Abstract
Aims: To elucidate psychosocial and ethical issues faced by adults at risk for alpha-1 antitrypsin deficiency (AATD) that have received little attention. Methods: Eleven individuals with AATD were interviewed in detail for 2 hours each. Results: Several specific aspects of AATD created critical, socially dynamic issues that shaped the patients' responses. The disease being relatively newly discovered, physicians do not know much about it and thus often do not consider or recommend testing for it. Hence, patients who may benefit from diagnosis and treatment are not always diagnosed. General practitioners, when they do diagnose patients, often refer them to specialists and thus remain inexperienced in treating the disorder. As a result, many individuals, too, remain unaware of this disease in their families and thus do not consider mentioning its possibility to doctors or family members. Thus, intrafamilial disclosures by patients become critical. Patients may be shocked and upset at diagnosis, as they might possibly already have transmitted the mutation to offspring, which further impedes disclosure to family members. Conclusions: These issues highlight how patients' interactions with doctors and others concerning genetics are critical, and need to be further explored and addressed. Several aspects of physician education and practice (e.g., regarding disclosures to at-risk family members) need to be improved.
Introduction
The literature on ethical, legal, and social issues in genetics has tended to focus on several disorders, and paid less attention to others that might be important in understanding the full implications of the rapidly expanding use of genetic tests. Researchers have identified genes for thousands of disorders, prompting testing for many of these conditions through clinicians and direct-to-consumer marketing on the internet. An important case in point is alpha-1 antitrypsin deficiency (AATD), the only disease for which the Equal Employment Opportunity Commission ruled that genetic discrimination has occurred (Oveson and Yarborough, 2001; Jones and Smith, 2003).
For several other adult-onset diseases, studies have explored how patients make decisions about testing—for example, for Huntington's disease (Klitzman et al., 2007a, 2007b, 2007c), breast cancer (Jacobsen et al., 1997; d'Agincourt-Canning, 2006), and hereditary nonpolyposis colorectal cancer (Aktan-Collan et al., 2000; Hadley et al., 2003). AATD may resemble, and/or differ from, these other conditions in critical ways—such as the timing and extent of clinical presentation, severity of symptoms, means of diagnosis, patterns of inheritance, and genetic expression—all of which can affect patient and health care provider making decisions about testing, treatment, and care.
AATD, a genetic disorder caused by defective production of alpha-1 antitrypsin, was discovered in 1963 (Laurel and Eriksson, 1963). This condition is autosomal codominant and affects the liver and lungs. Very low levels of antitrypsin predispose to the development of emphysema, which, if left untreated, can lead to early death. Individuals with phenotypes for severe deficiency (PiZZ or PiSZ) are also susceptible to liver disease and cirrhosis. Liver transplantation may be necessary in severe cases. Early detection of the disorder through genetic testing enables initiation of organ replacement, avoidance of environmental factors that can exacerbate illness (e.g., smoking and certain pollutants), and participation in genetic counseling and family planning programs (Hogarth and Rachelefsky, 2008).
In the United States, approximately 1/5000 individuals or about 60,000 individuals have phenotypes for severe AATD (Browne et al., 1996), yet its symptoms have been diagnosed in only about 5% of cases (Campos et al., 2005). Worldwide, approximately 10,000 patients (about 5000 in the United States) now receive treatment for this disorder (Stolk et al., 2006). Thus, in the United States, over 80% of patients who would benefit from treatment do not receive it. Many physicians remain unaware of problems caused by AATD (Stolk, 2005). Diagnosis itself can cause depression and anxiety. A study that screened Swedish neonates found that mothers of AATD children had significantly more anxiety, at least in the short term, than those of matched controls (Sveger et al., 1999; Sveger and Thelin, 2000). The World Health Organization (2006), The American Thoracic Society, and the European Respiratory Society have issued recommendations (ranked as to relative degree of importance) that in Europe and North America, given the relatively high prevalence of the disease, physicians should test patients, including those with emphysema, chronic obstructive pulmonary disease, untreatable asthma with air flow obstruction, and unexplained liver disease, and siblings and relatives of patients with AATD (American Thoracic Society, 2003; Hogarth and Rachelefsky, 2008). However, it is not known how far these recommendations are effective in improving the situation.
Despite the increased attention given to AATD since its discovery, between 1968 and 2003 diagnoses of the disease by physicians did not improve, and in fact the length of time between symptoms and diagnosis increased (Campos et al., 2005). Moreover, patients often report insufficient genetic counseling (Sveger et al., 1999; Stolk, 2005).
