Abstract
BACKGROUND
Psychological stress may impair premenopausal ovarian function and contribute to risk for chronic disease. Soy isoflavones may also influence ovarian function and affect health. Here, we report the effects of a psychological stressor (subordinate social status) and dietary soy on reproductive function and related health indices in female monkeys. We hypothesized that reproductive compromise and adverse health outcomes would be induced in subordinate when compared with dominant monkeys and be mitigated by exposure to soy.
METHODS
Subjects were 95 adult cynomolgus monkeys (Macaca fascicularis) housed in social groups of five or six. Animals consumed a soy-free, animal protein-based diet during an 8-month Baseline phase and then, during a 32-month Treatment phase, consumed either the baseline diet or an identical diet that substituted high-isoflavone soy protein for animal protein.
RESULTS
Across more than 1200 menstrual cycles, subordinate monkeys consistently exhibited ovarian impairment [increased cycle length (P < 0.02) and variability (P < 0.02) and reduced levels of progesterone (P < 0.04) and estradiol (P < 0.04)]. Subordinate status was confirmed behaviorally and was associated with elevated cortisol (P < 0.04) and relative osteopenia (P < 0.05). Consumption of the soy diet had no significant effects.
CONCLUSIONS
(i) Psychological stress adversely affects ovarian function and related health indices in a well-accepted animal model of women's health; (ii) Similar effects may extend to women experiencing reproductive impairment of psychogenic origin; (iii) soy protein and isoflavones neither exacerbate nor mitigate the effects of an adverse psychosocial environment; and (iv) this study was limited by an inability to investigate the genetic and developmental determinants of social status.
Keywords: functional hypothalamic anovulatory syndrome, stress, reproduction, diet
Introduction
Emerging evidence suggests that the chronic and degenerative diseases comprising the major part of the post-menopausal health burden begin developing during the premenopausal decades and progress along a trajectory that depends in part on the quality of premenopausal ovarian function (Kaplan and Manuck, 2004; Speroff, 2007; Kaplan and Manuck, 2008). Specifically, studies have linked substantial ovarian insufficiency (e.g. premature ovarian failure, ovariectomy, hypothalamic amenorrhea) to bone loss, cognitive impairment, movement disorders, cardiovascular disease and premature death (Sowers et al., 1998; Rocca et al., 2006; Gallagher, 2007; Rocca et al., 2007). It has been suggested that less severe premenopausal ovarian disruption (e.g. luteal phase deficits, anovulation) might also adversely affect health (Kaplan and Manuck, 2008). Here, we report the results of an experiment in monkeys designed to assess two factors that could influence ovarian function and perhaps thereby affect the premenopausal risk of chronic disease: (i) exposure to psychological stress, as represented by the occupation of a low position in a hierarchy of social status; and (ii) consumption of a diet high in soy protein and isoflavones. We hypothesized that reproductive function and related outcomes would be adversely affected in subordinate monkeys relative to their dominant counterparts, but that such effects would be at least partially mitigated by chronic exposure to soy and isoflavones.
Epidemiologic evidence in women and experimental data in non-human primates suggest that psychological stress—alone or in interaction with energy deficiency induced by physical activity and/or insufficient caloric intake—can disrupt ovarian function (Drew, 1961; Berga, 1996; Cameron, 1997; Ferin, 1999; Newton and Philhower, 2003; Williams et al., 2007; Kaplan and Manuck, 2008). In turn, even relatively minor ovarian disruptions have been associated with an increase in the extent of coronary artery disease in women (as documented by angiography) and linked to the precocious acceleration of coronary artery atherosclerosis in subordinate (low status) monkeys (Hanke et al., 1997; Kaplan et al., 2002a; Bairey-Merz et al., 2003). That estrogens may play a role in these phenomena is supported indirectly by the observation that oral contraceptive exposure inhibits atherosclerosis in subordinate monkeys (Kaplan et al., 1995). Furthermore, although the effects of oral contraceptive use on heart disease are still not known with certainty, reduced calcium content and reduced stenosis in the coronary arteries have been observed in women taking these compounds (Merz et al., 2006; Snell-Bergeon et al., 2008).
In women, reproductive dysfunction of psychogenic origin is often referred to as functional hypothalamic anovulatory syndrome (FHAS), occurring along a continuum from mild luteal phase deficits to anovulation and amenorrhea (Jones, 1949; Ginsburg, 1992; Berga, 1996). This syndrome has been related in part to an inability to cope with life's daily challenges and stressful life circumstances in general, and often is associated with generalized neurohormonal dysregulation (Berga and Yen, 2004). Although inherent psychological traits of individuals may modulate vulnerability to this syndrome, epidemiological research makes clear that the degree of reproductive impairment increases in relation to stress imposed by the environment (Drew, 1961; Berga, 1996). Importantly, the condition can be ameliorated by treatment with cognitive behavioral therapy or environmental changes that reduce stress, further emphasizing the importance of the psychosocial environment (Reifenstein, 1946; Drew, 1961; Berga et al., 2003). Nonetheless, ovulatory and related neurohormonal disruptions in women are often difficult to ascribe to a single factor owing to the frequent co-occurrence of metabolic and psychological stressors (Berga, 1996; De Souza et al., 2003; Loucks and Thuma, 2003; Williams et al., 2007). The situation is less ambiguous in non-human primates, where low social status or other psychosocial stressors have been linked reliably to indicators of reproductive dysfunction (including reduced fertility) in both experimental and natural groupings (Sade et al., 1976; Dittus, 1977, 1980; Adams et al., 1985; Harcourt, 1987; Cameron, 1997; Xiao et al., 2002; Speroff, 2007). Importantly, it has been observed in non-human primates that—as in women—reproductive impairment reverses following an improvement in psychological circumstances (e.g. if low status animals become high ranking; Shively and Clarkson, 1994; Adams et al., 1985).
Soy isoflavones are estrogen-like compounds found in soy protein and widely used in dietary supplements (Kurzer, 2002). Because they bind to estrogen receptors, soy isoflavones could alter endogenous hormonal activity, ovarian hormone profiles, menstrual cyclicity and ultimately health (Miksicek, 1994; Wang et al., 1996). Regarding health outcomes, Asian populations consuming large amounts of dietary soy protein and isoflavones are also characterized by a reduced incidence of cardiovascular disease, osteoporotic fracture and breast cancer, providing indirect evidence consistent with the hypothesis that soy isoflavones may influence disease processes known to be associated with the estrogen status of tissues (Beaglehole, 1990; Parkin et al., 1992; Ursin et al., 1994; Adlercreutz and Mazur, 1997; Vitolins et al., 2002). These observations also establish the possibility that soy isoflavones may mitigate or reverse the adverse consequences of psychological stress on ovarian function or risk of disease. However, investigations evaluating the reproductive effects of soy protein or isoflavones in women have had inconsistent outcomes. For example, short-term exposure to soy protein or isoflavones in studies with small numbers of women suggests that soy may reduce the length of the follicular phase and increase follicular estrogen concentrations (Cassidy et al., 1994), increase the overall length of the menstrual cycle (Jakes et al., 2001), increase luteal estrogen without altering follicular estrogen (Wu et al., 2000) or reduce estrogen concentrations at mid cycle and in the luteal phase (Lu et al., 1996; Nagata et al., 1997). In contrast, investigations involving longer exposures of larger numbers of women indicate minimal hormonal effects and no effects on the length of the menstrual cycle or any of its components (Duncan et al., 1998; Maskarinec et al., 2004). To our knowledge, no studies have systematically evaluated the effect of soy protein and isoflavones on ovarian function in an Old World monkey or ape, which uniquely share with women a menstrual cycle and menopause, and resemble women closely in their ovarian hormone profiles and responses to psychological stress (Zuckerman, 1930; Zuckerman and Parks, 1932; Wilks et al., 1976; Williams and Hodgen, 1982; Knobil, 1988; Kaplan and Manuck, 2004).
