Abstract
Background
To determine whether a Web-based diabetes case management program based in an electronic medical record can improve glycemic control (primary outcome) and diabetes-specific self-efficacy (secondary outcome) in adults with type 1 diabetes, a pilot randomized controlled trial was conducted.
Methods
A 12-month randomized trial tested a Web-based case management program in a diabetes specialty clinic. Patients 21–49 years old with type 1 diabetes receiving multiple daily injections with insulin glargine and rapid-acting analogs who had a recent A1C >7.0% were eligible for inclusion. Participants were randomized to receive either (1) usual care plus the nurse-practitioner-aided Web-based case management program (intervention) or (2) usual clinic care alone (control). We compared patients in the two study arms for changes in A1C and self-efficacy measured with the Diabetes Empowerment Scale.
Results
A total of 77 patients were recruited from the diabetes clinic and enrolled in the trial. The mean baseline A1C among study participants was 8.0%. We observed a nonsignificant decrease in average A1C (−0.48; 95% confidence interval −1.22 to 0.27; P = 0.160) in the intervention group compared to the usual care group. The intervention group had a significant increase in diabetes-related self-efficacy compared to usual care (group difference of 0.30; 95% confidence interval 0.01 to 0.59; P = 0.04).
Conclusions
Use of a Web-based case management program was associated with a beneficial treatment effect on self-efficacy, but change in glycemic control did not reach statistical significance in this trial of patients with moderately poorly controlled type 1 diabetes. Larger studies may be necessary to further clarify the intervention's impact on health outcomes.
Introduction
Despite substantial advances in the technological and pharmacological sophistication of treatment for type 1 diabetes, many patients do not achieve the optimal levels of glycemic control known to reduce the risk of future complications. Recent evidence from National Health and Nutrition Examination Survey in 2003–2004 suggests that only 55.7% of all individuals with diagnosed diabetes had “good” glycemic control1,2 and less than one-quarter of those with type 1 diabetes achieve A1C <7%.
Given this difficulty to achieve optimally tight glycemic control, efforts have begun to fundamentally alter the approach to chronic disease care. Inspired by the Institute of Medicine's Crossing the Quality Chasm report,3 the Chronic Care Model (CCM)4–6 proposes substantial changes throughout the delivery system to improve care processes and outcomes. Among the 31 studies of CCM-based diabetes interventions included in a recent meta-analysis,7 the overall effect size was equivalent to a reduction in A1C of 0.30–0.47%.
Among CCM-based approaches, case management interventions tend to show better-than-average improvements in glycemic control, with meta-analyses reporting average effect sizes of −0.53% across general diabetes case management interventions and even larger effects (−0.8%) among interventions in which nurse or pharmacist case managers can make medication adjustments without awaiting physician authorization.8,9
The “Living with Diabetes” (LWD) program developed at the University of Washington in Seattle expands on traditional case management approaches with several novel information technology resources designed to provide more efficient access to information for both patients and their case managers. The intervention utilizes a Web-based interface to improve the clinical case management support of self-care activities of patients with diabetes and to bolster patient activation. This multifaceted Web-based collaborative care program has shown success in early studies with type 2 patients in general internal medicine clinics,10 but it is unclear if it will add value to diabetes care among patients with type 1 diabetes.
The present trial sought to test the LWD program with a sample of moderately poorly controlled type 1 diabetes patients in an academic diabetes clinic. We hypothesized that intervention arm patients would experience larger reductions in A1C after 12 months of internet-based collaborative care compared to those receiving usual care. We also hypothesized a priori that intervention patients would report larger positive changes in diabetes-specific self-efficacy than patients receiving usual care. Early results of this study were presented previously in abstract form at the 2008 American Diabetes Association Scientific Sessions, San Francisco, CA.11
Research Design and Methods
Study design
The study was designed as a 12-month open-label, randomized, pretest-posttest trial comparing usual care alone with usual care plus a Web-based collaborative care program. The trial protocol was approved by the University of Washington's Institutional Review Board, and all participants provided written informed consent.