Thus, critical questions arise about specific obstacles to earlier and more frequent testing of patients at risk. In general, physicians have limited understanding of genetics (Menasha et al., 2000; Sifri et al., 2003; Wideroff et al., 2003). With other diseases, communication between physicians and patients is often deficient as well (Klitzman, 2006a, 2006b, 2008). In the case of Huntington's disease (Klitzman et al., 2007b) and HIV-AIDS (Klitzman and Bayer, 2003), critical obstacles also exist to disclosure of diagnoses by patients to others who may be at risk and to health care providers. Indeed, among individuals recruited through an AATD patient organization, one-third of whom requested a testing kit and returned it, and about half (56%) of whom also then completed a questionnaire, most said they planned to tell family members, though not all (only 82.9% of homozygotes for mutations, and 59.9% of carriers) would tell their physician (Strange et al., 2004). However, the number of individuals who actually did tell their family members was not ascertained. Moreover, this subsample of respondents is unusual in already being connected to a patient advocacy group, and being motivated to both undergo testing and complete the questionnaire. But disclosures to both family and providers may still be suboptimal.
Clearly, many psychological, social, and ethical issues faced by adult patients at risk for AATD have received little or no attention—for example, concerning whether, when, and how physicians and patients end up deciding to pursue testing (how they view and make these decisions, and what additional factors, may be involved), and how patients decide whether, when, what, and to whom to disclose their test results. Moreover, much of the work above has been done in Scandinavia, not in the United States, and systems of health care in these two parts of the world vary radically (World Health Organization, 2006). In Sweden, the condition is much more common (about 1/1500). Universal health coverage may dramatically alter attitudes and approaches toward making decisions about testing, disclosure, and health care among both providers and patients.
From a theoretical perspective, the Health Belief Model suggests that perceived susceptibility, disease severity, and costs and benefits may affect an individual's health behaviors (Rosenstock et al., 1988). Such a model has been applied to decisions about testing for other genetic diseases (Klitzman et al., 2007b), but has not yet been examined with regard to AATD. The Stages of Change Model (Prochaska et al., 1992) suggests that individuals go from being unaware and unengaged to making decisions about whether to follow a health behavior (e.g., testing) or not. This model has also been applied to other diseases (Klitzman et al., 2007b), but not AATD, where it may be relevant, too.
In short, it is important to examine the processes through which individuals at risk for AATD decide whether to undergo testing, when to disclose to family members and others, and what roles physicians may play in these decisions—for example, what obstacles are involved, how they operate, and what can be done to address them.
Materials and Methods
Sample
Eleven individuals with AATD (seven women and four men) were interviewed in detail for 2 hours each. Table 1 provides background information on the participants, including employment status, years since diagnosis of the disorder, and treatment taken.
Table 1.
Background Info on Alpha-1 Antitrypsin Deficiency Interviewees
| |
|
|
|
|
Treatment |
|
|---|---|---|---|---|---|---|
| ID # | Sex | Age | Years since diagnosis | Employment status | Prolastin | Other |
| 1 | F | 57 | 12 | Unemployed | Yes | |
| 2 | F | 59 | 4 | Professional | Yes | |
| 3 | F | 48 | 10 | Unemployed | Yes | Double lung transplant |
| 4 | M | 61 | 11 | Professional | Yes | |
| 5 | F | 61 | 5 | Professional | Yes | |
| 6 | F | 65 | 15 | Professional | Yes | |
| 7 | F | 70 | 4 | Professional | Yes | |
| 8 | F | 59 | 7 | Professional | Yes | Portable oxygen |
| 9 | M | 59 | 23 | Professional | Yes | |
| 10 | M | 54 | 12 | Professional | Yes | |
| 11 | M | 71 | 4½ | Professional | Yes | |
Information about the study was distributed through an AATD clinic, an AATD organization, an AATD newsletter, and word of mouth. Individuals interested in participating contacted the principal investigator (PI), who conducted a confidential in-depth semistructured interview with each concerning experiences of having, or being at risks for, AATD. Interviews were conducted in the PI's office. The Columbia University Department of Psychiatry Institutional Review Board approved the study, and all participants gave informed consent. Table 2 presents sample questions from the semistructured interview guide. We sought to obtain detailed descriptions of the participants' views and decisions concerning testing and related issues.
Table 2.