The current study extends prior investigations assessing the effects of social status on indices of reproduction and health (Kaplan et al., 1995; Kaplan et al., 2002a) by providing comprehensive longitudinal evaluations in a large number of socially housed monkeys who were fed diets in which most of their protein was derived from either high-isoflavone soy or from a combination of animal sources (casein and lactalbumin—CL). The data thus address key gaps in knowledge regarding the extent to which psychological stress and diet act independently or interactively to affect menstrual cyclicity, ovarian hormone profiles, cortisol responsiveness [an index of hypothalamic–pituitary–adrenocortical (HPA) status], and one potentially estrogen-dependent health measure—degree of osteopenia as indicated by bone mineral content and density. Regardless of outcome, observations derived from an anthropoid primate that resembles women in both reproductive function and susceptibility to cardiovascular disease, osteoporosis and other chronic disorders have considerable potential relevance for understanding the factors affecting women's health across their lifespan.
Materials and Methods
Animals
Subjects were 95 female cynomolgus monkeys (Macaca fascicularis) imported from Indonesia and having no known previous exposure to soy. Prior to shipment, all animals were radiographed and only those individuals exhibiting evidence of complete epiphyseal closure at the distal radius, ulna and the proximal tibia were accepted for use in the study. This stage of development generally occurs by 9 or 10 years of age in cynomolgus monkeys, at which time individuals are approximately equivalent to 27–30-year-old women (Bachrach, 2001; Kaplan and Manuck, 2004).
Study design
Following importation, monkeys were placed into 16 social groups of five or six animals each; the groups were approximately equivalent in body weight gradient. The following 8 months comprised the Baseline phase of the study during which all animals consumed a soy and isoflavone-free diet containing 19% of calories from protein, 35% of calories from fat, 46% of calories from carbohydrate and 0.28 mg cholesterol/kcal (reduced to 0.20 mg/kcal in the latter stages of the study). These dietary components were chosen to model those typically consumed by Americans and thought to increase the risk of chronic disease (Craig and Mangels, 2009; Dall et al., 2009).
At the end of the Baseline phase, social groups of monkeys were assigned for 32 months to one of two treatment conditions using a stratified randomization procedure that matched the conditions for total serum cholesterol and high-density lipoprotein cholesterol responses to the baseline (isoflavone-free) diet. Monkeys in the two treatment conditions were fed diets differing only in the major protein source: (i) SOY, derived from isolated soy protein (SUPRO©, Solae Corporation, St. Louis, MO, USA) and (ii) CL, using casein and lactalbumin (the same diet consumed by all animals during the Baseline phase). Approximately 20% of protein fed to each condition was derived from wheat flour. The SOY diet contained 1.88 mg total isoflavones/g protein, and was fed at a concentration designed to provide the equivalent of a woman's daily consumption of ∼140 mg aglycone units of isoflavone, assuming a daily intake of ∼1800 kcal (i.e. 0.0775 mg isoflavone/kcal). Monkeys were fed 120 kcal of diet/kg body weight and, therefore, consumed ∼9.3 mg isoflavone/kg body weight. This caloric adjustment of dose accounts for the higher metabolic rate in monkeys compared with human subjects. The SOY and CL diets were formulated to be isocaloric for macronutrients [protein (19% of kcal), carbohydrate (46%) and fat (35%)], and comparable for cholesterol (0.20 mg/kcal), vitamins and minerals (Walker et al., 2008).
The study was primarily designed to determine the effect of soy protein and isoflavones on cardiovascular and reproductive health. Findings relating to iliac artery atherosclerosis and inflammation (Walker et al., 2008), breast proliferation (Stute et al., 2004; Wood et al., 2006) and dietary (not status) effects on bone mineral content (Lees et al., 2002) have already been reported. During the Treatment phase, nine animals died from a variety of causes unrelated to the experimental treatments. All animal manipulations were performed in accordance with State and Federal regulations and with approval of our Institutional Animal Care and Use Committee. Wake Forest University is fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
Isoflavone concentrations
Serum samples were collected on three occasions from each monkey for determination of circulating isoflavone concentrations. These collections occurred at Baseline and 7 and 19 months following initiation of the Treatment phase. Animals were fed in the morning and then sedated 4 h post-feeding for blood collection. Blood was immediately processed, frozen and protected from light until analysis. Serum isoflavones were analyzed by liquid chromatography photo-diode array tandem mass spectrometry using electrospray ionization slightly modified from a previously established method to include equol in the panel of isoflavanoids (genistein, dihydrogenistein, daidzein, dihydrodaidzein, glycitein and O-desmethylangolensin) and isotopically labeled internal standards (Franke et al., 2002; Walker et al., 2008). Detection limits were previously found to be 1–15 nmol/l depending on the analyte, and inter-assay coefficients of variation (CV) 8–22% at levels below 20 nmol/l; 7–14% at levels 20–100 nmol/L; and 3–12% at levels over 100 nmol/l.
Dominance determinations and behavioral observations
The social status of each animal relative to others in her group was based on data collected during weekly, 30-min observations conducted across the Baseline phase. Dominance and subordination were determined by the outcomes of fights, which are highly asymmetric in this species and yield clear winners and losers as judged by specific facial expressions, postures and vocalizations (Sade, 1973; Bernstein, 1981; Kaplan and Manuck, 1998). The female in each group that defeated all others was designated the first-ranking monkey. The female that defeated all but the first-ranking monkey was designated second-ranking monkey, and so forth. Ranks tend to be stable under the experimental conditions described (Kaplan et al., 1995). In the current investigation, ranks over the Baseline period first were averaged to yield numbers from 1.0 to 6.0 (for groups containing six individuals). For purposes of analysis, these rankings were then divided into tertiles of social status. A similar approach to status identification and statistical analysis has been used extensively in prior studies (Kaplan et al., 1995, 2002a; Kaplan and Manuck, 2004).
In addition to rank determinations, the social behavior of all animals was observed across an 8-week period beginning 19 months after initiation of the Treatment phase. Behavioral observations consisted of three, 30-min periods per week during which all episodes of affiliative (groom, play, being groomed, passive body contact, sitting close), agonistic (attack, flee) and non-social (sitting alone) behavior were recorded on an electronic tablet (Noldus© Observer 3.0). Discrete behavioral acts (for example, bites, grabs or other forms of aggression; grimaces, cowers or other forms of submission) were counted electronically to provide frequencies of interaction. The duration of each episode was measured by noting the start and end of behavioral states [for example, grooming, being groomed and sitting close (within touching distance), in contact, or alone (not within touching distance)]. Frequency data were converted to a rate per hour per individual while the duration data were converted to a percentage of total time spent by individuals in each behavioral state.
