zeissig et al., 2007). This reduction was also reflected in a decrease of the claudin family of proteins, including occludin and ZO-1. Certain anesthetics, including isoflurane, have immunomodulatory effects; therefore it is possible that these experiments may underestimate the degree of intestinal permeability present following TBI in non-anesthetized animals (Fuentes et al., 2006). To our knowledge, our study is the first to show that TBI attenuates tight junction protein expression. Whether these post-TBI tight junction protein changes are caused by a release of systemic and/or local tissue inflammatory cytokines or an increase in epithelial apoptosis is unknown. What is also unknown is the molecular signaling mediating an increase in intestinal permeability from a local TBI insult. We recognize that general tissue injury, including burns, torso injury, and TBI, may decrease intestinal tight junction proteins by several mechanisms, including a heightened adrenergic state. However, the concept of the neuro-enteric axis is emerging as an interesting physiologic mechanism in the pathogenesis of gastrointestinal disease. In an important study, Bush and associates reported that inhibiting enteric glia, marked by a decrease in glial fibrillary acid protein, caused severe inflammation and necrosis of the small intestine in mice models (Bush et al., 1998). Ma and colleagues recently described the histologic juxtaposition of enteric glial cells to gut-associated lymphoid tissue in small bowel samples from mice, including the presence of muscarinic acetylcholine receptors at the junction of the nerve endings, suggesting central nervous system regulation of intestinal inflammatory cells (Ma et al., 2007). In a mouse model of chronic inflammatory arthritis, Wu and co-workers demonstrated that glial cells can actively secrete IL-1 and prostaglandin E2, causing a marked decrease in both occludin and ZO-1, and increasing permeability of the leptomeninges (Wu et al., 2008). The physiologic similarities and differences between intestinal and central nervous system glial cells have yet to be determined. It is plausible that enteric glia may represent a direct pathway for central nervous system control of gut physiology. Future investigations will be needed to better identify these pathways that ultimately lead to gut barrier breakdown after TBI.
In summary, we have observed an increase in intestinal permeability and marked changes in intestinal histology 6 h following TBI. Intestinal extracts show that expression of the tight junction proteins ZO-1 and occludin decrease following TBI compared to sham animals. Levels of intestinal tight junction proteins may be an important factor in increased intestinal permeability following TBI.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Anderson J.M. Van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Baguley I.J. Heriseanu R.E. Cameron I.D. Nott M.T. Slewa-Younan S. A critical review of the pathophysiology of dysautonomia following traumatic brain injury. Neurocrit. Care. 2008;8:293–300. doi: 10.1007/s12028-007-9021-3. [DOI] [PubMed] [Google Scholar]
- Bush T.G. Savidge T.C. Freeman T.C. Cox H.J. Campbell E.A. Mucke L. Johnson M.H. Sofroniew M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Chen G. Shi J. Jin W. Wang L. Xie W. Sun J. Hang C. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann. Clin. Lab. Sci. 2008b;38:65–74. [PubMed] [Google Scholar]
- Chen G. Shi J. Qi M. Yin H. Hang C. Glutamine decreases intestinal nuclear factor kappa B activity and pro-inflammatory cytokine expression after traumatic brain injury in rats. Inflamm. Res. 2008a;57:57–64. doi: 10.1007/s00011-007-7101-7. [DOI] [PubMed] [Google Scholar]
- Costantini T.W. Loomis W.H. Putnam J.G. Drusinsky D. Deree J. Choi S. Wolf P. Baird A. Eliceiri B. Bansal V. Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: Effects on tight junction structural proteins. Shock. 2008;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S. Chatterjee P.K. Mazzon E. Dugo L. De Sarro A. Van de Loo F.A. Caputi A.P. Thiemermann C. Role of induced nitric oxide in the initiation of the inflammatory response after postischemic injury. Shock. 2002;18:169–176. doi: 10.1097/00024382-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Feighery L. Smyth A. Keely S. Baird A.W. O'Connor W.T. Callanan J.J. Brayden D.J. Increased intestinal permeability in rats subjected to traumatic frontal lobe percussion brain injury. J. Trauma. 2008;64:131–137. doi: 10.1097/TA.0b013e3181568d9f. discussion 137–138. [DOI] [PubMed] [Google Scholar]