Semistructured Interview Guide (Sample Questions)
| When did you first find out you were at risk of Alpha, and what was your reaction at that time? |
| How did you find out you were at risk? |
| Have you undergone genetic testing for Alpha? Why or why not? |
| How did you make this decision? |
| Whom have you told or not told about having/being at risk of the disease? How did you make these decisions? |
On theoretical grounds, Geertz (1973) has advocated studying aspects of individuals' lives and social situations by trying to understand their experiences and drawing on their own words and perspectives to obtain a “thick description,” rather than by imposing external theoretical structures. Hence, we used qualitative methods to fully understand the various factors and issues that may be involved in genetic testing decisions.
In our methods, we adapted elements from grounded theory, as described by Strauss and Corbin (1990), because we were interested in understanding a complex social process. We have used these methods in several other studies involving genetics (Klitzman et al., 2007a, 2007b, 2007c) and other aspects of health behavior and doctor-patient relationships and communications (Klitzman et al., 2006a, 2006b). Specifically, grounded theory involves both inductive and deductive thinking, building inductively from the data to an understanding of themes and patterns within the data, and deductively drawing on frameworks from previous research and theories. For example, interviewees introduced topics such as interactions with providers, which were then explored further in these and other interviews. Our approach was informed constant comparison, in which data from different individuals were compared for similarities and differences to see whether these suggested hypotheses. Transcriptions and initial analyses of interviews were done during the period in which the interviews were being conducted and helped guide subsequent interviews. Interviews were conducted until “saturation” was reached (i.e., “the point at which no new information or themes are observed in the data”) (Strauss and Corbin, 1990; Guest et al., 2006).
Once the full set of interviews was completed, subsequent analyses were conducted in two phases, primarily by the PI together with a research assistant who had social science training. At several points during the analysis, we also received input from another senior expert in qualitative research. In phase one of the coding process, the PI and the research assistant independently examined a subset of interviews to assess factors that shaped participants' experiences, identifying categories of recurrent themes and issues that were subsequently given codes. These two coders assessed similarities and differences between participants, examining themes and categories that emerged, ranges of variation within categories, and variables that may be involved. They systematically coded blocks of test to assign “core” codes or categories. While reading the interviews, a topic name (code) was inserted beside each excerpt of the interview to indicate the themes being discussed. The coders then worked together to reconcile their independently developed coding schemes into a single scheme, developing a coding manual and examining areas of disagreement until reaching consensus between them (i.e., when each coder's view of a phenomenon or theme raised by a participant was reconciled into a coherent understanding of it). New themes that did not fit into the original coding framework were discussed, and modifications were made in the manual when deemed appropriate.
In the next phase of the analysis, the thematic categories were subdivided into secondary themes or subcodes, and then refined and merged, when suggested by associations or overlap in the data. Codes and subcodes were then used in analysis of all of the interviews. Major codes (or categories) of text included, for example, occasions when providers gave input about the disease, and when patients informed family members. Subcodes (or subthemes) were conceptual and thematic subdivisions of these larger categories and included reactions and behaviors of physicians once they diagnosed patients (e.g., referrals to specialists, discussions with patients or family members). To ensure coding reliability, the two coders analyzed all interviews. Areas of disagreement were examined to reach consensus. To ensure trustworthiness, an external senior qualitative researcher was frequently consulted at multiple points and the data triangulated with existing literature on other genetically associated diseases. These data also have a certain face validity that further substantiates their trustworthiness. We have presented below text from the interviews to allow readers to judge these data for themselves as well.
Results
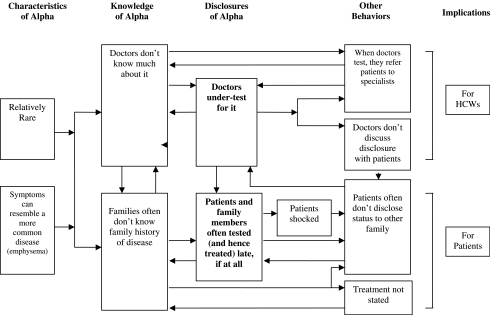
As illustrated in Figure 1, several key unique characteristics of AATD shaped the decisions knowledge and behaviors of individuals, which then proved interrelated, forming a complex, interdynamic system that contributed to undertesting and underdiagnosis of the disease.
FIG. 1.
Interacting themes concerning characteristics of AATD and their implications for providers and patients.
The following are such characteristics of the disease: First, it is relatively rare. Second, it is an autosomal recessive condition, and—unique from several other genetic diseases—heterozygotes for the mutation, if they smoke, can potentially have symptoms (though not as severely as those who are homozygote for the mutation) that may or may not be related to the condition. Third, enzyme replacement is possible.
As the disease is uncommon and its genetic basis relatively new and hence less known, doctors and health care workers (HCWs) do not know much about AATD. This leads to undertesting and underdiagnosis of the disease, as in the following instance.