Menstrual cyclicity
Animals were trained to move from their social pens into a holding cage for daily vaginal swabbing, a process that generally took <45 min for the entire cohort. Beginning in the Baseline phase and continuing for 6 months, all monkeys were swabbed 7 days per week and all instances of vaginal bleeding were recorded. Seven months after the start of the Treatment phase, animals were again subjected to daily vaginal swabbing, this time for 10 months. More than 1200 menstrual cycles could be assessed for length and variability (∼400 during Baseline and 800 during Treatment). Investigators blinded to treatment independently graded each monkey's history of vaginal bleeding to determine the beginning and end of every cycle. The onset of menses each month was assigned as cycle Day 1. No arbitrary limit was set on cycle length except that inter-menstrual periods of <20 days were not considered as cycles. Also, the final cycle from each sample period was discarded to reduce bias associated with the arbitrary termination of sampling on the same day for all individuals. From these data we derived the average and maximum cycle length and cycle variability (the SD in cycle length) for each animal in the Baseline and Treatment phases. Preliminary analysis indicated that amenorrhea was virtually non-existent in this population.
Ovarian hormone profiles
Using the techniques that were applied for vaginal swabbing, monkeys were subjected to blood sampling in order to assess progesterone concentrations, three times per week (Mondays, Wednesdays and Fridays) without anesthesia during both the Baseline and Treatment phases. The extensive amount of training required for awake bleeding along with the substantial number of clinical assessments conducted during the Baseline phase precluded collection of ovarian profiles for the complete cohort at this time. However, just prior to the end of the Baseline phase a subset of 55 animals was sampled three times per week for ∼10 weeks. Then, beginning ∼9 months after the start of the Treatment phase, all monkeys were subjected to blood sampling three times per week for ∼16 weeks. Finally, beginning in Month 14 of the Treatment phase, every animal was sampled for both 17β estradiol (E2) and progesterone across a single cycle on Days 5, 14 and 22 (determined by the first day of bleeding for that cycle in that monkey).
Samples were frozen at −70°C. All hormone assays were performed at the Yerkes Biomarker Core Laboratory (Atlanta, GA, USA). Progesterone was measured using a modification of a previously described assay that employs a commercially available kit from DPC (Siemens/DPC; Los Angeles CA, USA; Wilson, 1998). Samples (125 µl) were extracted with 2.5 ml of anesthesia grade ether and the organic layer evaporated to dryness under a stream of N2. The sample was reconstituted in 125 µl of the assay buffer and replicates (50 µl) were assayed following the kit protocol. The assay has a sensitivity of 0.10 ng/ml with inter- and intra-assay CVs of 8.14 and 7.73%, respectively. Sample values of progesterone were corrected for extraction efficiencies, which exceeded 95%. E2 was assayed using a modification of a previously validated assay (Siemens/DPC; Los Angeles CA, USA; Pazol et al., 2004). The assay has a sensitivity of 5 pg/ml and intra- and inter-assay CVs of 4.9 and 9.3%, respectively.
The two highest progesterone concentrations from the luteal phase of each cycle were identified and averaged; values for each cycle were then averaged across the entire sampling period (∼10 weeks during Baseline and 16 weeks during Treatment) to provide a mean progesterone value for each monkey. The highest progesterone concentration observed across each sampling period was taken as that animal's peak progesterone value. Although representing a truncated sample number, the Baseline subset was included in the overall hormonal analysis for two reasons: (i) to identify any baseline hormonal differences between animals later assigned to the SOY and CL conditions; and (ii) to determine whether social status influenced ovarian hormones during the Baseline phase and if any such differences persisted across time.
Cortisol response following dexamethasone suppression
Elevations in serum cortisol are commonly observed as a concomitant of subordinate social status in macaques and baboons (Kaplan et al., 1986; Abbott et al., 2003) and represent part of the general hormonal dysregulation that comprises FHAS (Berga and Yen, 2004). To evaluate the relationships among social status, soy treatment and cortisol, we exposed all animals to a dexamethasone suppression test near the end of the Treatment phase (15 months after the hormonal and menstrual cyclicity samples). Monkeys were captured at 22:00 h and a 3 ml blood sample was taken without anesthesia. The order of treatment was counterbalanced. Specifically, half of the animals were given an i.m. injection of dexamethasone (0.25 mg/kg) whereas the remainder were injected with a similar quantity of saline. The following day, 3 ml blood samples were collected at 08:00 and 12:00 h, again without use of anesthesia. Two weeks later the procedure was repeated, with the treatments reversed. All samples were centrifuged to separate the serum, which was then frozen at −20°C. After completion of all tests, frozen sera were sent in a single batch to the Yerkes Biomarker Laboratory. Serum levels of cortisol were determined by radioimmunoassay with a commercially available kit (Beckman-Coulter/DSL, Webster, TX, USA) previously described for rhesus monkeys (Wilson et al., 2005). Using 25 µl, the assay has a range from 0.5 to 60 µg/dl with inter- and intra-assay CVs of 4.9 and 8.7%, respectively.
Bone mineral content, body weight and BMI
Whole body bone mineral content (WBBMC) (g) and spinal bone mineral density (BMD) (g/cm2) were used as an index of general bone health. Measurements were made at baseline (∼6 months prior to starting the experimental diet), and 13 and 31 months after start of diet using a Norland (XR-46, software package 3.9.6b) dual X-ray absorptiometry (DXA) machine. The monkeys were sedated with ketamine (10 mg/kg) and then given isoflurane. Measurements were made of the whole body using techniques described previously (Jayo et al., 1991; Lees et al., 2002). The CVs were 1.7 and 2.4% for WBBMC and spinal BMD, respectively. The body weights reported here were taken at the same time that animals were sedated for the DXA studies (scale Model #815, Chatillon, Largo, FL, USA). Body length (defined as the distance between the suprasternal notch and pubic symphysis) in these skeletally mature animals was measured on a single occasion during the Baseline phase. For purposes of analysis, we calculated the BMI as the body weight (kg)/body length (m2). Although we previously reported that soy protein and isoflavones did not influence bone mineral content (Lees et al., 2002), the current study is the first to consider the interaction between bone indices and social status in relation to diet.
Statistical analyses
This study was a randomized trial that considered not only experimental treatment (diet) and a behavioral outcome (social status), but also accounted for the housing of the animals in 16 distinct social groups. Hence, data from monkeys within the same social group were expected to be correlated (either positively or negatively) with each other. A mixed effect modeling approach was used to account for these intra-class correlations and the correlation between repeated measures for each monkey in the assessment of the main study outcomes (menstrual cyclicity, mean and peak luteal progesterone). Mixed models are also flexible when handling missing data, which allow the use of mean and peak luteal progesterone from both the Baseline and the Treatment phases. The models included the main effect of diet, phase (Baseline or Treatment) and dominance status as well as all the two-way interaction terms. Additionally, we evaluated the effect of body weight and BMI as a covariate on the hormone profiles. Neither body weight nor BMI was significant in the models and both were therefore excluded in further analyses based on parsimonious principles. The diet-by-phase interaction evaluates whether the pattern of change over time in the outcome differs by dietary group, and was therefore retained in the model regardless of statistical significance. No diet-by-status interaction was found and this term was removed from subsequent data analyses. When the status-by-phase interaction was not significant, the least squares mean for each rank averaged over time for indices of menstrual cyclicity and ovarian hormone profiles was reported. Similar formulation of mixed models was also used for E2 and progesterone concentrations observed during the Treatment phase across a single cycle at Days 5, 14 and 22 and for BMD outcomes (WBBMC and spinal BMD) measured at baseline, 13 months and 31 months after the start of dietary treatment. The effects of diet treatment and dominance status were examined longitudinally with and without adjustment for the corresponding body weight or BMI at each time point.