Study setting and participants
The study was conducted at the Diabetes Care Center (DCC), a subspecialty clinic located 1 mile from the main University of Washington Medical Center. The DCC's multidisciplinary practice team includes physicians, nurse practitioners, on-site pharmacists, nurse educators, nutritionists, and mental health professionals.
Potential study participants were identified in the clinic's electronic medical record (EMR). Patients between the ages of 21 and 49 years were eligible for inclusion in the study if they carried a diagnosis of type 1 diabetes, had two or more clinical encounters at the DCC and at least one A1C test result in the previous 12 months, had a most recent A1C value ≥7%, and resided within King or Snohomish County.
Full medical records of patients meeting these inclusion criteria were further screened against additional exclusion criteria. Potential participants were deemed ineligible during record review if they did not receive multiple daily injection therapy with insulin glargine, were currently receiving continuous subcutaneous insulin infusion (or were transitioning to pump therapy), were terminally ill, had documentation of significant mental illness or substance abuse in their charts, or did not speak and read English.
Patients meeting these eligibility criteria were recruited via letter and telephone. During the recruitment call, potentially eligible patients were further screened to ensure that they had a home computer with internet access. Interested and eligible patients were then scheduled for an enrollment appointment at the clinic.
Patients were randomly assigned to the two study arms based on an allocation sequence using a 1:1 ratio in blocks of 10. The allocation sequence was developed by the study statistician and programmed into an electronic database to conceal allocation from other study staff during recruitment efforts. Although the allocation assignment was known to the study coordinator during the enrollment visit, this assignment was not disclosed to participants until written informed consent had been given and all baseline data collection had been completed.
During enrollment, we had higher attrition in the intervention arm because of six participants who did not complete software installation and testing. As a result, we changed the allocation ratio to 4:2 (intervention to control) for the final three blocks of recruitment to allow for equal groups in subgroup analyses of program users versus control.
Intervention
Participants randomized to the control arm received usual care from their DCC practice team. Those patients randomized to the intervention arm continued to receive usual care, but were also provided access to a nurse case manager and a disease management module comprising the five related Websites described in Table 1.
Table 1.
Living with Diabetes Intervention: Component Websites
| Website | Features |
|---|---|
| My Health Record | Allows patients to view their entire EMR, including all clinical encounters, clinician notes, and lab test results from January 1994 forward |
| My Upload Meter | Allows patients to remotely upload blood glucose readings stored in a digital meter to a health record viewable by both patients and case managers |
| My Diabetes Daily Diary | Allows patients and their case managers to enter medication, nutrition, and exercise data into an online daily diary and view trended displays of these data and uploaded blood glucose values |
| My Action Planner | Allows patients to collaboratively generate action plans intended to enhance self-efficacy and improve self-management activities |
| LWD Patient Education Website | Provides patients with an array of diabetes-related information content and links sanctioned by the Medical Director of the DCC |
Detailed descriptions of the individual Website components of the LWD program have also been provided previously by Goldberg et al.12 and Ralston et al.13
The nurse case manager for this study (G.L.) is an advanced registered nurse practitioner with 25 years of experience as a certified diabetes educator and 10 years of experience as a primary care practitioner in diabetes. Patients assigned to the intervention arm received an initial 1-h consultation with the case manager and one-on-one instruction with the trial's Web module from the study coordinator.
During this initial orientation, the study coordinator (K.P.M.) provided patients with study reference materials and provided a brief hands-on introduction to the LWD Websites. During the consultation with the case manager, patients discussed areas of concern with their diabetes self-management, performed “live” data entry and upload to the LWD system, and worked with the case manager to develop an individualized action plan.
Following the initial clinic visit, all remaining intervention activities took place remotely via e-mail and Web resources. The case manager reviewed patient-uploaded data weekly and initiated weekly e-mail contact with patients during the first month, after which she initiated contact based on individual patient goals (with a minimum of once per month) but continued to review records weekly and provide feedback to patients uploading information or initiating e-mail contact.
