Abstract
In the brains of humans and other mammals, there are two principal groups of cholinergic nuclei aside from those forming the cranial motor nuclei. One group lies in the forebrain and includes the nucleus basalis of Meynert. The second group lies in the hindbrain and includes the nucleus tegmenti pedunculopontinus (NPP), identified by Mesulam et al. [Mesulam, M.-M., Mufson, E. J., Wainer, B. H. & Levey, A. I. (1983) Neuroscience 10, 1185-1201] as cholinergic cell group Ch5. The basal forebrain cholinergic cell groups, which innervate widespread areas of the neocortex, undergo degeneration in Alzheimer disease and also in parkinsonism associated with dementia. We here report that the hindbrain NPP Ch5 cell group, thought to innervate many nuclei of the extrapyramidal motor system, the superior colliculus, and the substantia innominata, undergoes degeneration in idiopathic Parkinson disease and in the parkinsonian syndrome of progressive supranuclear palsy. These findings strongly suggest that degeneration in the brainstem in Parkinson disease is not confined to catecholamine-containing neurons, but that cholinergic neurons of the NPP are also vulnerable. The findings further raise the possibility that certain symptoms of Parkinson disease and progressive supranuclear palsy have their genesis in pathology of these cholinergic neurons.
Full text
PDF
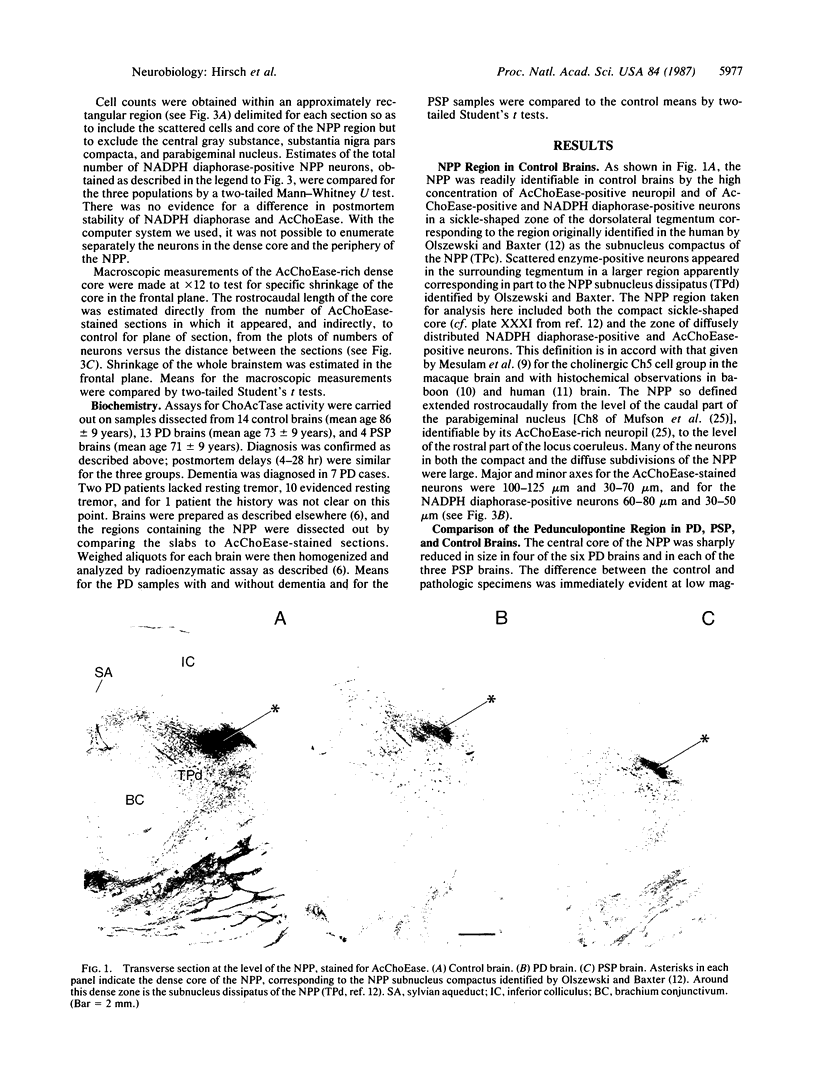

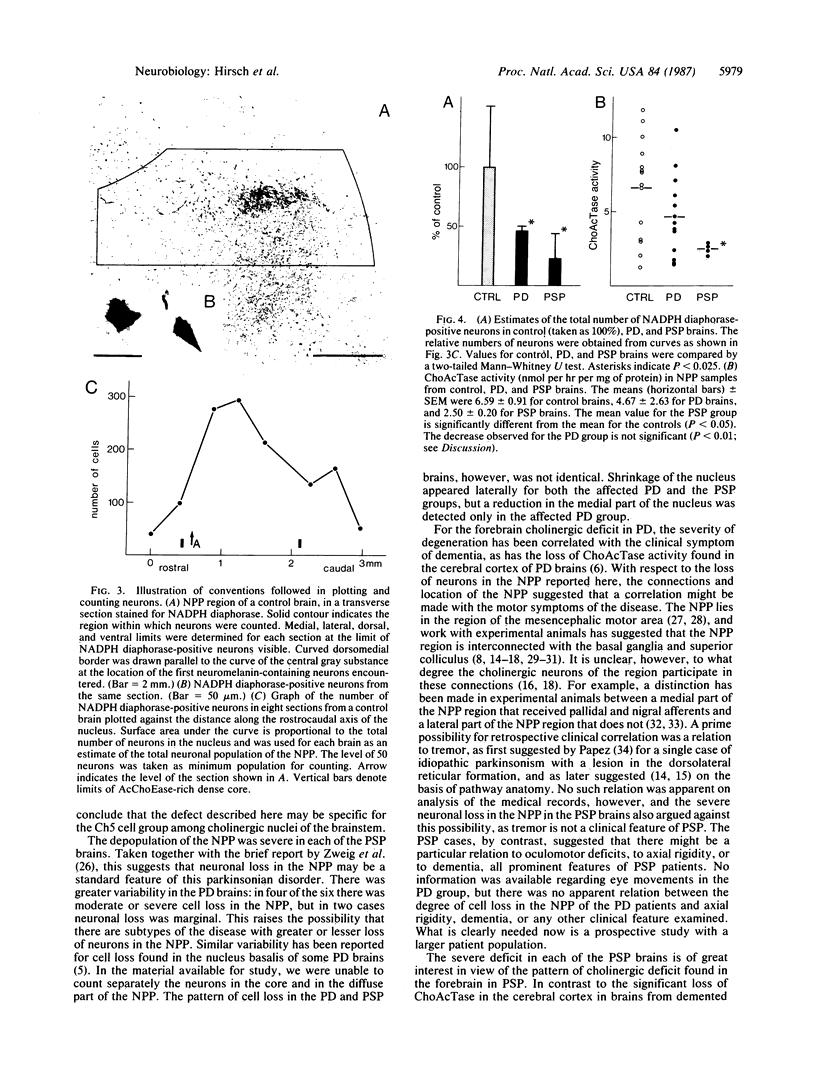

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agid Y., Javoy-Agid F., Ruberg M., Pillon B., Dubois B., Duyckaerts C., Hauw J. J., Baron J. C., Scatton B. Progressive supranuclear palsy: anatomoclinical and biochemical considerations. Adv Neurol. 1987;45:191–206. [PubMed] [Google Scholar]
- Beninato M., Spencer R. F. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol. 1986 Nov 22;253(4):525–538. doi: 10.1002/cne.902530409. [DOI] [PubMed] [Google Scholar]
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Candy J. M., Perry R. H., Perry E. K., Irving D., Blessed G., Fairbairn A. F., Tomlinson B. E. Pathological changes in the nucleus of Meynert in Alzheimer's and Parkinson's diseases. J Neurol Sci. 1983 May;59(2):277–289. doi: 10.1016/0022-510x(83)90045-x. [DOI] [PubMed] [Google Scholar]
- Dubois B., Hauw J. J., Ruberg M., Serdaru M., Javoy-Agid F., Agid Y. Démence et maladie de Parkinson: corrélations biochimiques et anatomo-cliniques. Rev Neurol (Paris) 1985;141(3):184–193. [PubMed] [Google Scholar]