Social behavior was an ancillary measure collected in the Treatment phase. Most social behaviors were highly skewed, with 15–20% of the data clustered around 0. We first used a rank-based semi-parametric approach to analyze the effect of diet, dominance status and their interaction (Conover and Iman, 1981). The main effect of diet and the diet-by-status interactions were not statistically significant. Thus, the medians for the tertile ranks and the associated P-values from Kruskal–Wallis tests were presented.
The dexamethasone suppression test was performed as a crossover experiment. We used a linear mixed effects model to determine whether the cortisol response differed in animals challenged by saline first from those challenged by dexamethasone first (period effect). Because the period effect was not significant, we pooled the data from both periods and stratified our analyses by exposure to saline or dexamethasone. For each stratified analysis, a mixed model was fit to assess the main effect of diet, dominance status and their interactions with time (20:00, 08:00 and 12:00 h). All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA). P-values of <0.05 were considered statistically significant.
Throughout the Results and Discussion section, ‘CL' designates females that consumed the casein and lactalbumin-based diet during the Treatment phase while ‘SOY' designates females that consumed the soy protein and isoflavone-based diet during the Treatment phase.
Results
Confirmatory measures: serum isoflavones, status-associated social behavior, body weight and BMI
As expected, isoflavones were present only in trace amounts during the Baseline phase (9.75 ± 7.66 nmol/l in CL versus 3.58 ± 1.30 nmol/l in SOY, NS). Consumption of the SOY diet resulted in high concentrations of total isoflavones across the study while trace amounts were again measured in animals assigned to the CL condition (21.97 ± 6.64 nmol/l in CL versus 798.12 ± 62.21 nmol/l in SOY, P < 0.0001). Values for the CL condition fall at or near the levels of detection.
Table I details the social behavior of animals by tertile of rank. As expected, status was associated with clear behavioral distinctions, with dominant monkeys displaying more aggression and less submission than subordinates and also spending a greater percentage of time being groomed. Diet had no significant effect on any aspect of social behavior (data not shown), nor were there any significant diet-by-status interactions.
Table I.
Behavior and tertiles of social status in group-dwelling female cynomolgus monkeys.
| Behaviors | Rank tertile |
Pa | ||
|---|---|---|---|---|
| 1 (n = 26) | 2 (n = 32) | 3 (n = 30) | ||
| Total aggression/h | 17.17 | 8.88 | 1.54 | <0.0001 |
| Total submission/h | 0.46 | 7.71 | 14.42 | <0.0001 |
| % Time grooming | 0.07 | 0.04 | 0.02 | 0.09 |
| % Time being groomed | 0.06 | 0.04 | 0.01 | 0.01 |
| % Time within touching distance | 0.11 | 0.10 | 0.08 | NS |
| % Time in body contact | 0.08 | 0.11 | 0.08 | NS |
| % Time alone at a distance | 0.59 | 0.64 | 0.68 | NS |
aAll P's determined by Kruskal–Wallis.
Table II shows body weight and BMI by tertile of rank and diet. It can be seen that although suggestive, there was no significant association between tertiles of rank and either body weight or BMI. Nor was dietary treatment associated significantly with either body weight or BMI. Although not shown in these tables, the average body weight (n = 93) did increase significantly over time [Baseline: 2.93 ± 0.05 (SEM) kg; 13 months: 3.11 ± 0.05 kg; 31 months: 3.50 ± 0.05 kg; F2,170 = 104.3, P < 0.001], as did BMI (Baseline: 40.72 ± 1.11 kg/m2; 13 months: 43.20 ± 1.10 kg/m2; 31 months: 48.49 ± 1.11 kg/m2; F2,170 = 111.27, P < 0.001).
Table II.
Body weight and BMI in female monkeys consuming soy (SOY) or CL.
| Tertiles of status | Body weight (kg ± SE)a |
BMI (kg/m2 ± SE)b |
||
|---|---|---|---|---|
| CL (n = 49) | SOY (n = 46) | CL (n = 48) | SOY (n = 45) | |
| 1. High | 3.24 ± 0.12 | 3.22 ± 0.12 | 45.49 ± 1.82 | 42.38 ± 2.02 |
| 2. Middle | 3.23 ± 0.12 | 3.35 ± 0.12 | 45.29 ± 1.79 | 45.58 ± 1.90 |
| 3. Low | 3.05 ± 0.12 | 2.99 ± 0.12 | 45.60 ± 1.88 | 40.47 ± 1.87 |
aBody weight was not associated significantly with status (F2,170 = 2.88, P = 0.06), diet (F1,170 = 0.04, P = 0.84) or their interaction (F2,170 = 0.33, P = 0.72).
bBMI was not associated significantly with status (F2,170 = 1.58, P = 0.21), diet (F1,170 = 1.55, P = 0.21) or their interaction (F2,170 = 2.01, P = 0.14).
Menstrual cyclicity
Table III contains the menstrual cycle data arranged by experimental phase (Baseline, Treatment) and diet (SOY, CL). It can be seen from this table that diet had no significant effect on any of the measured cycle characteristics. Cycles did become significantly less variable in the Treatment phase compared with Baseline, an effect that was independent of dietary treatment.
Table III.
Menstrual cyclicity in cynomolgus monkeys consuming SOY or CL.
| Cycle characteristics | Baseline |
Treatment |
||
|---|---|---|---|---|
| CL (n = 49) | SOY (n = 46) | CL (n = 48) | SOY (n = 45) | |
| Average cycle length (Days ± SE)a | 33.2 ± 1.1 | 33.6 ± 1.1 | 32.8 ± 1.1 | 32.9 ± 1.1 |
| Variability (SD) in average cycle length (Days ± SE)b | 8.5 ± 1.1 | 8.8 ± 1.1 | 5.7 ± 1.1 | 6.0 ± 1.1 |
| Maximum cycle length (Days ± SE)a | 42.4 ± 2.2 | 44.5 ± 2.3 | 41.6 ± 2.2 | 43.8 ± 2.3 |
aMixed model analysis revealed no significant effects of Phase or Treatment on average cycle length or maximum cycle length (all F's < 1.0, NS).
bTreatment had no effect on variability in cycle length (F < 1.0, NS); however, cycles were less variable in the experimental phase than in baseline (F1,87 = 8.55, P < 0.01), an effect that was independent of dietary treatment (F1,87 = 0.0, NS).
