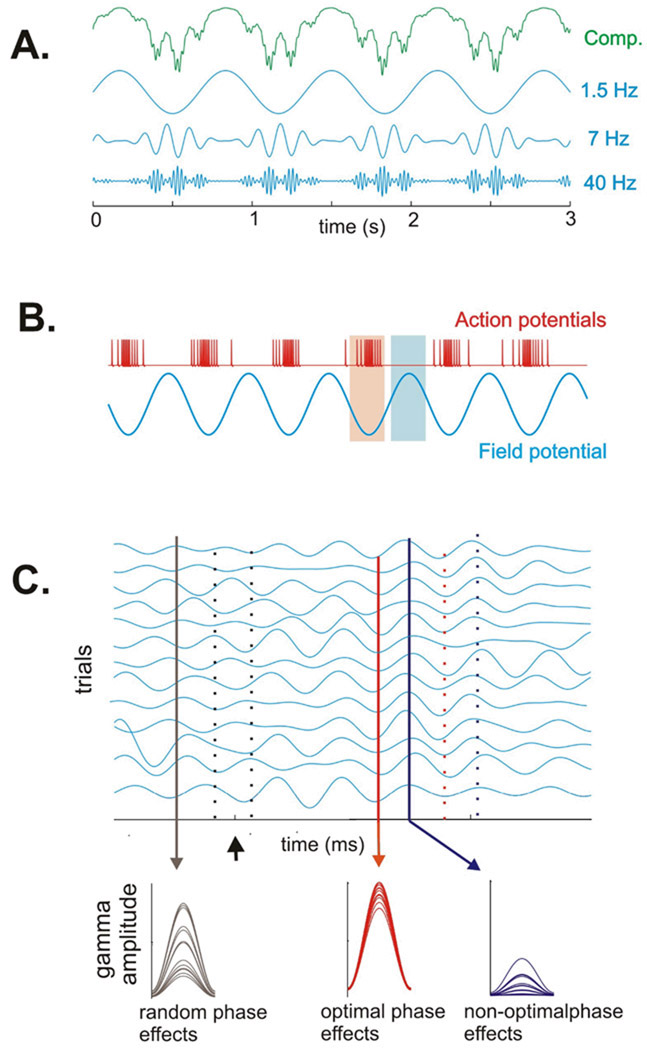
While the involvement of gamma band (30–100 Hz) oscillations in perceptual “binding,” attentional amplification of representation and cross-areal communication, and several elaborate aspects of information encoding may be arguable, it does seem clear that gamma enhancement reflects a state of high neuronal excitability and synchrony, that is conducive or even critical for brain operations (Fries et al., 2007). This notion sets gamma up as a “master” or executor process that determines whether an input is effectively integrated and an effective output is generated. However, gamma amplitude (generally taken as synchrony) is often coupled to the phase of lower frequency delta or theta oscillations (Buzsaki and Draguhn, 2004;Canolty et al., 2006;Lakatos et al., 2005), which would make gamma a “slave” to lower frequency activity. This relationship is illustrated schematically in Figure 1a. Here, we discuss conditions in which gamma oscillations dynamically couple and de-couple with lower frequencies, in accord with task demands.
Fig. 1.
A) Schematic of cross-frequency (phase-amplitude) coupling between delta, theta and gamma frequencies. The top (green) trace illustrates the typical observation: oscillations recorded in the brain are normally complex mixtures of components at different frequencies. The traces below illustrate the individual oscillatory components in the delta, theta and gamma band that comprise the composite waveform. We and others have noted (see text) that in normal systems, there is strong “phase-amplitude” coupling between frequencies, and it has a hierarchical organization. Gamma oscillatory amplitude varies with the phase of the underlying theta oscillation, and theta oscillatory amplitude varies with the phase of the underlying delta oscillation. B) A schematic of the relationship between excitability as indexed by action potential firing rate (red trace above) and the phase of oscillation in the local neuronal ensemble, as indexed by a local field potential (blue tracing below). Based on this type of experimental observation (Lakatos et al., 2005;Lakatos et al., 2007) we have proposed that ongoing neuronal oscillations have optimal (high excitability) and non-optimal (low excitability) phases. C - top) A series of simulated single trial responses representing activity in A1 as affected by visual inputs. When the system is at rest and unengaged (baseline prestimulus period to the left of zero) oscillations within a given frequency have a high degree of phase-variability across trials (see black drop line on left). A modulatory or phase-resetting event (arrow) can cause a phase re-set of the ongoing oscillations, such that the oscillation develops strong phase coherence across trials; under these conditions, the optimal phases (red drop lines) and non-optimal phases (blue drop lines) align separately and at predictable times. C-bottom) Sensory inputs arriving in random phase with the ongoing oscillation (black drop line in C) generate highly variable response amplitudes. Inputs arriving during the optimal phase (red drop line in C) are amplified, while those arriving during the non-optimal phase (blue line in C) are suppressed. Over time, the cross trial coherence dissipates, and the system goes back to its resting (random phase) state.