“I saw 20 doctors; none of them tested me for Alpha, or knew what it was. I still come across doctors who don't know what it is. They ask me, ‘Any medical history?’ I say I've got Alpha One. ‘What is that?’ ” (Table 1, ID #3)
These patients felt that certain types of specialists, in particular, should know about AATD, but often do not.
“Any pediatrician, pulmonary doctor, or doctor that deals with asthma, should be up to date and fully aware of this disease.” (Table 1, ID #3)
Yet, the rareness of the disease contributes to doctors being less aware of it. This problem may also arise for other relatively rare disorders for which single-point mutations are nevertheless found.
“If a disease has been around for many years, everybody knows about it. Alpha is so new and rare. Doctors don't know about it, therefore it's missed. I went back to two of those doctors to get it off my chest. I expected, ‘I'm sorry, I wish I had known, or had more knowledge.’ But one made a smart remark. I called him an asshole.” (Table 1, ID #3)
Doctors may know the name of the disease, but little else, if anything, about it.
“I liked one doctor a lot, but when she had my file she obviously knew the term, but not really a whole lot about it. Nor should she. I was hospitalized for pneumonia a couple of years ago, and was close to my main Alpha doctor, but other residents would come in. One young intern is going to be an excellent doctor. She said, ‘You're my first Alpha, I read all about this in medical school.’ I look pretty much like everyone else, except if you see my x-rays.” (Table 1, ID #9)
As a result, “There are a lot of people who know more about this than the doctors do,” (Table 1, ID #9), which can in turn create stresses and strains in receiving treatments and interacting with providers.
Providers' low knowledge of a disease, especially a relatively unusual one, can also lead them to dispense inaccurate information about testing and other aspects of the illness. Without much understanding, physicians may give patients incorrect prognoses and advice.
“Some Alphas are mad that their doctor didn't know squat, and just told them they're going to die. One person was diagnosed early on, in the early 80s, and told not to exercise: ‘You only have so much function left. Don't use it up with exercise’—which turns out is exactly the wrong advice. He got a lung transplant 9 years ago, and died 2 years ago, but was told he was going to die in ’81!” (Table 1, ID #10)
Owing to lack of knowledge about the condition, doctors do not test for it as often as they should. Often, only the patient's persistence prompts eventual assessment and testing. Doctors may at first miss the diagnosis and later send the patient to specialists. Yet such secondary or tertiary referrals may serve to keep knowledge or experience about AATD low among general internists and other less specialized doctors, furthering a self-perpetuating cycle.
“Before I caught Alpha-1, when I just had plain old emphysema, I was tested in my local hospital, which is pretty good. But did the respiratory therapist or doctor say, ‘Maybe you should test for Alpha’? No. I would think that would have been the first thing they'd say. But that was back in the 80s. I don't know what they knew. I was seeing a family practice doctor. I said, ‘I need to see you because I'm not getting any better.’ He finally did diagnose me, but then got me mad because he didn't want to see me as a patient. He sent me to New York, because he didn't have any Alpha patients, and didn't know squat about it.” (Table 1, ID #10)
Referral to specialists may also require further travel and cause logistical difficulties for patients.
“It turns out, looking back, that was the exact right thing for him to do. But at the time, I was upset. I didn't want to come to New York. It's a pain in the ass.” (Table 1, ID #10)
Patients may feel frustrated, not understanding the need for a referral, and realizing only in retrospect that such referral may be the best. They may see only the final outcome as justifying the extra effort. For rare diseases, reliance on specialists may enhance the care, but it would minimize other physicians' experience in treating future patients and the motivation to gain such experience.
Family histories of the diagnosis are also often not known. The presence of the disease may not be recognized because of poor prior diagnoses, or because a family history is communicated poorly, if at all. Moreover, relatives may have had undiagnosed AATD.
“My father's sisters and brothers could have had it. A cousin died at 35 of emphysema. At that time, they didn't know what Alpha was, so it could have been.” (Table 1, ID #3)
In fact, known only since 1963, AATD was often missed before that.
“My cousin's father was possibly a carrier. He was turned down during World War II. I think it's because he had asthma or something like that. Alpha wasn't discovered until 1963.” (Table 1, ID #8)
Indeed, several participants said they were the first in their family to be diagnosed—which may frequently be the case for codominant or recessive disorders. Hence, entry into the Alpha community may be further impeded or delayed.
