Abstract
Background
Unequivocal eradication of donor leukocyte microchimerism from recipients of long-surviving organ transplants has never been reported. Here we describe a drastic attempt to accomplish this objective.
Methods
In control experiments, a rank order of microchimerism and of associated donor specific nonreactivity was produced in Brown-Norway (BN) rats by transplantation of Lewis (LEW) liver, bone marrow cell (BMC) and heart allografts under a brief course of tacrolimus. The degree of microchimerism at 60 and 110 days was estimated with semiquanitative immunocytochemical and PCR techniques. Tolerance at 110 days was assessed in the different control groups by challenge transplantation of naive LEW hearts. In parallel experimental groups, an attempt was made to eliminate microchimerism from the BN recipients. The animals were submitted at 60 days to 9.5-Gy total body irradiation (TBI), reconstituted immediately with naïve BN BMC, and tested for donor specific nonreactivity by LEW heart transplantation at 110 days.
Results
After the TBI-reconstitution at 60 days, microchimerism was undetectable in BMC recipients at 110 days, significantly reduced in heart recipients, and least affected in liver recipients. Except in liver recipients, abrogation of LEW-specific nonreactivity was demonstrated by rejection of the priming grafts, or by rejection of the challenge heart grafts, and by in vitro immune assay.
Conclusions
It is difficult to eliminate microchimerism in organ recipients once the donor cells have settled into tissue niches.
Keywords: Microchimerism, Tolerance, Passenger leukocytes, Liver transplantation, Bone marrow cells
It is generally accepted that the acute migration of multilineage leukocytes from the organ graft into the host (1–6) has profound immunologic implications. However, the role, if any, of persistent small numbers of donor cells (microchimerism) in the maintenance of organ alloengraftment has been controversial (7–13). An ideal way to assess the biologic significance of such microchimerism would be to study the consequences of eliminating the donor leukocytes from recipients of stable transplanted organs. We report here an effort to remove microchimerism in Brown Norway (BN) rat recipients of Lewis (LEW) heart, liver, or bone marrow cell (BMC) allografts.
To generate controls, we used a previously standardized model, in which a spectrum of microchimerism-associated donor specific nonreactivity (tolerance) is reproducibly induced under a short course of tacrolimus (14). In addition to histopathologic analysis of the primary graft, the extent of the tolerance is assessed in this model by transplantation of a challenge LEW heart at 110 days. In the present study, the highly reproducible microchimerism in such experiments and the associated donor-specific nonreactivity were compared with the outcomes when the allograft-primed BN recipients were subjected to total body irradiation (TBI, 9.5 Gy) after 60 days, and reconstituted immediately with an infusion of naïve BN BMC. Even with this drastic procedure, it was not possible in organ recipients to completely eliminate the microchimerism.
MATERIALS AND METHODS
Animals
LEW (RT1l) donor and BN (RTln) recipient rats weighing 150–250 g (Harlan Sprague Dawley, Indianapolis, Indiana) were kept in a specific pathogen-free environment, fed a standard diet, and provided water ad libitum. The guidelines were observed of the Council on Animal Care at the University of Pittsburgh and the National Research Council’s Guide for the humane Care and Use of Laboratory Animals.
Organ/Cell Transplantation Procedures
Orthotopic liver transplantation (OLTx) without arterial reconstruction and heterotopic heart transplantation (HTx) into the abdomen were carried out as previously described (14). Rejection of heart and liver allografts was defined as the cessation of heartbeat and animal survival, respectively.
BMC were obtained by flushing the tibias and femurs with RPMI 1640, supplemented with 25 mM HEPES buffer, 2 mM L-glutamine, and 10 μg/ml gentamicin (all from Life Technologies, Grand Island, NY). BMC (2.5×108 cells/animal) with >95% viability were injected intravenously via the penile or jugular vein.
Immunosuppression
Tacrolimus (Fujisawa Pharmaceutical Co., Osaka, Japan) was given at a daily intramuscular dose of 1.0 mg/kg on days 0 to 6 after transplantation. A single supplemental injection of the same dose was given on days 13 and 20.
Experimental Design
Male or female BN rats received male LEW heart (Group 1, Fig. 1), liver (Group 2), or BMC (Group 3) grafts under a short course tacrolimus immunosuppression. In Groups 4–6, depletion of persisting donor passenger leukocytes was attempted at 60 days after primary transplants. BN recipients of LEW heart (Group 4), liver (Group 5) or BMC allografts (Group 6) were subjected to 9.5 Gy TBI (137Cs Gammacell 40), and infused on the same day with 5×107 female naïve BN unfractionated bone marrow cells for hema-tolymphoid reconstitution. At 110 days after priming (50 days after TBI and reconstitution), the recipients were challenged with naïve LEW heart grafts. Animals were followed until the rejection of primary or challenge allografts or for 100 days after challenge heart transplantation.