- Edley S. M., Graybiel A. M. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983 Jun 20;217(2):187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol. 1977 Sep 1;175(1):37–78. doi: 10.1002/cne.901750105. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Hirsch E. C., Agid Y. A. Differences in tyrosine hydroxylase-like immunoreactivity characterize the mesostriatal innervation of striosomes and extrastriosomal matrix at maturity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):303–307. doi: 10.1073/pnas.84.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M. Neurochemically specified subsystems in the basal ganglia. Ciba Found Symp. 1984;107:114–149. doi: 10.1002/9780470720882.ch7. [DOI] [PubMed] [Google Scholar]
- Grillner S., Shik M. L. On the descending control of the lumbosacral spinal cord from the "mesencephalic locomotor region". Acta Physiol Scand. 1973 Mar;87(3):320–333. doi: 10.1111/j.1748-1716.1973.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Jackson A., Crossman A. R. Basal ganglia and other afferent projections to the peribrachial region in the rat: a study using retrograde and anterograde transport of horseradish peroxidase. Neuroscience. 1981;6(8):1537–1549. doi: 10.1016/0306-4522(81)90223-2. [DOI] [PubMed] [Google Scholar]
- Jones B. E., Yang T. Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985 Dec 1;242(1):56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Mehler W. R. The basal ganglia-circa 1982. A review and commentary. Appl Neurophysiol. 1981;44(5-6):261–290. [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984 Jul;12(3):669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Wainer B. H., Levey A. I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983 Dec;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mizukawa K., McGeer P. L., Tago H., Peng J. H., McGeer E. G., Kimura H. The cholinergic system of the human hindbrain studied by choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Brain Res. 1986 Jul 30;379(1):39–55. doi: 10.1016/0006-8993(86)90253-2. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Martin T. L., Mash D. C., Wainer B. H., Mesulam M. M. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res. 1986 Apr 2;370(1):144–148. doi: 10.1016/0006-8993(86)91114-5. [DOI] [PubMed] [Google Scholar]