Over time, cultural and familial norms and taboos about discussing diagnoses have also shifted. In the past, more reticence reigned. On the other hand, the hereditary nature of the disease in and of itself could help prompt disclosure. One woman said about the major differences between genetic and other diseases:
“I wonder if my grandparents had breathing problems. My father's mother died when he was very young. They don't know what she died from. People didn't talk about things then.” (Table 1, ID #5)
Yet patients may first learn about their own risk through relatives'—even offspring's—experiences. Since physicians may have little clinical exposure to the disease, they may not apply their past-acquired factual knowledge in clinical contexts.
“I had been sick for a while, not responding to antibiotics. Then, my son came for Christmas and had pneumonia, which he's been prone to since a young child. He was on an antibiotic. But I sent him down to my doctor, and made him promise to have tests done when he got back to his own home. He did that and a couple of months later was sick again. I asked him, ‘Did you ever see your doctor?’ He said, ‘Well, yeah, as a matter of fact, I have something with a missing enzyme. They said it's not a concern right now.’ A month or so later, my family doctor asked me if he had had testing done. I said, ‘Yeah, they told him he had some kind of missing enzyme,’ and the light bulb went on for my doctor. He said, ‘Alpha Antitrypsin! My God, I'll bet that's what you have!’ ” (Table 1, ID #6)
Yet a doctor may not tell a patient to disclose the diagnosis to at-risk family members.
“My son's doctor did not say, ‘You should tell your parents to get themselves checked out.’ It was a gift from the gods that the scenario played out for me the way it did. If I hadn't asked, I wouldn't have found out. I don't know that he even told him it was genetic, just that it was an enzyme deficiency.” (Table 1, ID #6)
Poor communication may have resulted, too, from avoidance or denial concerning the disease.
“My son is in tremendous denial about this. He's a heavy smoker.” (Table 1, ID #6)
Disclosure within families is thus particularly important, but may encounter significant obstacles. Family members may also be estranged. If the condition were not genetic, some felt they would not have to communicate with, or tell, these other individuals at all.
“You have to speak to, and reach out to family members that you probably wouldn't speak to.… If it wasn't genetic, you wouldn't bother even telling them.” (Table 1, ID #3)
Yet, diagnosis with a potentially lethal disease can also shock relatives, impeding their disclosure of the illness to other relatives.
“I felt depressed, scared, told I have a lethal disease—that more than likely, I will not live a normal life span.” (Table 1, ID #10)
Many are shocked, too, suddenly confronted with complex information about a disease they had never heard of.
“We were numb. The doctor taped the meeting—though we didn't want to let him. But we went back to the house, and sat down and listened to it several times, and tried to figure out what it was all about.” (Table 1, ID #8)
The unexpected nature of the process of diagnosis—the fact that patients are not psychologically prepared and genetic counseling may not have been given—can lead to confusion, which then prevents patients from disclosing this information to other relatives who may also be at risk and could potentially benefit from genetic testing and treatment.
When diagnosed, individuals are suddenly confronted by a large amount of new, complex, and threatening information about diagnosis, prognosis, and treatment, including often seeing further patients with more advanced disease.
“I went to a specialist and saw all these gray people carrying these oxygen canisters. I was really knocked out. It took me a year to really get back. In my town, I know how to deal with everything. Not as stressful as going to a strange place in a wheelchair, because it's hard to walk. And with a sinus infection I can't do anything. Once you get used to it, it isn't as bad. But I never thought this way. When you're in an unfamiliar place and can't breathe, it's more stressful.” (Table 1, ID #8)
Nonetheless, the diagnosis can be a relief as it provides an answer to the patient's problem and can lead to initiation of beneficial prevention and treatment.
“I was so pleased to get an actual diagnosis that matched, because I didn't feel I fit emphysema … I felt better knowing.” (Table 1, ID #2)
Questions arose as to whether doctors should encourage patients to have family members tested and, if so, when, how, and to what degree. Some felt that physicians should indeed do so. Yet the question remained, to what degree.
“It was up to the doctor … to either write it down for [the patient] or tell him: ‘This is what you have. You are a carrier. You've got this gene from one of your parents. Have your parents tested.’ ” (Table 1, ID #5)
Thus, doctors do not always communicate about the importance of disclosure and testing issues. This patient may have been told to tell his parents, but for various reasons may not have either fully heard or been able to follow the advice.
Discussion
These data show how several specific aspects of AATD—primarily its rareness, and the resemblance of its symptoms to more common diseases such as asthma or chronic obstructive pulmonary disease—lead to circumstances that profoundly shape the responses of health care providers and patients. Specifically, its newness leads to physicians not knowing much about it, and thus often not considering or ordering genetic tests for it. Consequently, patients who may benefit from diagnosis and treatment are not tested or diagnosed. Moreover, primary providers, when they do diagnose a patient, then often refer that patient to a specialist for treatment. General practitioners thus do not acquire the added experience that might be helpful.