- Fuentes J.M. Talamini M.A. Fulton W.B. Hanley E.J. Aurora A.R. De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin. Vaccine Immunol. 2006;13:281–288. doi: 10.1128/CVI.13.2.281-288.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.H. Wagner S. Liu K.F. Hu X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- Habgood M.D. Bye N. Dziegielewska K.M. Ek C.J. Lane M.A. Potter A. Morganti-Kossmann C. Saunders N.R. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur. J. Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- Hang C.H. Shi J.X. Li J.S. Li W.Q. Wu W. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J. Surg. Res. 2005a;123:188–193. doi: 10.1016/j.jss.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Hang C.H. Shi J.X. Li J.S. Li W.Q. Yin H.X. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J. Gastroenterol. 2005b;11:1149–1154. doi: 10.3748/wjg.v11.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang C.H. Shi J.X. Li J.S. Wu W. Yin H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003;9:2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang C.H. Shi J.X. Sun B.W. Li J.S. Apoptosis and functional changes of dipeptide transporter (PepT1) in the rat small intestine after traumatic brain injury. J. Surg. Res. 2007;137:53–60. doi: 10.1016/j.jss.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Han X. Fink M.P. Uchiyama T. Yang R. Delude R.L. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G126–G136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- Jin W. Wang H. Ji Y. Hu Q. Yan W. Chen G. Yin H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 2008;44:135–140. doi: 10.1016/j.cyto.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Kao C.H. ChangLai S.P. Chieng P.U. Yen T.C. Gastric emptying in head-injured patients. Am. J. Gastroenterol. 1998;93:1108–1112. doi: 10.1111/j.1572-0241.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Kemp C.D. Johnson J.C. Riordan W.P. Cotton B.A. How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am. Surg. 2008;74:866–872. [PubMed] [Google Scholar]
- Ma B. von Wasielewski R. Lindenmaier W. Dittmar K.E. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat. Histol. Embryol. 2007;36:62–74. doi: 10.1111/j.1439-0264.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- Mazzon E. Cuzzocrea S. Role of TNF-alpha in ileum tight junction alteration in mouse model of restraint stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G1268–G1280. doi: 10.1152/ajpgi.00014.2008. [DOI] [PubMed] [Google Scholar]
- Mazzon E. Cuzzocrea S. Thalidomide treatment reduces the alteration of paracellular barrier function in mice ileum during experimental colitis. Shock. 2006;25:515–521. doi: 10.1097/01.shk.0000209556.31457.e7. [DOI] [PubMed] [Google Scholar]
- McGarry L.J. Thompson D. Millham F.H. Cowell L. Snyder P.J. Lenderking W.R. Weinstein M.C. Outcomes and costs of acute treatment of traumatic brain injury. J. Trauma. 2002;53:1152–1159. doi: 10.1097/00005373-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Osborn T.M. Tracy J.K. Dunne J.R. Pasquale M. Napolitano L.M. Epidemiology of sepsis in patients with traumatic injury. Crit. Care Med. 2004;32:2234–2240. doi: 10.1097/01.ccm.0000145586.23276.0f. [DOI] [PubMed] [Google Scholar]
- Schneeberger E.E. Lynch R.D. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Shohami E. Younis F.M. Kariya K. Otto V.I. Lenzlinger P.M. Grosjean M.B. Eugster H.P. Trentz O. Kossmann T. Morganti-Kossmann M.C. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Wu Z. Tokuda Y. Zhang X.W. Nakanishi H. Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiol. Dis. 2008;32:543–551. doi: 10.1016/j.nbd.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Zeissig S. Bojarski C. Buergel N. Mankertz J. Zeitz M. Fromm M. Schulzke J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S. Burgel N. Gunzel D. Richter J. Mankertz J. Wahnschaffe U. Kroesen A.J. Zeitz M. Fromm M. Schulzke J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]