FIGURE 1.
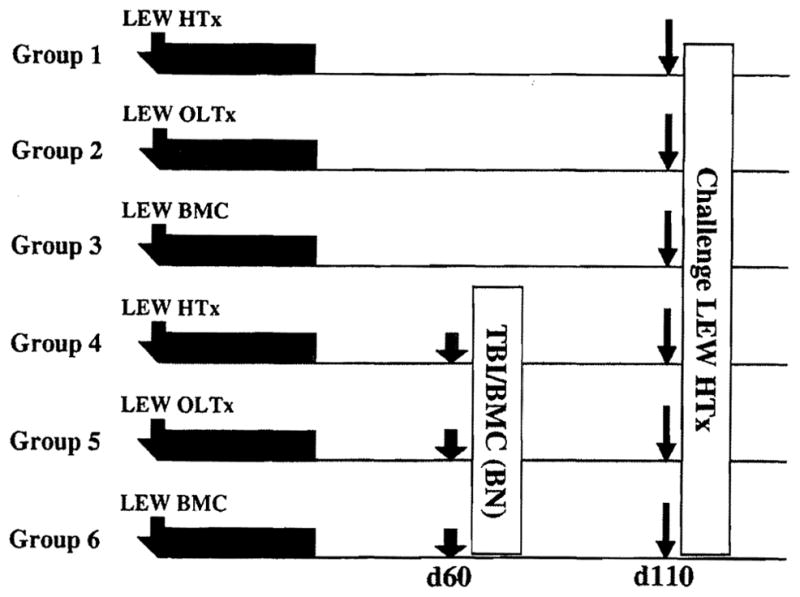
Experimental design in which all six groups of BN recipients were given LEW priming allografts under a short course of tacrolimus (TAC) immunosuppression and tested for donor-specific reactivity 110 days later by challenge LEW heart transplantation (bold arrows). No other treatment was given to the animals of groups 1–3. In Groups 4–6, it was attempted to remove donor leukocyte micro-chimerism by 9.5 Gy TBI and immediate reconstitution with 5×107 naïve BN BMC (shaded arrows). Transplantations were of heterotopic hearts (HTx), orthotopic livers (OLTx), and infused bone marrow cells (BMC).
Two sets of control animals were studied. In one group, naïve BN rats were irradiated with 9.5 Gy TBI, but not reconstituted with BMC. All died with a mean survival of 12.2 days (n=9). Therefore, TBI with 9.5 Gy is considered to be lethal A second group demonstrated that TBI-syngeneic BM transplantation procedure was succeeded by prompt recovery of vigorous host immune responsiveness. In these experiments, naïve BN recipients were irradiated with 9.5 Gy TBI and reconstituted with syngeneic BN BMC. After 50–77 days, LEW → BN heart transplantation was carried out. The LEW hearts were rejected with a median survival of 9 days (n=3), the same as in naïve animals. The result suggested that the reconstituted immune system did not have a major deficit.
At autopsy, the liver or heart allografts, as well as the recipient liver, heart, kidney, spleen, thymus, skin (tongue), and cervical lymph nodes were sectioned and fixed in 10% neutral buffered formalin for routine histopathology. Other tissues were embedded in optimum cold temperature compound for immunohistochemical studies, and/or snap-frozen in liquid nitrogen for DNA extraction. In addition, recipient cervical lymph nodes were taken for flow cytometry and mixed leukocyte reactions (MLR).
Pathologic Studies
The formalin-fixed heart and liver grafts were embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin. Histopathological changes in heart allografts were evaluated by the presence or absence of obliterative arteriopathy (OA) and endocardial, pericardial, interstitial and periarterial mononuclear inflammation with patchy interstitial fibrosis (15). In the liver allografts, particular attention was directed to the presence or absence of bile duct damage and/or loss, bile ductular proliferation, subendothelial mononuclear infiltration in the portal or central veins, and severity of portal tract inflammation (16).
In Vitro Immunological Analyses
Mixed Leukocyte Reaction
Anti-donor reactivity of BN recipients was assayed with one-way mixed leukocyte reaction (MLR) as previously described (14). Triplicate cultures of responder cervical lymph node lymphocytes (1.75×105) and irradiated stimulator cells (2000 rad, 3×105) were incubated in a humidified atmosphere of 5% C02 in air for 4 days at 37°C. One μCi of 3H-thymidine was added to each well 16 hr before the termination of the culture. Cultures were harvested and 3H-thymidine uptake was determined by liquid scintillation.