All these factors contribute to individuals being unaware of this disease in their family and thus not mentioning the possibility of the diagnosis to their doctors. Disclosures by patients to other family members thus becomes extremely important. Yet physicians may or may not tell patients, when diagnosed, to inform other kin. Newly tested patients may be shocked and upset (i.e., anxious and depressed) at being diagnosed with a potentially fatal genetic disease, the mutation for which they might already have passed onto their children or even grandchildren. Treatment is costly, and organ transplants may be needed, which are difficult to obtain. These may further impede disclosure within a family.
Thus, providers and patients form a vicious cycle—a complex set of positive feedback loops that impede testing and subsequent treatment. Low knowledge among both providers and patients can be mutually reinforcing, and hamper discourse and discussions between patients on the one hand and providers and at-risk family members on the other.
To alter these dynamics requires careful efforts to improve knowledge and behavior of patients and providers. At first glance, this problem may seem inevitable, and difficult to remedy except through broad population-based screening. Yet increased awareness of these difficulties among medical educators and providers may help ameliorate it. This study thus highlights the importance of examining these complex social interactions and dynamics—for example, between providers and patients—involved in the testing and disclosure of genetic diseases.
From a theoretical perspective, these data highlight the degrees to which social interactions shape perceived awareness and susceptibility to a disease—key components of the Health Belief Model and Stages of Change Model, respectively. Stages of change, going from being unaware to being engaged in making a decision, are very much molded by dynamic social processes, with social inputs from providers, family members, and others. One at-risk individual can tell others, who can then inform more individuals. Thus, it is critical that providers understand and appreciate these interactions. At the same time, these data underscore both the limitations of these two rational choice models and the need to develop more nuanced frameworks that examine and take into account these complex social systems and dynamic processes. For example, various characteristics of immediate and extended families as social entities may affect whether, when, what, and how information is communicated within them. These characteristics may prove critical in influencing whether, when, and to what extent individuals become aware of their susceptibility to genetic disease.
The fact that these dynamics and characteristics of this genetic disease shape the specific issues that arise has critical implications for other genetic tests being pursued. Indeed, mutations may in fact well be identified disproportionately for rare versus more common diseases.
Genetic research may also lead to the identification and labeling of other new and rare diseases, and possibly of entirely new diagnostic categories and disorders. Indeed, in the past, nosologies have been altered by technological interventions that permit more precise and sophisticated differentiation between disease states that were formerly lumped together. Such other new disorders will pose similar challenges for the education of both medical trainees and established providers, and for clinical practice. Practitioners will need education to deal with increasing complex decisions regarding testing, counseling, and disclosure. Physicians and medical educators need to give more attention to addressing whether, how, and when physicians and patients do or do not interact and communicate concerning disclosures of genetic diagnoses to family members who may also be at risk. Yet, here, ethical questions and tensions arise. For example, patients may not be in touch with at-risk family members. Patients may want to tell children who are cognitively or emotionally too immature to comprehend or handle the information adequately.
Patients may also want to arrange to test family members. But these relatives may also be too young or unable to fully respond to the information. Alternatively, physicians may suggest in passing that family members be tested, but not sufficiently follow-up or encourage them to be effective. Doctors and other health care providers need to be fully prepared to deal with these issues and be comfortable doing so. Physicians may want to refer patients to genetic counselors to handle these issues. But, unfortunately, there are only a little over 2000 genetic counselors in the United States (American Board of Genetic Counseling, 2008), not all of whom are working at any one time. Hence, physicians need to be able to provide counseling information. Fortunately, AATD follows fairly straightforward Mendelian principles.
In general, physicians lack the training and ability to deal with such uncertainties and communicate them to patients in an effective way—even to the extent of not knowing whether to use verbal or numerical approaches (and if numerical, percentages vs. proportions). HCWs may assign similar quantitative probabilities to qualitative descriptions of odds but differ from patients in interpreting these terms (e.g., “likely” vs. “unlikely”) (Kong et al., 1986). Patients may assess rates differently than proportions (Grimes and Snively, 1999) and be affected by the order in which risks and benefits are presented (Bergus et al., 2002). Medical education thus needs to be better prepared to handle these issues.