Study measures
Clinical end point
The difference in mean A1C change from baseline to 12-month follow-up between the intervention and control groups was the primary clinical end point. All A1C values used in the study dataset were “rapid” immunoassay tests performed using a Bayer DCA-2000+ analyzer (Siemens Medical Solutions, Tarrytown, NY). The range for normal values was 4.0–6.0%.
Baseline A1C values were identified from the EMR. Selection rules defined the baseline value as the last value in the patient's record during the 12 months prior to enrollment in the trial. The median baseline value was collected 3 months prior to enrollment (SD = 3.78).
“Follow-up” A1C values were identified with pre-established selection rules that utilized two distinct time windows. We identified the first test result in the EMR in the 13th through 18th study month. If there was no result available in this window, we used the last result appearing in the EMR during the 10th through 12th month of the study. The median follow-up value included in the dataset was collected 13 months after enrollment (SD = 2.00). Participants without any eligible follow-up values in the EMR were contacted by phone, and one eligible A1C test performed outside of the DCC was subsequently included in the study dataset.
Self-efficacy
We utilized baseline and 1-year follow-up administration of the short-form Diabetes Empowerment Scale (DES)14 to assess patients' psychosocial self-efficacy with diabetes care. Created by Anderson et al.,15 the DES was developed alongside intervention modules to improve overall patient empowerment in diabetes self-management. It has been further validated with representative samples of diabetes patients and has been used extensively in diabetes research and education activities as a broad measure of psychosocial self-efficacy.
Additional measures
In addition to standard demographic measures, we assessed patients' frequency of e-mail/Web use and presence of a broadband connection at home. We also assessed health literacy at baseline using the Rapid Estimate of Adult Literacy in Medicine (REALM)16,17and depression symptoms using the Patient Health Questionnaire (PHQ-9).18,19
Usage audits were performed to describe the level of engagement with the Web-based LWD resources among intervention arm patients. For e-mail communications, we used single messages as the metric. For EMR use, we counted the number of times each patient logged into his or her medical record and had one or more Web page views. Finally, we also tracked the total number of home glucose meter uploads performed.
Study data collection
Lab values for A1C tests, patient demographics, and other clinical information were obtained directly from the EMR. The REALM was administered in person upon enrollment. The other survey measures (DES and PHQ-9) were programmed into a Web-based questionnaire, which patients completed at baseline on a computer in the enrollment appointment room.
E-mail invitations to complete the follow-up Web survey were sent to all patients after their 12th study month, at which time the survey was completed online from home. All participants were provided a $25 gift certificate at the conclusion of the enrollment appointment and a $50 certificate after completing the follow-up Web survey.
Statistical analysis
Baseline demographics/descriptive statistics
We described the distribution of baseline demographic and clinical characteristics within each study group with means and SDs (for continuous variables) or counts and percentages (for categorical variables). For both the primary (A1C) and secondary (DES score) outcomes, we computed change scores for each participant by subtracting the baseline value from the follow-up value. Analyses were performed in STATA version 9 (Stata Corp., College Station, TX).
Primary outcome: change in A1C values
We addressed the primary research question of the intervention's effect on A1C using a multivariate linear regression model to adjust for potential clustering of patients within providers in the DCC and to control for baseline A1C, age, and sex, variables seen to be associated with glycemic control in prior literature. Approximately 17% of patients were missing follow-up A1C values, and 8% failed to complete the follow-up survey measure. Because a complete case approach excluding these individuals could potentially bias the analyses in the presence of differential drop-out between groups, primary analyses of both A1C and DES outcomes were performed with multiple imputation of missing values using the “ICE” procedure in Stata.20,21
Sensitivity analyses compared the primary multiple-imputation analyses to a complete case approach and two commonly employed conservative single imputation strategies. The first single imputation approach assumed that there was no change in glycemic control from baseline for the 13 individuals lost to follow-up, while the second approach replaced each of the missing values with the mean change observed in the control group.