Flow Cytometry
Lineages of lymphocytes obtained from cervical lymph nodes were analyzed by two-color flow cytometry using PE or FITC-conjugated monoclonal antibodies (mAbs) OX8 (CD8α), W3/25 (CD4), and OX39 (CD25, IL-2R) (Pharmingen, San Diego, CA or Serotec, Kidlington, Oxford, UK). Isotype-matched nonspecific antibodies were substituted for the primary reagents in the negative controls. The samples were fixed and analyzed on a Counter Elite ESP.
Leukocyte Chimerism Determination
Immunohistopathology
Donor MHC class II+ cells were looked for in 4 μm tissue sections stained with a routine indirect avidin-biotin complex method using mAb L21–6 (mouse IgG1), that reacts with class II MHC antigens of LEW but not BN (17). Isotype matched non-specific antibody was substituted in controls.
Y-chromosome PCR
In male → female transplant experiments, quantitation of male DNA in host tissues was performed by conventional PCR with Y-chromosome specific Southern hybridization and by SYBR Green real-time (quantitative) PCR. Genomic DNAs were prepared from recipient tissues using QIAamp kit (Qiagen Inc., Chatworth, CA) as described by the manufacturer, and the concentration of isolated DNA was measured by OD260/280 using Thermo Electron Spectroscopy (Thermo, Madison, WI).
In conventional PCR method, PCR was performed with 1.5 μg genomic DNA in 50 μl of total reaction mixture containing 1.25U Taq DNA polymerase and 1 μl each of 25 μM rat sex determining region-Y (Sry) specific oligonucleotide primers (5′-GAGAGAGGCACAAGTTGGC-3′ and 5′-GCCTCCTGGAAAAAGGGCC-3′) as previously described (18). The PCR products were then fractionated in 1.5% agarose gels and transferred onto nylon membranes for Southern blotting and semiquantitation. Sry-specific probe was prepared by extraction and purification of PCR product of male LEW spleen DNA and multiprime-labeled with alpha-32P dCTP. After hybridization with the P32-labeled probe, membranes were exposed to Storage Phosphor Screen (Molecular Dynamics, Sunnyvale, CA), and the radioactivity was measured by Phosphorlmager (Molecular Dynamics) (18).
SYBR Green real time PCR for rat Sry genes was performed using oligonucleotide primers (5′-AAGTCAAGCGCCCCATGA-3′ and 5′-TGAGCCAACTTGTGCCTCTCT-3′). For the internal control, rat GAPDH gene oligonucleotide primers (5′-ATGCTGGTGCTGAGTATGTCG-3′ and 5′-GTGGTGCAGGATGCATTGCTGA-3′) were used. The reactions were performed in duplicate by SYBR Green PCR mix (Applied Biosystems, Foster City, CA) using an ABI7000 Prism Sequence Detection System (Perkin Elmer, Foster City, CA). The thermal cycler was configured as following: incubation (95°C, 10 min), up to 40 cycles of denaturation (95°C, 15 sec), and annealing and extension (60°C, 60 sec).
In both PCR methods, the level of chimerism in each sample was calculated with a standard curve prepared by known concentrations of male DNA. As we previously reported (18), it was possible to detect the male DNA concentration ~0.001% using conventional PCR and Southern hybridization method. In the real-time PCR method, the detection sensitivity was also ~0.001%, similar to levels reported in other human/mouse studies using real-time quantitative Y-chromosome-specific PCR methods (19–21). For each assay, standards and negative control without template were included. Donor DNA was considered as nondetectable when experimental samples showed below the value of control female samples.
Statistical Analysis
Results were reported as mean ± SD. Student t test was used for the analyses of flow cytometry and MLR results. P value less than 0.05 was considered significant.
RESULTS
TBI-Reconstitution Eliminated Microchimerism
Confirming previous observations (14), sparse and het-erogeneously distributed LEW MHC class II+ mononuclear leukocytes were found in the tissues of BN recipients that had been primed 110 days earlier with all three kinds of allografts (data not shown). The rank order concentration of donor cells after the three kinds of priming was liver (highest) → BMC → heart. Also confirming previous studies (5, 15, 22–24), the organ allografts appeared to be privileged sanctuaries (i.e., the donor cells were more highly concentrated in the organ allografts; Fig. 2A) than in recipient tissues. Donor MHC class II+ cells persisting in the liver grafts were mostly dendritic cells located in the portal triad and some resident sinusoidal Kupffer cells with a frequency of 19.2± 15.0 cells/high power field (HPF, X400) (5,23).