- Nauta W. J., Mehler W. R. Projections of the lentiform nucleus in the monkey. Brain Res. 1966 Jan;1(1):3–42. doi: 10.1016/0006-8993(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Riopelle R. J. Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci. 1986 Aug;6(8):2312–2321. doi: 10.1523/JNEUROSCI.06-08-02312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberg M., Javoy-Agid F., Hirsch E., Scatton B., LHeureux R., Hauw J. J., Duyckaerts C., Gray F., Morel-Maroger A., Rascol A. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol. 1985 Nov;18(5):523–529. doi: 10.1002/ana.410180503. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Ploska A., Javoy-Agid F., Agid Y. Muscarinic binding and choline acetyltransferase activity in Parkinsonian subjects with reference to dementia. Brain Res. 1982 Jan 28;232(1):129–139. doi: 10.1016/0006-8993(82)90615-1. [DOI] [PubMed] [Google Scholar]
- Russchen F. T., Amaral D. G., Price J. L. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985 Dec 1;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res. 1982 Dec 9;252(2):367–372. doi: 10.1016/0006-8993(82)90404-8. [DOI] [PubMed] [Google Scholar]
- Satoh K., Fibiger H. C. Distribution of central cholinergic neurons in the baboon (Papio papio). I. General morphology. J Comp Neurol. 1985 Jun 8;236(2):197–214. doi: 10.1002/cne.902360205. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Sims K. L., Kauffman F. C., Johnson E. C., Pickel V. M. Cytochemical localization of brain nicotinamide adenine dinucleotide phosphate (oxidized)-dependent dehydrogenases. Qualitative and quantitative distributions. J Histochem Cytochem. 1974 Jan;22(1):7–19. doi: 10.1177/22.1.7. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Hattori T. Organization and efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience. 1984 Apr;11(4):931–946. doi: 10.1016/0306-4522(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Satoh K., Armstrong D. M., Fibiger H. C. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett. 1983 Dec 23;43(1):31–36. doi: 10.1016/0304-3940(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Satoh K., Armstrong D. M., Panula P., Vale W., Fibiger H. C. Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience. 1986;17(1):167–182. doi: 10.1016/0306-4522(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Hedreen J. C., White C. L., 3rd, Price D. L. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol. 1983 Mar;13(3):243–248. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- Woolf N. J., Butcher L. L. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986 May;16(5):603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]