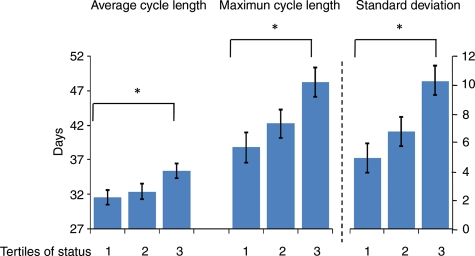
In contrast to the absence of significant effects related to dietary treatment, Fig. 1 shows that Status (by tertile) significantly affected average cycle length, maximum cycle length and variability in the number of days per cycle. Importantly, the effect of status on these three significant outcomes did not fluctuate by phase (all F values of < 1.0, P > 0.05). Hence, dominant monkeys had shorter (closer to 30 days on average) cycles and cycles that were less variable than those of their subordinate counterparts across all animals and in both phases.
Figure 1.
Menstrual cycle characteristics in female cynomolgus monkeys (n = 93) exhibit a significant effect for linear trend across tertiles of social status: mean cycle length ± SE (F2,92 = 3.29, P = 0.04); maximum cycle length ± SE (F2,92 = 4.84, P = 0.01); variability (standard deviation) in cycle length ± SE (F2,91 = 6.77, P < 0.01).
Ovarian hormones
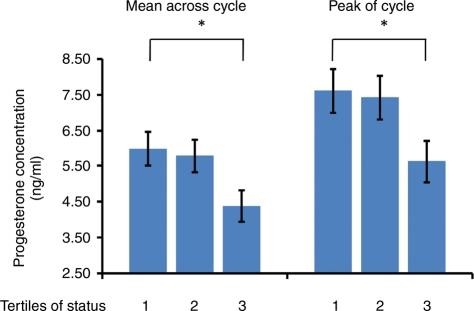
Table IV contains mean and peak luteal progesterone concentrations by diet and study phase. There were no significant effects of diet (CL, SOY) or the diet-by-phase (Baseline, Treatment) interaction on either measure of progesterone. However, there was an independent effect of phase on both mean and peak progesterone, which increased significantly between the Baseline and Treatment phases. Figure 2 contains the combined Baseline and Treatment phase mean and peak progesterone concentrations, with the estimated values determined by the mixed model procedure that included a subset of animals during Baseline (n = 55) and the complete cohort in the Treatment phase (n = 95). Analysis of the combined data set revealed that there was an overall (phase-independent) effect of status on both mean and peak progesterone, with dominant animals having higher progesterone concentrations than subordinates. However, for both measures, there also was a tendency for the disparities between dominants and subordinates to be more pronounced during the Treatment phase, relative to Baseline (data not shown). A separate analysis conducted only on the Treatment phase data confirmed that the differences between dominant and subordinate monkeys—evaluated across the complete cohort during the Treatment phase only—were significant for both mean (F2,73 = 4.44, P < 0.02) and peak (F2,73 = 4.96, P < 0.01) progesterone concentrations.
Table IV.
Luteal phase serum progesterone concentrations in cynomolgus monkeys consuming SOY or CLa.
| Baseline |
Treatment |
|||
|---|---|---|---|---|
| CL (n = 26) | SOY (n = 29) | CL (n = 48) | SOY (n = 44) | |
| Mean luteal phase progesterone (ng/ml ± SE) | 4.78 ± 0.49 | 4.58 ± 0.47 | 5.99 ± 0.38 | 6.37 ± 0.40 |
| Peak luteal phase progesterone (ng/ml ± SE) | 6.04 ± 0.65 | 6.01 ± 0.62 | 7.62 ± 0.51 | 7.94 ± 0.54 |
aMixed model analysis revealed no significant effects of dietary treatment on mean or peak luteal phase progesterone concentrations during either study phase (all F's < 1.0, including the Treatment × Phase interaction). However, both mean and peak progesterone increased significantly from the Baseline to the Treatment phase (Mean, F1,51 = 21.5, P < 0.001; Peak, F1,51 = 15.9, P < 0.001), effects that were independent of dietary treatment (all F's < 1.0, NS).
Figure 2.
Mean ± SE and peak ± SE serum progesterone concentrations in female cynomolgus monkeys exhibit a significant effect for linear trend across tertiles of social status using the combined estimate for the Baseline (n = 55) and Treatment phases (n = 93): mean progesterone ± SE (F2,88 = 3.47, P < 0.04); peak progesterone ± SE (F2,88 = 3.37, P < 0.04).
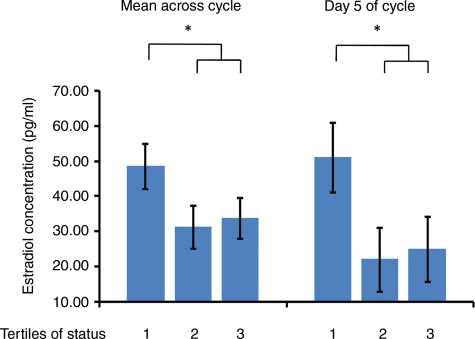
A similar pattern with respect to both E2 and progesterone was observed in Treatment phase samples taken from all animals during a single cycle at Days 5, 14 and 22. Specifically, dominant monkeys exhibited higher E2 concentrations than subordinates across all three sample days, although this difference achieved statistical significance only when the dominant tertile was contrasted with Tertiles 2 and 3 combined (Fig. 3). Figure 3 also shows that monkeys in the dominant tertile had higher E2 than their subordinate counterparts when the comparison is limited to the early follicular phase (Day 5). Finally, the luteal phase (Day 22) progesterone concentrations of the dominant animals were higher than those of subordinates, matching the tertile pattern observed in the more extended sample collections (F1,174 = 5.57, P = 0.02).
Figure 3.
Serum estradiol measured at Days 5, 14 and 22 of a single menstrual cycle; female cynomolgus monkeys in the most dominant tertile (1) differ significantly from the remainder (Tertiles 2 and 3) when considered across all days (F1,171 = 5.32, P = 0.02), and at Day 5 (early follicular; F1,171 = 3.93, P < 0.05).
Serum cortisol response to dexamethasone and saline
Order of treatment (saline first, dexamethasone first) had no significant effect on the outcomes, so we report here the combined responses to saline and the combined responses to dexamethasone. Table V depicts the cortisol concentration results by diet (CL, SOY) at the three time points, beginning the prior night and ending the next day (22:00, 08:00 and 12:00 h). The data demonstrate that dietary treatment had no significant effect on any outcome.
Table V.
Cortisol responses at baseline and following exposure to either saline or dexamethasone in cynomolgus monkeys consuming SOY or CL.
| Diet group | Salinea |
Dexamethasoneb |
||||
|---|---|---|---|---|---|---|
| 20:00 h | 08:00 h | 12:00 h | 20:00 h | 08:00 h | 12:00 h | |
| CL (n = 42) | 17.9 ± 1.60 | 24.17 ± 1.6 | 21.49 ± 1.6 | 19.93 ± 0.90 | 3.68 ± 0.89 | 4.03 ± 0.89 |
| SOY (n = 44) | 20.41 ± 1.7 | 26.14 ± 1.7 | 24.61 ± 1.7 | 19.25 ± 0.91 | 3.79 ± 0.91 | 4.93 ± 0.91 |
Data are serum cortisol concentrations expressed in µg/dl ± SE.
aDiet, F1,163 = 1.46, P = 0.23; time, F2,163 = 28.19, P < 0.01; diet × time, F2,163 = 0.25, NS.
bDiet, F1,163 = 0.01, NS; time, F2,163 = 351.12, P < 0.001; diet × time, F2,163 = 0.68, NS.