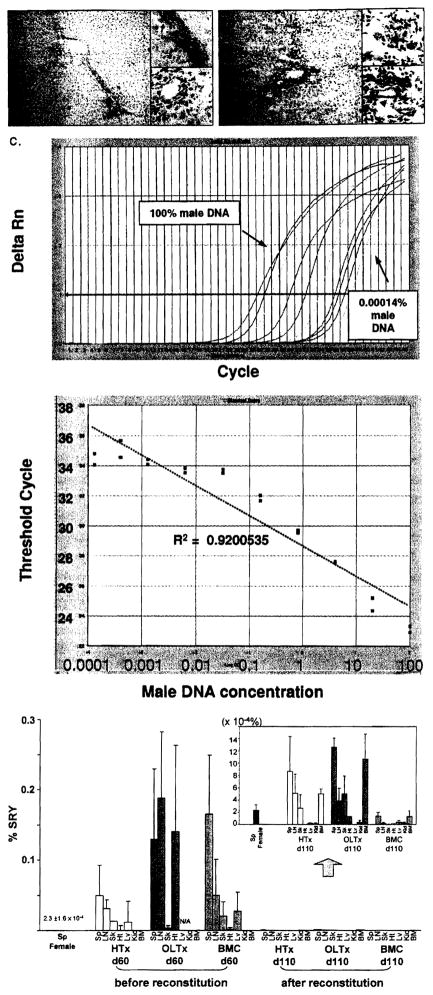
FIGURE 2.
Microchimerism after 110 days in female BN recipients primed with male LEW allografts before and after TBI reconstitution. (A) Transplanted LEW liver after 110 days in non-irradiated BN recipients (see Group 2, Fig. 1). LEW MHC class II+ cells in the periportal area are stained brown by the L21–6 mAb (arrows, insert, upper). Note that biliary epithelial cells remain negative for L21–6 (insert, lower). Original magnification, X200 and X600. (B) Transplanted LEW liver after 110 days in irradiated and reconstituted BN recipients (see Group 5, Fig. 1). Most of the donor MHC class II+ cells are missing, but some remain (arrows, insert, upper). Faint staining of biliary epithelial cells (arrow heads, insert, lower) suggests the possibility of subtle biliary injury that was not detectable with conventional histopathology. Three animals in each group were analyzed and representative picture is shown. Original magnification, X200 and X600. (C) Standardized amplification curve made with artificial mixtures of serial male DNA concentrations. Artificial mixtures were made with serial male DNA concentrations (10 serial dilutions of 100, 20, 4, 0.8, 0.16, 0.032, 0.0064, 0.00128, 0.000427, 0.000142% male DNA) and analyzed with SYB1 green real-time PCR. Upper panel shows Sry-specific marker ΔRn curves for the 10 samples. Regular positive amplification curves were observed with ΔRn curve shift to the right as male DNA concentration decreased. Lowe panel shows standardized amplification curve plotted from these results. Cycle threshold values linearly correlated with the log of male DNA concentration (R2=0.9200535). (D) Male DNA concentrations (microchimerism) before (60 days) and after (110 days) TBI-reconstitution in tissues of female recipients of male heart, liver, and BMC allografts (Groups 4–6 in Figure 1, n=3 in each with SYBR green real-time PCR). At 60 days before TBI-reconstitution, ail primed recipients with LEW heart, liver and BMC showed ~0.1 % donor male DNA. After TBI-reconstitution, the male DNA found in liver recipients (~0.001%) was present in smaller amounts in heart recipients, but was not identifiable in BMC recipients (insert). BM, bone marrow; LN, lymph nodes; Sp, spleen; Kid, kidney; Sk, skin; Ht, heart; Lv, liver.
TBI-reconstitution greatly reduced the number of MHC class II+ LEW cells detected with the L21–6 mAb, including in the highly privileged site of the priming hepatic allograft (Fig. 2B). Although few LEW MCH class II faintly positive cells (0.31 ±0.70 cells/HPF, n=3) were found in the host tissues of the liver-primed and irradiated recipients at 110 days, they could rarely be identified with certainty in the irradiated heart-primed recipients and were never seen in the BMC-primed recipients.