Given the enrolled sample size, the trial had sufficient statistical power (80%) to detect a difference in A1C change of 0.65% between study groups and 99% power to detect a treatment difference of 1.0%, similar to that seen in an earlier trial of the intervention in patients with type 2 diabetes.10
Secondary outcomes: change in DES
We sought to test the hypotheses that the intervention would lead to positive changes in diabetes-related self-efficacy as assessed by the DES. We used linear regression to estimate the mean change scores between treatment groups while adjusting standard errors to account for clustering of patients within providers.
Results
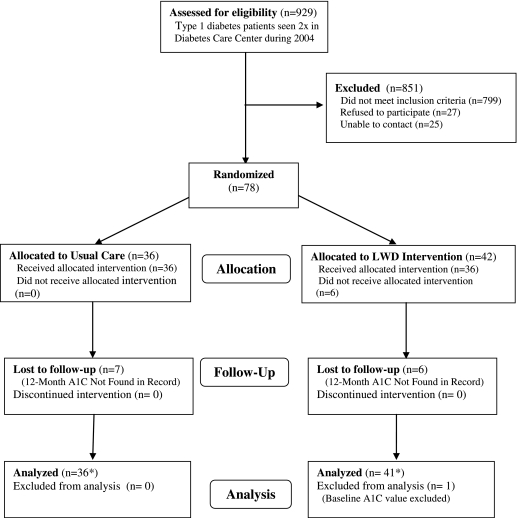
Figure 1 shows the progress of participants through the trial. We identified 929 patients with type 1 diabetes and at least two DCC encounters during 2004, the majority (86%) of whom did not meet additional eligibility criteria to enter the trial. Among the 799 patients deemed ineligible, 276 (35%) had recent A1C values below 7.0%, 210 (26%) were outside the age range, 132 (17%) were on insulin pumps or were not prescribed multiple daily injection, 90 (11%) had not had an A1C in the previous 12 months, and 91 (11%) were excluded for other reasons, including relocation out of the area, lack of a computer at home, terminal illness, lack of English, and death.
FIG. 1.
Flow of participants through the trial. *Primary analysis of A1C change was performed with an intent-to-treat approach using multiple imputation of missing follow-up values.
Among the 130 eligible patients, 27 were contacted but refused to participate, and study staff was unable to contact another 25. The remaining 78 patients were enrolled into the trial between May 2005 and April 2006.
Of patients randomized, 13 (17%) did not have an A1C value during the period 10–18 months after baseline and were considered lost to follow-up, and one individual was excluded because this individual's baseline A1C value was conducted on the high-performance liquid chromatography analyzer and thus was not comparable to the other participants.
The two treatment groups were similar for almost all measured characteristics at baseline, but the control group was 1 year older on average and included slightly fewer women than the intervention group (Table 2). Assessment of follow-up outcomes was also similar in the LWD and control groups, with average follow-up A1C occurring at 14.0 (SD = 1.9) and 13.6 (SD = 2.1) months, respectively.
Table 2.