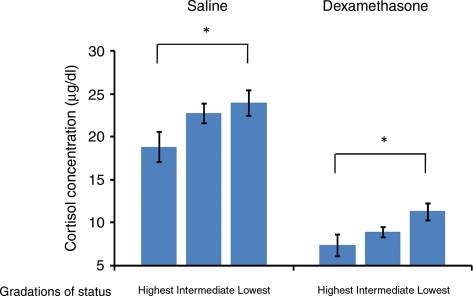
Using the same tertiles that were used for all other analyses, there was a significant Time-by-Status interaction at the 12:00 h time point for cortisol concentrations following dexamethasone infusion; this result indicated a more rapid escape from suppression in subordinate, relative to dominant, monkeys (Tertile 1: 3.53 ± 1.08 µg/dL, Tertile 2: 3.25 ± 1.06 µg/dL, Tertile 3: 6.65 ± 1.06 µg/dL, F2,163 = 3.22, P = 0.04). No such effect was observed following saline infusion (Tertile 1: 21.2 ± 1.58 µg/dl; Tertile 2: 25.0 ± 1.57 µg/dl; Tertile 3: 22.9 ± 1.56 µg/dl, NS). However, inspection of the data indicated that the relationship between cortisol and social status might be strongest at the extremes. For this reason, a second analysis was conducted, using a modified, three-part distribution: (i) ‘most dominant’, containing only the highest ranking animals (average ranks 1–1.5, n = 12); (ii) ‘most subordinate’ containing only the lowest ranking animals (average ranks 4.5–6.0, n = 19); and (iii) ‘intermediate’, containing the remaining individuals (average ranks 1.5–4.5, n = 55). These ranks correlated with the baseline ranks at r = 0.74. There were two primary outcomes using this more extreme distribution: (i) subordinates had elevated cortisol concentrations relative to dominants in both the saline and dexamethasone conditions, averaged across all time points (see Fig. 4); and (ii) there again was a significant Time-by-Status interaction at the 12:00 h time point following dexamethasone infusion (most dominant: 2.46 ± 1.62 µg/dl, intermediate: 3.86 ± 0.77 µg/dl, most subordinate: 7.60 ± 1.29 µg/dl, F2,163 = 4.13, P = 0.02) with no similar effect observed following saline infusion (most dominant: 20.2 ± 2.17 µg/dl; intermediate: 23.2 ± 1.27 µg/dl; most subordinate: 24.3 ± 1.81 µg/dl, NS).
Figure 4.
Mean ± SE serum cortisol concentrations collapsed across time in female cynomolgus monkeys across the highest (n = 12), intermediate (n = 55) and lowest ranking (n = 19) monkeys during the saline and dexamethasone treatments. There are significant effects for linear trend across groupings in relation to samples collected both following saline (F2,163 = 3.46, P = 0.03) and dexamethasone (F2,163 = 3.52, P = 0.03) infusion.
Bone indices
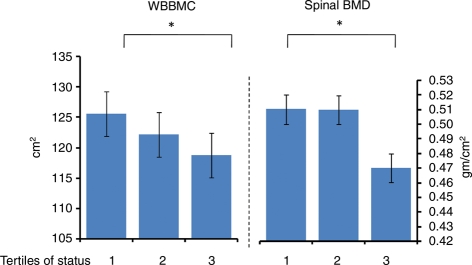
Table VI shows WBBMC and spinal BMD measured across time. It can be seen that all animals experienced a significant increase in WBBMC and spinal BMD despite having completed long bone growth (as determined by epiphyseal closure measured pre-experimentally). The table also shows that dietary treatment had no significant impact on these measures. However, as shown in Fig. 5, high-ranking monkeys had significantly greater WBBMC and spinal BMD than did their lower ranking counterparts. These effects were consistent across the study as indicated by the absence of a significant Status X Time interaction (WBBMC: F4,93 = 0.93, P = 0.45; Spinal BMD: F4,93 = 0.99, P = 0.42). The Status effect on WBBMC was unaffected by adjustment for body weight or BMI. As indicated in Fig. 5, adjustment for body weight (but not BMI) somewhat reduced the significance of difference in spinal BMD; however, the pattern of difference among the tertiles was unchanged as demonstrated by the significant differences between the highest and lowest tertiles (t93 = 2.08, P = 0.04, with body weight as covariate).
Table VI.
Whole body bone mineral content (WBBMC) and spinal bone mineral density (BMD) in cynomolgus monkeys by diet and time.
| Time of measurement | WBBMC (g ± SE)a |
Spinal BMD (g/cm2 ± SE)b |
||
|---|---|---|---|---|
| CL (n = 49) | SOY (n = 46) | CL (n = 48) | SOY (n = 45) | |
| Baseline | 108.8 ± 2.9 | 111.5 ± 3.0 | 0.45 ± 0.01 | 0.46 ± 0.01 |
| 13 Months after diet initiation | 116.9 ± 2.9 | 122.0 ± 3.0 | 0.49 ± 0.01 | 0.51 ± 0.01 |
| 31 Months after diet initiation | 126.1 ± 3.4 | 133.8 ± 3.5 | 0.52 ± 0.01 | 0.54 ± 0.01 |
aWBBMC was not associated significantly with diet (F1,93 = 1.50, P = 0.22) but was associated with time (F2,93 = 111.10, P < 0.001). The diet × time interaction was not significant (F2,93 = 1.76, P = 0.18).
bSpinal BMD was not associated significantly with diet (F1,93 = 1.36, P = 0.25) but was associated with time (F2,93 = 205.23, P = 0.001). The diet × time interaction did not reach conventional significance (F2,93 = 2.82, P = 0.065).
Figure 5.
Whole body bone mineral content (WBBMC) and spinal bone mineral density (BMD) for female cynomolgus monkeys of high (Tertile 1), middle (Tertile 2) and low (Tertile 3) dominance rank, collapsed across time. There are significant effects for linear trend across tertiles (WBBMC: F2,93 = 3.84, P = 0.025; Spinal BMD: F2,93 = 4.03, P = 0.02). The results remained largely unchanged after inclusion of body weight or BMI in the model (WBBMCbw: F2,93 = 3.33, P = 0.04; WBBMCBMI: F2,93 = 3.59, P = 0.03; Spinal BMDbw: F2,93 = 2.98, P = 0.056; Spinal BMDBMI: F2,93 = 3.24, P = 0.04).
Discussion
This is the most comprehensive experimental study to date assessing the effects of social status on reproductive function and related health indices in a premenopausal monkey model of women's health. Additionally, the study simultaneously considered the relative impact of soy protein and isoflavones—widely used by women as health supplements—on the same outcome measures. The results demonstrate that the social status gradient affected both menstrual cyclicity (average length, variability, maximum length) and ovarian hormones (E2 and progesterone) significantly, with subordinate individuals having longer and more variable cycles and lower serum E2 and luteal progesterone concentrations than dominant monkeys. Because amenorrhea was virtually non-existent, the ovarian impairment observed here could be considered ‘subclinical’. The effects relating status to menstrual cyclicity and progesterone concentrations were seen at Baseline as well as in the Treatment phase of the study (E2 was measured only during Treatment). Furthermore, subordinate monkeys relative to dominants were characterized by increased cortisol concentrations and a more rapid escape from dexamethasone—an indication of social stress and a condition often observed in women with FHAS (Berga and Yen, 2004). Subordinates were also characterized by reduced WBBMC and spinal BMD, effects that were present at Baseline and persisted throughout the study. Notably, the data provide initial experimental evidence that consumption of a typical American diet that is also rich in soy protein and isoflavones—either alone or in interaction with social status—does not significantly affect menstrual cyclicity, ovarian hormone profiles, cortisol concentrations or bone indices. These results extend prior observations in two ways: (i) by demonstrating the co-occurrence of social subordination, reproductive impairment and cortisol hyperresponsivity in a pattern analogous to that observed in women experiencing reproductive abnormalities of psychogenic origin; and (ii) by indicating that even modest reproductive impairment may be associated with relative osteopenia and thus potentially pose a previously unrecognized threat to the health of premenopausal women affected by FHAS.