These results with immunocytochemical staining were consistent with polymerase chain reaction studies in female BN recipients of male LEW allografts who were irradiated and reconstituted with naïve female BN bone marrow (Fig. 2C and D). Probes for male DNA revealed unequivocal widely-distributed male DNA in all primed recipients before TBI-reconstitution (n=3 for each group). Liver-primed recipients tended to have higher levels of donor male DNA than HTx- or BMC-primed recipients. After TBI-reconstitution, male DNA signals were essentially zero in BMC-primed recipients. Trace quantities of male DNA were found in heart-primed recipients (Fig. 2D and insert). The most male DNA was in liver recipients (Fig. 2D and insert).
Leukocyte Lineage Composition Was Not Altered by TBI-Reconstitution
The percentages of CD4+, CD8+ and CD4+CD25+ lymphocytes in naive BN rats were 54.0±7.9, 5.5±0.7, and 6.4±0.4%, respectively. Sixty days after priming transplantation, the CD4+ population was significantly reduced (P<0.01) to about the same extent in all three kinds of organ recipients: 35.5±7.3% (BMC recipients), 37.3 ±7.0% (liver recipients) and34.4±4.4% (heart recipients). The percentage of CD8+ and CD4+/CD25+ cells that have been identified as potential immunoregulatory candidates (12,25) were no different after 60 days in three groups of animals than in naive animals. Interestingly, the leukocyte profile that evolved in the first 60 days (up to the time of TBI-reconstitution) was essentially the same as that 50 days later (i.e., the time of challenge heart transplantation at 110 days).
Mixed Leukocyte Reaction Studies Showed That TBI Reconstitution Caused a Change of Donor Specific Nonreactivity toward Reactivity
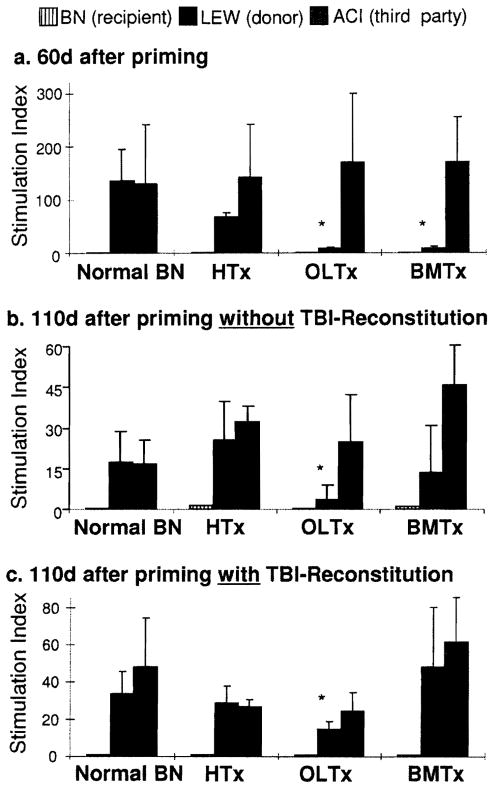
Sixty days after priming transplantation, antidonor proliferation was markedly suppressed in liver and BMC recipients (P=0.03), but only moderately so in heart recipients (P=0.07; Fig. 3A). In the control animals of Groups 1–3, the donor specific suppression was still evident by 110 days but was significant only in the liver-primed recipients (Fig. 3B). TBI-reconstitution at 60 days completely eliminated the LEW-specific nonreactivity in the BMC- and heart-primed recipients, but not in the liver-primed recipients (Fig. 3C).
FIGURE 3.
One-way MLR of BN cells (from cervical nodes) to irradiated stimulator cells. (A) 60 days: before TBI/reconstitution. (B) 110 days: without TBI/reconstitution. (C) 110 days: after TBI/reconstitution at 60 days. All results are expressed as mean stimulation index ± SD n=3 each group. *P<0.05 vs. normal BN response to LEW stimulator cells. HTx, heart transplantation; OLTx, orthotopic liver transplantation; BMTx, bone marrow cell transplantation.
TBI Reconstitution Frequently Caused Rejection of Priming Heart But Not Priming Liver Allografts
As expected, all of the control LEW heart (Group 1) and liver grafts (Group 2) survived in non-irradiated BN recipients until transplantation of a challenge LEW heart at 110 days, and for another 100 days until termination of the experiments (Table 1). Although the priming heart allografts continued to beat, they all developed characteristic chronic rejection (CR): OA, patchy interstitial fibrosis, and mononuclear infiltration in interstitial and periarterial areas. However, the priming liver allografts were normal at 210 days, except for occasional bile duct proliferation and mild lymphocytic infiltrates in the portal triad.
TABLE 1.