Participant Characteristics at Baseline, Stratified by Study Arm
| Characteristic | Control (n = 36) | Intervention (n = 41) | Total (n = 77) |
|---|---|---|---|
| Mean (SD) age (years) | 37.8 (7.67) | 36.8 (8.51) | 37.3 (8.09) |
| Percentage female | 27.80% | 36.60% | 32.50% |
| Percentage Caucasian | 97.20% | 95.10% | 96.10% |
| Mean (SD) years of education | 15.2 (1.95) | 15.6 (2.17) | 15.4 (2.08) |
| Percentage married | 52.20% | 56.0% | 54.20% |
| Insurance status | |||
| Commercial | 86.10% | 84.60% | 85.30% |
| Medicaid | 5.60% | 5.10% | 5.30% |
| Medicare | 2.80% | — | 1.30% |
| Private (self-pay) | 5.60% | 2.60% | 4.00% |
| Other | — | 7.70% | 4.00% |
| Mean (SD) REALM score | 64.53 (1.54) | 64.65 (1.59) | 64.59 (1.56) |
| Frequency of internet use | |||
| Less than once weekly | 2.90% | 2.40% | 2.60% |
| 1–2 times weekly | 5.70% | 4.90% | 5.30% |
| 3–4 times weekly | 5.70% | 4.90% | 5.30% |
| 5+ times weekly | 85.70% | 87.80% | 86.90% |
| Percentage with broadband at home | 85.70% | 82.90% | 84.20% |
| Mean PHQ-9 severity score | 5.26 (5.3) | 4.85 (4.9) | 5.04 (5.07) |
| Mean (SD) baseline A1C | 8.05 (1.32) | 7.99 (1.05) | 8.02 (1.19) |
| Glycemic control at baseline | |||
| A1C 7–8% | 66.70% | 63.40% | 64.90% |
| A1C >8% | 33.30% | 36.60% | 35.10% |
Glycemic control
Over the course of the trial, the intervention group experienced an average decrease in A1C test values of 0.37%, while the control group experienced a slight increase of 0.11%. The between-group difference of −0.48% was not significant (Table 3). Patterns of missing A1C outcomes were similar in the LWD and control arms (15% and 19% missing, respectively). While there were variations of up to 0.21% in the estimated group difference across the three additional missing data approaches, all failed to reach significance, and the overall conclusion was not meaningfully altered by the approach used for missing data.
Table 3.
Changes in Value of Outcome Variables Between Baseline and 12-Month Follow-Up
| |
|
|
|
Group differenceb |
|
|---|---|---|---|---|---|
| Control (n = 36) | Intervention (n = 41) | Mean group differencea | 95% CI | P value | |
| Primary outcome: A1C | |||||
| Mean (SD) baseline A1C | 8.05 (1.3) | 7.99 (1.1) | |||
| Mean (SD) follow-up A1C | 8.16 (1.5) | 7.62 (1.4) | |||
| Mean (SD) change in score | +0.11 (1.4) | −0.37 (1.3) | −0.48 | −1.22 to 0.27 | 0.160 |
| Secondary outcome: DES | |||||
| Mean (SD) baseline DES score | 4.08 (0.59) | 4.00 (0.60) | |||
| Mean (SD) follow-up DES score | 3.92 (0.63) | 4.14 (0.60) | |||
| Mean (SD) change in score | −0.16 (0.62) | +0.14 (0.62) | 0.30 | 0.01 to 0.59 | 0.044 |
Effect size estimate for A1C analysis adjusted for baseline A1C, age, and sex.
Confidence intervals (CIs) and P values for all analyses are based on standard error estimates adjusted for clustering by the primary DCC physician.
DES
Participants' responses to the DES measure of psychosocial self-efficacy in diabetes revealed a significant group difference (effect size of 0.30; 95% confidence interval 0.01 to 0.59; P = 0.04; Table 3). Results were consistent across the primary multiply imputed analysis and the three other missing data approaches examined in sensitivity analyses.
Program usage
Nearly two-thirds (65.9%) of intervention participants completed at least one meter upload from home, with a mean of 3.8 (SD = 4.8) uploads per patient. Sixty-one percent accessed their EMR from home, with a mean of 3.3 (SD = 3.65) logins and 37.9 (45.1) total pageviews per patient. Less than half (44%) sent e-mail to their case manager, with a mean of 5.0 (SD = 4.8) messages sent. Table 4 displays characteristics of program users compared to those of nonusers.
Table 4.