Among other results, progesterone concentrations increased and menstrual cycles became somewhat less variable and shorter across time, outcomes that were independent of both social status and diet treatment. These particular changes may have reflected the increasing accommodation of this previously free-living population to the conditions of captivity. Serum isoflavone concentrations confirmed that monkeys consumed their assigned diets, as isoflavones were substantially elevated in SOY animals and only trace amounts were observed in the CL individuals. Behaviorally, animals ranked more dominant or more subordinate on the basis of fight outcomes also displayed significant differences in their patterns of social interaction, with dominants exhibiting more aggression, less submission and more time being groomed than subordinates. Taken together the results indicate that low social status had a consistent negative impact on reproductive and related health indices and that consumption of relatively large amounts of soy protein and isoflavones did not mitigate these effects.
With respect to the dietary manipulation, a recent meta-analysis suggested that neither soy nor isoflavones significantly influenced primary measures of reproductive function in premenopausal women, including E2, estrone, progesterone or sex hormone-binding globulin concentrations (Hooper et al., 2009). Although this meta-analysis indicated that soy and isoflavones are associated with significant declines in FSH or LH and a significant increase in average menstrual cycle length, such effects disappeared when potentially biased studies were removed from the analysis. Hooper et al. (2009) suggested that any effects of soy in premenopausal women are modest and lack clear clinical implications, a conclusion consistent with the observations on monkeys reported here. Our studies are also consistent with those on the effects of soy on reproductive end-points in non-human primates. Hence, soy and isoflavones are not estrogenic in cynomolgus monkeys, even at relatively high doses (Stute et al., 2004; Wood et al., 2006; Cline and Wood, 2009). Soy isoflavones may dampen estrogen-induced proliferative responses of the breast and endometrium, but the mechanism for such observations remains unknown (Cline and Wood, 2009).
It is perhaps surprising that the soy diet had such minimal effects on the outcomes reported here. One potentially positive implication of the results is that high-isoflavone soy supplementation is probably neutral with respect to effects on fertility. Moreover, the results do not preclude beneficial effects on other bodily systems. For example, we have reported elsewhere that soy inhibits the development of atherosclerosis and reduces arterial inflammation in monkeys (Walker et al., 2008). The failure of soy to inhibit the relative osteopenia observed in subordinate individuals may relate to the absence of a soy effect on cortisol responsivity, a factor that may mediate the adverse influence of low social status on bone (see below, and Misra and Klibanski, 2006).
In contrast to the inconsistent and relatively minor effects of soy or isoflavones on reproductive phenomena in premenopausal women, it is suggested that psychological stress contributes prominently to reproductive abnormalities such as FHAS (Berga, 1996; Williams et al., 2007). The current study demonstrates that one such factor—low social status—adversely affects menstrual cycle characteristics and ovarian hormones in macaque monkeys, which have long been used to model the reproductive biology of women. Such clear associations relating behavioral factors to reproductive impairment are often difficult to establish in women because metabolic (exercise, dieting) and psychogenic (stress) insults frequently co-occur (De Souza et al., 2003; Loucks and Thuma, 2003). Moreover, experimental studies evaluating the effect of psychosocial stress are generally precluded in women by ethical and logistical concerns. However, one investigation in premenopausal women focusing specifically on social status demonstrated that the risk for cycle irregularity increased monotonically with increasing submissiveness (Newton and Philhower, 2003). In our study, increased cycle irregularity was similarly associated with increasing degrees of submissiveness across tertiles. Among female monkeys, subordinate individuals are notable for the lack of control they exert over their environment in terms of access to space and preferred social partners, and the ability to maintain a daily routine unfettered by harassment from individuals more dominant to them (Silk, 2002; Kaplan and Manuck, 2004; Kaplan and Manuck, 2008). It has been suggested that women with FHAS are also characterized, at least in part, by a similar lack of control over their circumstances, conceptually linking their situation to that encountered by subordinate female monkeys (Berga et al., 2003).
The primary reproductive impairment that is induced by psychogenic or metabolic insults is hypothalamic in origin, involving alterations in the activity of the GnRH ‘pulse generator' and subsequent attenuation of LH release, in both amount and pulse frequency. However, women with FHAS and their normal controls are also often differentiated by a more global hormonal dysregulation (Ripley and Papanicolaou, 1942; Reifenstein, 1946; Berga et al., 1989; Berga, 1996; Berga and Yen, 2004). Among such alterations, HPA activation is often accorded a primary role in disruption of the GnRH pulse generator (Chrousos et al., 1998; Breen and Karsch, 2006; Michopoulos et al., 2009). In this regard, our observation that low-ranking monkeys were relatively hypercortisolemic in comparison to high-ranking monkeys (as indicated both by a more rapid escape from dexamethasone suppression and, at the extremes of rank, by higher average cortisol concentrations) suggests that subordinate monkeys were stressed by their social environment. Such HPA activation could have mediated the adverse changes in menstrual cyclicity and ovarian hormones exhibited by subordinate monkeys, thus possibly representing a further parallel between subordinate monkeys and women with FHAS.
Our observation that low social status is also associated reliably with reduced WBBMC and spinal BMD suggests that impaired bone health may represent a further consequence of even relatively modest stress-induced ovarian dysfunction. Importantly, a recent study demonstrated that women with functional hypothalamic amenorrhea (a form of FHAS characterized by complete cessation of menses) have lower bone density than normal controls and are characterized by hypercortisolemia (Lawson et al., 2009); it was hypothesized that activation of the HPA system mediated the loss of bone in these reproductively compromised women, a suggestion supported by several other studies in women (e.g. Misra and Klibanski, 2006). If so, it is possible that an adverse effect on bone might also extend to women with FHAS who are not amenorrheic, yet nonetheless have impaired ovarian function and have elevated cortisol concentrations: characteristics which resemble those of the monkeys described here.