Survival of primary allografts and challenge donor heart allografts
| Group | Primary transplant | TBI/reconstitutiona | Primary grafts |
Challenge heart grafts |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Survival (days) | Median | Rejection | n | Survival (days) | Median | Rejection | |||
| 1 | HTx | None | 6 | >210×6 | 210 | Chronic | 6 | >100×6 | 100 | Chronic |
| 2 | OLTx | None | 10 | >210×10 | 210 | None | 10 | >100×10 | 100 | None |
| 3 | BMC | None | 10 | N/A | N/A | NA | 10 | > 100×10 | 100 | Chronic |
| 4 | HTx | Yes | 12 | 73,87,87,88, 88,89,93 110×2, >210×3 | 91 | Acute/chronic | 3 | > 100×3 | 100 | Chronic |
| 5 | OLTx | Yes | 7 | >210×7 | 210 | None | 7 | > 100×7 | 100 | None |
| 6 | BMC | Yes | 16 | NA | N/A | NA | 16 | 6,6,7,7,7, 8,8,8,8, 10,13, 25,25,28 > 100X2 | 8 | Acute/chronic |
Median survival of LEW hearts in naive BN recipients is 9.0 days.
On 60 days after primary transplantation, recipients were irradiated with 9.5-Gy total body irradiation (TBI) and then were reconstituted 5 × 107 naive BN bone marrow cells intravenously.
TBI and reconstitution altered the outcome. Instead of the universal survival of the original hearts seen in Group 1, 9 of the 12 priming heart grafts of the irradiated and reconstituted Group 4 ceased to beat at 73–110 days (Table 1) and had histopathologic findings of acute and/or chronic rejection. The 3 exceptional priming hearts that survived until 110 days continued to beat after challenge heart transplantation and had findings of severe CR at 210 days.
In contrast, all comparably irradiated and reconstituted liver recipients (Group 5) retained their priming liver grafts until challenge heart transplantation at 110 days and for the following 100 days (Table 1). With blinded analysis of conventional histopathologic sections, the priming hepatic allografts at 210 days were indistinguishable from the priming livers in the non-irradiated Group 2. Of interest, there was up-regulation of MHC class II on biliary epithelial cells in liver tissues of Group 5 stained with the mAb L21-6, suggesting subtle biliary injury caused by rejection (Fig. 2B).
TBI Reconstitution Usually Abrogated the Protection Afforded Challenge Hearts by Priming Heart and Priming BMC, but not that Afforded by Priming Liver Allografts
All 26 challenge hearts survived for 100 days after transplantation to non-irradiated recipients who had been primed 110 days earlier with heart (n=6), liver (n=10), and BMC (n=10) (Table 1). By day 210, the challenge hearts in the heart- (Group 1) and BMC-primed recipients (Group 3) had developed moderate to severe CR, while the hearts transplanted to liver-primed recipients (Group 2) were normal (Fig. 4).
FIGURE 4.
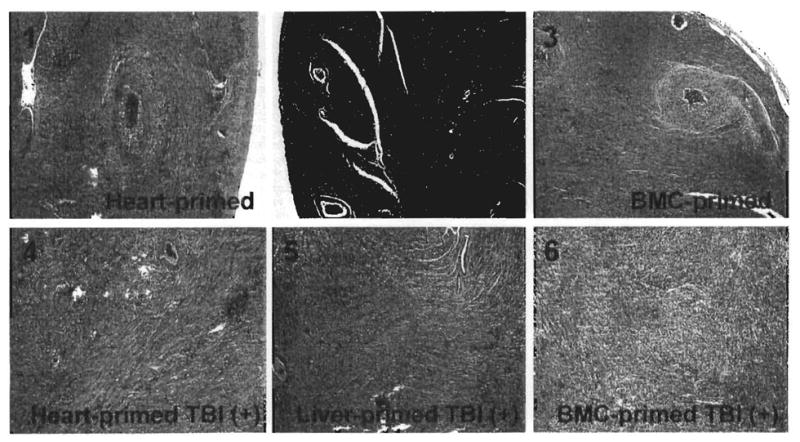
Histopathological findings of challenge heart allografts after 100 days (210 days after primary transplantation). The six panel numbers correspond to the six groups shown in Figure 1 and histological findings in Table 1. H&E stain, original magnification × 100.
In the three heart-primed and irradiated recipients of the original 12 whose primary grafts were still beating at 110 days (Group 4, Table 1), the challenge hearts survived until day 210 and had developed severe CR (Fig. 4). All of the LEW hearts transplanted into liver-primed and irradiated recipients beat until 210 days (Group 5), and were CR-free (Fig. 4).