Characteristics of Program Users Versus Nonusers (Intervention Arm Only)
| Characteristic | Nonusers (n = 16) | Users (n = 25) |
|---|---|---|
| Mean (SD) age (years) | 36.7 (7.36) | 36.8 (9.33) |
| Percentage female | 43.75% | 32.00% |
| Percentage Caucasian | 93.75% | 96.00% |
| Mean (SD) years of education | 15.43 (2.22) | 15.76 (20.17) |
| Percentage married | 33.33% | 68.75% |
| Insurance status | ||
| Commercial | 80.00% | 87.50% |
| Medicaid | 6.67% | 4.17% |
| Medicare | — | — |
| Private (self-pay) | 6.67% | — |
| Other | 6.67% | 8.33% |
| Mean (SD) REALM score | 64.13 (1.96) | 64.96 (1.27) |
| Frequency of internet use | ||
| Less than once weekly | — | 4.00% |
| 1–2 times weekly | 12.50% | — |
| 3–4 times weekly | 12.50% | — |
| 5+ times weekly | 75.00% | 96.00% |
| Percentage with broadband at home | 81.25% | 84.00% |
| Mean (SD) PHQ-9 severity score | 4.06 (4.49) | 5.38 (5.17) |
| Mean (SD) baseline A1C | 7.84 (0.97) | 8.08 (1.13) |
| Glycemic control at baseline | ||
| A1C 7–8% | 62.50% | 64.00% |
| A1C >8% | 37.50% | 36.00% |
Program “users” are defined as intervention group members who engaged at least one system resource on more than one occasion (i.e., by making at least two remote meter uploads, sending at least two e-mails to their case manager, or completing at least two logins to their EMR during the active study period).
Discussion
This pilot randomized trial sought to extend a diabetes case management intervention used previously among type 2 diabetes patients to a sample of younger adults with type 1 diabetes and to test whether the positive intervention effects observed in a general medicine clinic could be replicated in a subspecialty clinic.
This study found no treatment effect on A1C, although the observed trend was consistent with the study hypotheses. An effect of 0.48% would be clinically significant if replicated in a larger trial with greater statistical power, but it appears we were underpowered to detect such a difference. It is also important to consider the limited uptake of the intervention as potentially restricting its effect.
Although the majority (61%) of patients had some engagement, less than one-quarter of intervention arm participants used the study resources consistently. We believe more research is needed to clarify the extent to which this intervention can add value for patients in similar treatment settings, especially as technology continues to improve with real-time continuous glucose sensors.
We found the LWD intervention to have a statistically significant treatment effect on patients' self-efficacy. Previous research has shown self-efficacy constructs to be highly predictive of self-care behavior among younger type 1 diabetes patients both cross-sectionally and prospectively.22 As such, there is likely a benefit to increasing self-efficacy even in the absence of an effect on A1C.
There are several strengths to our approach. First, by completing a randomized trial with an intent-to-treat analysis, the observed effects are likely true impacts of the LWD program. Second, by standardizing the Websites and as using a single case manager, we limited several sources of potential bias in the delivery of the intervention.
There are also several limitations to our findings. The study selected a specific subsample of patients with diabetes whose disease was moderately to poorly controlled and who had a multiple daily injection regimen. As such, it is difficult to predict how the intervention might provide benefit for more tightly controlled patients or those receiving continuous subcutaneous insulin infusion.
Because of the small size of this pilot trial, we cannot tease apart which intervention components might have been more influential than others. We observed reasonably large number of patients who failed to fully engage with the intervention resources, but saw no significant differences in baseline demographic or clinical characteristics between users and nonusers and did not have large enough samples to directly quantify the differential effects of different “doses” of the intervention on study outcomes.
The study was further limited by the lack of prespecified collection dates for the A1C tests and reliance on pre-existing EMR data. While it appears as if missingness in the A1C variable did not bias our results, it is important to consider the added confidence that could be realized with lower levels of missing outcome data. Finally, the use of a single case manager implementing the intervention may limit generalizability.
In summary, we observed preliminary evidence in this pilot study that suggests internet-based case management may provide added benefit beyond usual care for patients with moderately to poorly controlled type 1 diabetes, even among patients receiving care at a subspecialty clinic. The greatest benefit observed in this study is a bolstering of diabetes-specific self-efficacy, which may help patients engage in more effective self-care behaviors.
While the lack of a convincing treatment benefit in glycemic control makes it difficult to recommend widespread use of this or similar interventions based on this trial alone, we believe the observed trends warrant larger trials to assess impact on glycemic control and other clinical outcomes (low-density lipoprotein, blood pressure). Larger trials should also seek to identify the most important intervention components and relevant populations for the intervention.