The burgeoning availability of genetic tests and the arrival of pharmacogenomics and direct-to-consumer marketing of genetic tests makes these issues even more critical. Patients and providers will increasingly need to deal with problems related to testing, diagnosis, disclosure, and testing of other family members, overcoming barriers that may exist. Physicians and other HCWs will no doubt need to assume some functions of genetic counselors, though at the moment they generally lack the necessary training to optimally do so. Moreover, major challenges exist, since even with training, physicians are not now reimbursed for such time-intensive counseling. Hence, policy makers need to consider how to address these systemic gaps—that is, whether to encourage or mandate reimbursement for these functions. This study thus has important policy implications as well.
These data indicate, too, the need for further research on these and other key aspects of genetic disorders. Though rare, and therefore often underresearched, such diseases serve as powerful and important precedents for future genetic assays. These data highlight the need to ensure that HCWs, patients, and their families are adequately prepared to confront the resultant challenges ahead.
In sum, physicians play critical roles, particularly when dealing with rare genetic diseases, and may need more training to handle the complex issues that arise. Given the shortage of genetic counselors, insurance companies need to reconsider their current reimbursement policies to be able to address the need for appropriate provider-patient interactions concerning testing and disclosure. Genetic testing involves not only a single patient at a time, but his or her family members who may be at risk as well. Given the importance of early treatment for many conditions, genetic tests for these will certainly be developed. Failure to address disclosure may mean that patients fail to inform at-risk family members, who may as a result end up getting treatment too late, if at all. But to address these issues will take time, and resources (e.g., appropriate reimbursement) that will need to be established. Such efforts are critical, however, for disease prevention and treatment.
Acknowledgments
I would like to thank Dr. Edward Eden and Lori Tartell for their assistance with this project, Wendy Chung for her comments, and Melissa Conley and Kristen Nelson for their help with preparing the manuscript. This research was supported by the NIH through a grant from NHGRI (#5-R01-HG002431-01).
Disclosure Statement
The author has no commercial associations or conflict of interest.
References
- Aktan-Collan K. Mecklin J. de la Chappelle A, et al. Evaluation of a counselling protocol for predictive genetic testing for hereditary non-polyposis colorectal cancer. J Med Genet. 2000;37:108–113. doi: 10.1136/jmg.37.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Board of Genetic Counseling. 2008. http://www.abgc.net/english/View.asp?x=1607. [Jun 16;2008 ]. http://www.abgc.net/english/View.asp?x=1607
- American Thoracic Society. American Thoracic Society/European Respiratory Society Statement: Standards for the Diagnosis and Management of Individuals with Alpha-1 Antitrypsin Deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- Bergus GR. Levin IP. Elstein AS. Presenting risks and benefits to patients: the effect of information order on decision making. J Gen Intern Med. 2002;17:612–617. doi: 10.1046/j.1525-1497.2002.11001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne BS. Rozalinde J. Mannino DM, III, et al. Alpha-1 antitrypsin deficiency deaths in the United States from 1979–1991: an analysis using multiple-cause mortality data. Chest. 1996;110:78–83. doi: 10.1378/chest.110.1.78. [DOI] [PubMed] [Google Scholar]
- Campos MA. Wanner A. Zhang G, et al. Trends in the diagnosis of syptomatic patients with alpha-1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128:1179–1186. doi: 10.1378/chest.128.3.1179. [DOI] [PubMed] [Google Scholar]
- d'Agincourt-Canning L. Genetic testing for hereditary breast and ovarian cancer: responsibility and choice. Qual Health Res. 2006;16:97–118. doi: 10.1177/1049732305284002. [DOI] [PubMed] [Google Scholar]
- Geertz C. Interpretation of Cultures: Selected Essays. Basic Books; New York: 1973. [Google Scholar]
- Grimes D. Snively G. Patients' understanding of medical risks: implications for genetic counseling. Obstet Gynecol. 1999;93:910–914. doi: 10.1016/s0029-7844(98)00567-5. [DOI] [PubMed] [Google Scholar]
- Guest G. Bunce A. Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18:59–82. [Google Scholar]