The worst results with challenge heart transplantation were in 16 irradiated BN recipients who had been primed with LEW BMC (Group 6, Table 1). Only 2 of the 16 LEW challenge hearts transplanted at 110 days beat until 210 days. Nine of the other 14 were acutely rejected in 6–8 days (7.2±0.8 days), significantly earlier than the 9.0±0.8 days of LEW hearts transplanted into naive unmodified animals (P<0.0001). Five more challenge hearts were lost to rejection between 10–28 days. The wide range of heart graft survival was reflected in a spectrum of histopathologic findings: severe acute rejection, CR, or a mixture.
DISCUSSION
Ko et al. (7) attempted to eliminate donor leukocyte microchimerism in a rat model in which heterotopically transplanted cardiac allografts remain free of chronic rejection (CR) for 200 days following a brief posttransplant course of cyclosporine. A dose of donor leukocyte-specific monoclonal antibody (mAb) administered on the day of transplant during the acute spread of graft passenger leukocytes resulted in allograft CR. In contrast, a dose injected at 18 days had no detrimental effect. Although the latter observation has been interpreted as evidence ruling out microchimerism as a factor in the perpetuation of organ alloengraftment, none of the animals infused with the mAb at 18 days had elimination of microchimerism (7). Thus, these experiments merely demonstrated the difficulty of eradicating microchimerism once it was established.
Although higher doses of irradiation or the addition of other myeloablative drugs may eliminate established microchimerism after organ transplantation, our data further demonstrate the difficulty of completely removing donor leukocyte microchimerism once it is established in recipient tissues. It is noteworthy that the passenger leukocytes of organs were less vulnerable to the chimerism-depleting effects of TBI-reconstitution than infused BMC alone. The explanation for this observation is speculative. We have suggested that the copresence of the transplanted organ facilitates the survival of its peripheralized passenger leukocytes by providing a syngeneic haven (22, 23). Similarly, Bingaman and Larsen et al. (26) have shown that transplantation of a syngeneic bone shell may aid the production of hematolympho-poietic chimerism.
It has been well-established that the migration of organ passenger leukocytes occurs by the same routes as those taken by infused BMC as well as by hematogenously spreading non-cytopathic microorganisms (2–6). The leukocyte traffic is selective at first to host lymphoid organs (6). After a few days or weeks, however, donor cells that escape destruction by the immune response they have induced move on to nonlym-phoid niches that are relatively inaccessible to cellular and humoral effector mechanisms (5, 18), or back to the graft itself (22–24). In these privileged nonlymphoid locations, the passenger leukocytes appear to have a survival advantage analogous to that of residual microorganisms following a systemic infection (27,28).
Despite the failure to completely eliminate microchimerism in our organ recipients, the study demonstrated that variable organ-induced partial tolerance was abrogated in rough proportion to the reduction of the microchimerism. The latter rinding is compatible with the hypothesis that microchimerism is essential for maintenance of clonal exhaustion-deletion that is induced by the maximal initial flood of passenger leukocytes during the first few weeks after transplantation (27,28). In this paradigm, however, persistent donor cells migrating from the protected sites to organized lymphoid collections may have the undesired opposite effect of sustaining or initiating protective immunity (29,30). Anderson and Matzinger (13) have demonstrated in mice how experimental variables can determine whether microchimerism results in one or the other outcome.
The observations in our experiments also could be accommodated in (or even used to bolster) competing immunologic hypotheses of alloengraftment. For example, it could be argued that the loss or reduction of tolerance following irradiation-reconstitution was simply due to purging of engraftment-facilitating immunoregulatory cells (25) or interdiction of other linked antigen-dependent or antigen-independent cytokine- or idiotypic antibody-dependent mechanisms (12). In addition, the phenomenon of homeostatic proliferation that includes resurgence of antidonor memory cells (31) could be invoked to explain the loss of tolerance. As discussed elsewhere (4), the resistance of the liver to antitolerance measures is particularly noteworthy and may involve locally secreted growth factors or other unique features of the hepatic microenvironment. Examination of these theories and alternative mechanisms was beyond the intent of our experiments which were designed to determine the difficulty and feasibility of completely eradicatingt issue microchimerism.
Acknowledgments
We gratefully acknowledge the help of Ms. Terry L. Mangan in the manuscript preparation.
This work was supported by National Institutes of Health Grants AI38899 and DK64207.