Natural sensory stimulation has pervasive rhythms. The natural auditory environment has a 1/f spectrum and auditory cortical oscillatory activity appears tuned to this spectrum (Garcia-Lazaro et al., 2006), as does oscillatory activity throughout the brain (Buzsaki and Draguhn, 2004). Obviously, explicit rhythms are absent in static visual and somatosensory stimuli. However, task-relevant somatosensory and visual stimulation is usually “acquired” through the observer’s motor behavior (e.g., eye/hand movements), which imposes a rhythmic pattern on the input stream. In large part, this is because motor behavior is itself patterned by oscillatory mechanisms like the 10 Hz “µ” rhythm (Pfurtscheller et al., 2000;Pineda, 2005). Moreover, as best exemplified by human speech, motor outputs have complex rhythm structures in which higher frequencies are “nested” in lower frequencies, just as is seen in the hierarchical phase-amplitude coupling of the cortical electroencephalogram (EEG) (Schroeder et al., 2008). Thus, biologically-generated stimuli such as body movements and vocalizations, even if passively received, can impart a great deal of rhythm.
In order to enable the generation of adaptive behavior, the brain’s sensory operations are tied by selective attention to specific or “relevant” environmental objects and events. When task-relevant stimuli occur in rhythmic patterns, and/or are sampled by motor behavior as described above, the brain can operate in a rhythmic mode, simply entraining its low frequency rhythms to external event rhythms, and attention contributes by enforcing oscillatory entrainment to the task-relevant event stream. The reason that this is beneficial is that each oscillation cycle has high and low excitability phases lasting from 10’s of milliseconds for beta and alpha to 100’s of milliseconds for theta/delta (Fig. 1b), and entrainment aligns these transient windows of high excitability with the relevant stimuli (Fig. 1c-top), amplifying the neuronal representation and facilitating both sensory discrimination and response speed and accuracy (Lakatos et al., 2008;Schroeder and Lakatos, 2008). Stimuli occurring out-of-phase with the task-relevant event stream generate inputs that arrive in the brain during low excitability phases of the entrained oscillations, and are thus suppressed (Fig 1C-bottom). A key component of the high-excitability phase is enhancement of gamma amplitude/synchrony, and because gamma amplitude is coupled to lower frequency phase, there is effectively a burst of gamma occurring at the time an input from a task relevant event is expected. This is an efficient use of resources as gamma band activity appears to be more metabolically-demanding than low frequency oscillations (Mukamel et al., 2005;Niessing et al., 2005), and because of hierarchical coupling, gamma activity is “rationed,” or selectively enhanced at critical time points, when a high excitability state is most useful.
In contrast, when there is no task relevant rhythm that the system can entrain to, low frequency oscillations are actually detrimental to processing, as they entail long periods of low excitability during which detection of a subtle random stimulus is less likely. Under these conditions a continuous (vigilance) mode of operation is implemented, and attention maximizes the sensitivity of the system by suppressing lower frequency oscillations and exploiting the advantages of extended continuous gamma band oscillations (Borgers and Kopell, 2008). One might think that a system could optimize itself by just staying in a continuous vigilance mode, but it turns out that in line with the common subjective experience, the vigilance mode is difficult to maintain for extended periods. For a variety of reasons (Schroeder and Lakatos, 2008), the rhythmic seems to be the preferred mode of the system, in which it spends the most time. Thus, gamma may be more often slave than master.
Reference List
- Borgers C, Kopell NJ. Gamma oscillations and stimulus selection. Neural Comput. 2008;20:383–414. doi: 10.1162/neco.2007.07-06-289. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1629. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. TINS. 2007;30(7):309–316. doi: 10.1016/j.tins.2007.05.005. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- Garcia-Lazaro JA, Ahmed B, Schnupp JW. Tuning to natural stimulus dynamics in primary auditory cortex. Curr Biol. 2006;16:264–271. doi: 10.1016/j.cub.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:1–14. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Oscillatory entrainment as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Kraut M. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin.Neurophysiol. 2000;111:1873–1879. doi: 10.1016/s1388-2457(00)00428-4. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating "seeing" and "hearing" into "doing". Brain Res Brain Res Rev. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low frequency oscillations as instruments of sensory selection. TINS. 2008 doi: 10.1016/j.tins.2008.09.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. TICS. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]