Acknowledgments
This clinical trial received financial support from Aventis Pharmaceuticals, Inc. through a research grant to H.I.G. and I.B.H. and through funds from grant T32 HS013853 from the Agency for Healthcare Research and Quality to K.P.M.
Author Disclosure Statement
I.B.H. and J.D.R. have received research funding from sanofi-aventis. I.B.H. is also a consultant to Eli Lilly, Johnsons & Johnson, and Roche Pharmaceuticals. No competing financial interests exist for the remaining authors.
References
- 1.Hoerger TJ. Segel JE. Gregg EW. Saaddine JB. Is glycemic control improving in U.S adults? Diabetes Care. 2008;31:81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 2.Saydah SH. Fradkin J. Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. Crossing the Quality Chasm. [PubMed] [Google Scholar]
- 4.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 5.Bodenheimer T. Wagner EH. Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 6.Bodenheimer T. Wagner EH. Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 7.Tsai AC. Morton SC. Mangione CM. Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478–488. [PMC free article] [PubMed] [Google Scholar]
- 8.Norris SL. Nichols PJ. Caspersen CJ. Glasgow RE. Engelgau MM. Jack L. Isham G. Snyder SR. Carande-Kulis VG. Garfield S. Briss P. McCulloch D. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22:15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 9.Shojania KG. Ranji SR. McDonald KM. Grimshaw JM. Sundaram V. Rushakoff RJ. Owens DK. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg HI. Hirsch IB. Hoath JI. Ralston JD. Mullen MA. Internet co-management of type 2 diabetes: a randomized controlled trial (RCT) [abstract] 66th Scientific Sessions Abstract Book, Diabetes. 2006;55(Suppl):A1147-P. [Google Scholar]
- 11.McCarrier KP. Hirsch IB. Lewis G. Martin DP. Ralston JD. Zimmerman FJ. Goldberg HI. The effects of a Web-based co-management program in younger adults with type 1 diabetes: a randomized controlled trial [abstract] 68th Scientific Sessions Abstract Book, Diabetes. 2008;57(Suppl):A1222-P. [Google Scholar]
- 12.Goldberg HI. Ralston JD. Hirsch IB. Hoath JI. Ahmed KI. Using an Internet comanagement module to improve the quality of chronic disease care. Jt Comm J Qual Saf. 2003;29:443–451. doi: 10.1016/s1549-3741(03)29053-5. [DOI] [PubMed] [Google Scholar]
- 13.Ralston JD. Revere D. Robins LS. Goldberg HI. Patients' experience with a diabetes support programme based on an interactive electronic medical record: qualitative study. BMJ. 2004;328:1159. doi: 10.1136/bmj.328.7449.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RM. Fitzgerald JT. Gruppen LD. Funnell MM. Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF) Diabetes Care. 2003;26:1641–1642. doi: 10.2337/diacare.26.5.1641-a. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RM. Funnell MM. Fitzgerald JT. Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self-efficacy. Diabetes Care. 2000;23:739–743. doi: 10.2337/diacare.23.6.739. [DOI] [PubMed] [Google Scholar]
- 16.Davis TC. Crouch MA. Long SW. Jackson RH. Bates P. George RB. Bairnsfather LE. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23:433–435. [PubMed] [Google Scholar]
- 17.Davis TC. Long SW. Jackson RH. Mayeaux EJ. George RB. Murphy PW. Crouch MA. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 18.Kroenke K. Spitzer RL. Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe B. Kroenke K. Herzog W. Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 20.Royston P. Multiple imputation of missing values: update. Stata J. 2005;5:1–14. [Google Scholar]
- 21.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 22.Johnston-Brooks CH. Lewis MA. Garg S. Self-efficacy impacts self-care and HbA1c in young adults with Type I diabetes. Psychosom Med. 2002;64:43–51. doi: 10.1097/00006842-200201000-00007. [DOI] [PubMed] [Google Scholar]