References
- 1.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339(8809):1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemlander A, Soots A, von Willebrand E, et al. Redistribution of renal allograft-responding leukocytes during rejection. II. Kinetics and specificity. J Exp Med. 1982;156:1087–1100. doi: 10.1084/jem.156.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel route for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetris AJ, Qian S, Sun H, et al. Early events in liver allograft rejection. Am J Pathol. 1991;138:609–618. [PMC free article] [PubMed] [Google Scholar]
- 5.Demetris AJ, Murase N, Fujisaki S, et al. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 6.Murase N, Demetris A, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko S, Deiwick A, Jager MD, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med. 1999;5(11):1292. doi: 10.1038/15248. [DOI] [PubMed] [Google Scholar]
- 8.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 9.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today. 1996;17(12):584. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 10.Hisanaga M, Hundrieser J, Boker K, et al. Development, stability, and clinical correlations of allogeneic microchimerism after solid organ transplantation. Transplantation. 2000;61:40–45. doi: 10.1097/00007890-199601150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Burlingham WJ, Grailer AP, Fechner JH, Jr, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59(8):1147. [PubMed] [Google Scholar]
- 12.Waldmann H, Cobbold S. Exploiting Tolerance Processes in Transplantation. Science. 2004;305:209–212. doi: 10.1126/science.1099538. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nature Med. 2001;7:2425. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 14.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation. 1995;60(2):158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demetris AJ, Murase N, Ye Q, et al. Analysis of chronic rejection and obliterative arteriopathy. Possible contributions of donor antigen-presenting cells and lymphatic disruption. Am J Pathol. 1997;150:563. [PMC free article] [PubMed] [Google Scholar]
- 16.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 17.Yagihashi A, Takahashi S, Murase N, et al. A monoclonal antibody (L21-6) recognizing an invariant chain expressed on the cell surface in rats with the exception of the BN (RTln): a study of tissue and strain distributions. Transplant Proc. 1995;27(2):1519. [PMC free article] [PubMed] [Google Scholar]
- 18.Terakura M, Murase N, Demetris AJ, et al. Lymphoid/non-lymphoid compartmentalization of donor leukocyte chimerism in rat recipients of heart allografts, with or without adjunct bone marrow. Transplantation. 1998;66:350–357. doi: 10.1097/00007890-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Bosio E, Lee-Pullen TF, Fragall CT, et al. A comparison between realtime quantitative PCR and DNA hybridization for quantitation of male DNA following myoblast transplantation. Cell Transplant. 2004;13(7-8):817–821. doi: 10.3727/000000004783983369. [DOI] [PubMed] [Google Scholar]
- 20.Wang LJ, Chen YM, George D, et al. Engraftment assessment in human and mouse liver tissue after sex-mismatched liver cell transplantation by real-time quantitative PCR for Y chromosome sequences. Liver Transpl. 2002;8(9):822–828. doi: 10.1053/jlts.2002.34891. [DOI] [PubMed] [Google Scholar]
- 21.Fehse B, Chukhlovin A, Kuhlcke K, et al. Real time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J Hematother Stem Cell Res. 2001;10(3):419–425. doi: 10.1089/152581601750289028. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto T, Ye Q, Lu L, et al. Donor hematopoietic progenitor cells in non myeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67:833–840. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa N, Demetris AJ, Starzl TE, et al. Donor and recipient leukocytes in organ allografts of recipients with variable donor-specific tolerance: with particular reference to chronic rejection. Liver Transplantation. 2000;6:686–702. doi: 10.1053/jlts.2000.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gassel HJ, Otto C, Klein I, et al. Persistence of stable intragraft cell chimerism in rat liver allografts after drug-induced tolerance. Transplantation. 2001;71:1848–1852. doi: 10.1097/00007890-200106270-00023. [DOI] [PubMed] [Google Scholar]
- 25.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 26.Bingaman AW, Waitze SY, Alexander DZ, et al. Transplantation of the bone marrow microenvironment leads to hematopoietic chimerism without cytoreductive conditioning. Transplantation. 2000;69:2491–2496. doi: 10.1097/00007890-200006270-00006. [DOI] [PubMed] [Google Scholar]
- 27.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. NATURE Reviews: Immunology. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivasai KS, Alevy TY, Duffy BF, et al. Peripheral blood microchimerism in human liver and renal transplant reciepients: rejection despite donor-specific chimerism. Transplantation. 1997;64:427–432. doi: 10.1097/00007890-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 30.Brant I, Halloran B, Melk A, et al. Microchimerism in sensitized renal patients. Transplantation. 1999;67:1381–1383. doi: 10.1097/00007890-199905270-00018. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nature Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]