- Hadley DW. Jenkins J. Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163:573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- Hogarth DK. Rachelefsky G. Screening and familial testing of patients for alpha-1-antitrypsin deficiency. Chest. 2008;133:981–988. doi: 10.1378/chest.07-1001. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB. Valdimarsdottir HB. Brown KL, et al. Decision-making about genetic testing among women at familial risk for breast cancer. Psychosom Med. 1997;59:459–466. doi: 10.1097/00006842-199709000-00001. [DOI] [PubMed] [Google Scholar]
- Jones NL. Smith AM. Report for Congress. Genetic Information: Legal Issues Relating to Discrimination and Privacy. 2003. http://ncseonline.org/NLE/CRSreports/03Jun/RL30006.pdf. [Jun 2;2008 ]. http://ncseonline.org/NLE/CRSreports/03Jun/RL30006.pdf
- Klitzman R. Improving education on doctor-patient relationships and communication: the views of doctors who become patients. Acad Med. 2006a;81:447–453. doi: 10.1097/01.ACM.0000222271.52588.01. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Views of risks and benefits among doctors who become patients. Patient Educ Couns. 2006b;64:61–68. doi: 10.1016/j.pec.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Klitzman R. When Doctors Become Patients. Oxford University Press; New York: 2008. [Google Scholar]
- Klitzman R. Bayer R. Mortal Secrets: Truth and Lies in the Age of AIDS. Johns Hopkins; Baltimore, MD: 2003. [Google Scholar]
- Klitzman R. Thorne D. Williamson J, et al. Decision-making about reproductive choices among individuals at-risk for Huntington's disease. J Gen Couns. 2007a;16:347–362. doi: 10.1007/s10897-006-9080-1. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Thorne D. Williamson J, et al. The roles of family members, health care workers and others in decision-making processes about genetic testing among individuals at risk for Huntington's disease. Gen Med. 2007b;9:358–371. doi: 10.1097/GIM.0b013e3180653c5a. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Thorne D. Williamson J, et al. Disclosures of Huntington's disease risk within families: patterns of decision-making and implications. Am J Med Gen. 2007c;143:1835–1849. doi: 10.1002/ajmg.a.31864. [DOI] [PubMed] [Google Scholar]
- Kong A. Barnett G. Mosteller F. Youtz C. How medical professionals evaluate expressions of probability. N Engl J Med. 1986;315:740–744. doi: 10.1056/NEJM198609183151206. [DOI] [PubMed] [Google Scholar]
- Laurel BC. Eriksson S. The electrophoretic alpha-1-globulin pattern of serum in alpha-1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–140. [Google Scholar]
- Menasha JD. Schechter C. Willner J. Genetic testing: a physicians' perspective. Mt Sinai J Med. 2000;67:144–151. [PubMed] [Google Scholar]
- Oveson L. Yarborough M. Aspen Report: Ethical Issues in Occupational Genetics. The Ramazzini Institute for Occupational and Environmental Health Research 2(2) 2001. www.ramazziniusa.org/apr01/geneticprofiles.htm. [May 21;2008 ]. www.ramazziniusa.org/apr01/geneticprofiles.htm
- Prochaska JO. DiClemente CC. Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM. Strecher VJ. Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Sifri R. Myers R. Hyslop T, et al. Use of cancer susceptibility testing among primary care physicians. Clin Genet. 2003;64:355–360. doi: 10.1034/j.1399-0004.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- Stolk J. Case detection of alpha-1 antitrypsin deficiency: does it help the patient or the doctor? Eur Resp J. 2005;26:561–652. doi: 10.1183/09031936.05.00091405. [DOI] [PubMed] [Google Scholar]
- Stolk J. Seersholm N. Kalsheker N. Alpha-1 antitrypsin deficiency: current perspective on research, diagnosis, and management. Int J Chron Obstruct Pulmon Dis. 2006;1:151–160. doi: 10.2147/copd.2006.1.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange C. Dickson R. Carter C, et al. Genetic testing for Alpha-1-antitrypsin deficiency. Genet Med. 2004;6:204–210. doi: 10.1097/01.gim.0000132669.09819.79. [DOI] [PubMed] [Google Scholar]
- Strauss A. Corbin J. Basics of qualitative research-techniques and procedures for developing grounded theory. Sage Publications; Newbury Park, CA: 1990. [Google Scholar]
- Sveger T. Thelin T. A future for neonatal Alpha-1-antitrypsin screening. Acta Paediatr. 2000;89:625–631. [PubMed] [Google Scholar]
- Sveger T. Thelin T. McNeil TF. Neonatal Alpha-1-antitrypsin screening: parents' views and reactions 20 years after the identification of the deficiency state. Acta Paediatr. 1999;88:315–318. doi: 10.1080/08035259950170097. [DOI] [PubMed] [Google Scholar]
- Wideroff L. Freedman AN. Olson L, et al. Physician use of genetic testing for cancer susceptibility: results of a national survey. Cancer Epidemiol Biomarkers Prev. 2003;12:295–303. [PubMed] [Google Scholar]
- World Health Organization. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. 2006. http://www.who.int/respiratory/copd/GOLD_WR_06.pdf. [Jun 16;2008 ]. http://www.who.int/respiratory/copd/GOLD_WR_06.pdf