Admittedly, there are limitations in the current study. First, the genetic and developmental determinants of individual differences in social status could not be investigated. Monkeys were moved directly from the single cage housing characteristic of quarantine to their permanent social groups. As we and others have suggested, the ranks established by monkeys under such conditions likely reflect a variety of factors, including their own behavioral states and traits and those of the other monkeys placed in the same group (Kaplan et al., 2002b). However, the initial randomization ensuring equivalence of physiological responses and body size across social group also likely mitigated at least some potential stratification of genetic and developmental origin. Another limitation was that the number of reproductive measures used as outcome variables was small and did not include gonadotrophins or pretreatment E2 levels. It is possible that exposure to the soy diet could have had important (though subtle) effects, especially on gonadotrophins. However, soy appears to have only a modest influence on reproductive function in women, with no significant impact on the primary ovarian hormones (estrone, E2, progesterone; Hooper et al., 2009). Moreover, we previously reported in this cohort that the soy manipulation had no significant effects on proliferation of epithelial area in the breast, an outcome that should have been sensitive to any hormonal influences of soy (Wood et al., 2006). We also note that monkeys assigned to the SOY condition were exposed to the human equivalent of 140 mg isoflavones/day, a higher dose than used in most human studies. Another limitation of this study was that progesterone data were obtained from only a subset of the animals during the Baseline period. Nonetheless, even accounting for the missing values during baseline, it is clear that the social status differences were present soon after the animals were placed in their social groups and were consistent across the investigation. Moreover, the same pattern of results (the absence of a significant effect of diet, the presence of a substantial effect of status) was obtained with respect to the menstrual cyclicity data, which were derived from the complete cohort during the Baseline as well as Treatment phase. Finally, we note that although social behavior, E2 and cortisol were not measured at baseline, the randomized design and the consistency of other outcomes provide some assurance that findings reported here are robust.
We have previously shown in this model that subordinate females exhibit a precocious acceleration of coronary artery atherosclerosis (Kaplan et al., 1995, 2002a, 2009; Kaplan and Manuck, 2004). The current study extends earlier observations by linking ovarian dysfunction, elevations in cortisol, and relative osteopenia to subordinate social status in a large, longitudinally studied cohort, providing additional evidence that the effects of mild ovarian impairment may extend beyond fertility to include an increased risk for chronic disease. The study further demonstrates that monkeys habitually consuming a typical American diet are relatively insensitive to the reproductive effects of high doses of soy isoflavones. This outcome also may extend to women in Western societies who, as in the experimental conditions described here, are not usually exposed to large amounts of isoflavone supplements until adulthood. In contrast to the absence of data linking soy and isoflavones significantly to reproductive changes, our data confirm that behavioral factors—such as social status—readily perturb menstrual cyclicity, ovarian hormones and the HPA axis in this commonly studied Old World primate. Such perturbations resemble the FHAS of women in etiology and presentation, suggesting that socially housed monkeys might be used to elucidate the mechanisms underlying reproductive impairment of psychogenic origin and to explore further the potential health sequelae of this phenomenon.
Authors’ roles
J.R.K.: Study design and conduct, primary author. H.C.: Study statistician and co-author. S.E.A., M.E.W.: Co-investigator, reproductive assessments and co-author. C.J.L.: Co-investigator, skeletal assessments and co-author. A.A.F.: Co-investigator, isoflavone assessments and co-author. S.L.B.: Study consultant, reproductive interpretation and co-author. S.B.M.: Study consultant, biobehavioral interpretation and co-author. T.B.C.: Co-investigator and co-author.
Funding
This work was supported in part by National Center for Research Resources S10 RR020890 and the National Heart, Lung, and Blood Institute P01 HL45666 and R01HL79421.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. doi:10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. doi:10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12:2228. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Bairey-Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson KT, Matthews KA, Pepine CJ, Ries SE, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. doi:10.1016/S0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- Beaglehole R. International trends in coronary heart disease mortality, morbidity, and risk factors. Epidemiol Rev. 1990;12:11–15. doi: 10.1093/oxfordjournals.epirev.a036048. [DOI] [PubMed] [Google Scholar]
- Berga SL. Functional hypothalamic chronic anovulation. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. Vol 1. Philadelphia: Lippencott Raven; 1996. pp. 1061–1075. [Google Scholar]
- Berga SL, Yen SSC. Reproductive failure due to central nervous system-hypothalamic-pituitary dysfunction. In: Strauss JF, Barbieri RL, editors. Reproductive Endocrinology. Physiology, Pathophysiology, and Clinical Management. 5th edn. Philadelphia: Elsevier Saunders; 2004. pp. 537–632. [Google Scholar]
- Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, Yen SS. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–308. doi: 10.1210/jcem-68-2-301. doi:10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity of women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. doi:10.1016/S0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance: the baby and the bathwater. Behav Brain Sci. 1981;4:419–457. doi:10.1017/S0140525X00009614. [Google Scholar]
- Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropins suppression. Front Neuroendocrinol. 2006;27:233–245. doi: 10.1016/j.yfrne.2006.03.335. doi:10.1016/j.yfrne.2006.03.335. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. doi:10.1055/s-2008-1067966. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell KDR. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–340. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Cline JM, Wood CE. Estrogen/isoflavone interactions in cynomolgus macaques (Macaca fascicularis) Am J Primatol. 2009;71:722–731. doi: 10.1002/ajp.20680. doi:10.2307/2683975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–129. doi:10.2307/2683975. [Google Scholar]
- Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets; American Dietetic Association. J Am Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. doi:10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Dall TM, Fulgoni VL, 3rd, Zhang Y, Reimers KJ, Packard PT, Astwood JD. Potential health benefits and medical cost savings from calorie, sodium, and saturated fat reductions in the American diet. Am J Health Promot. 2009;23:412–422. doi: 10.4278/ajhp.080930-QUAN-226. doi:10.4278/ajhp.080930-QUAN-226. [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Van Heest J, Demers LM, Lasley BL. Luteal phase deficiency in recreational runners: Evidence for a hypometabolic state. J Clin Endocrinol Metab. 2003;88:337–346. doi: 10.1210/jc.2002-020958. doi:10.1210/jc.2002-020958. [DOI] [PubMed] [Google Scholar]
- Dittus WPJ. The social regulation of population density and age-sex distribution in the toque monkey. Behaviour. 1977;63:281–322. doi:10.1163/156853977X00450. [Google Scholar]
- Dittus WPJ. The social regulation of primate populations: a synthesis. In: Lindburg DG, editor. The Macaques: Studies in Ecology, Behavior and Evolution. New York: Nostrand Reinhold Co.; 1980. pp. 263–283. [Google Scholar]
- Drew FL. Epidemiology: the epidemiology of secondary amenorrhea. J Chron Dis. 1961;14:396–407. doi: 10.1016/0021-9681(61)90138-2. doi:10.1016/0021-9681(61)90138-2. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1998;84:192–197. doi: 10.1210/jcem.84.1.5387. doi:10.1210/jc.84.1.192. [DOI] [PubMed] [Google Scholar]
- Ferin M. Clinical Review 105: stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. doi:10.1210/jc.84.6.1768. [DOI] [PubMed] [Google Scholar]
- Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:45–59. doi: 10.1016/s1570-0232(02)00216-7. doi:10.1016/S1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14(3 Pt 2):567–571. doi: 10.1097/gme.0b013e31804c793d. doi:10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- Ginsburg KA. Luteal phase defect: etiology, diagnosis, and management. Endocrinol Metab Clin N Am. 1992;21:85–104. [PubMed] [Google Scholar]
- Hanke H, Hanke S, Ickrath O, Lange K, Bruck B, Mück AO, Seeger H, Zwirner M, Voisard R, Haasis R, et al. Estradiol concentrations in premenopausal women with coronary heart disease. Coron Artery Dis. 1997;8:511–515. [PubMed] [Google Scholar]
- Harcourt AH. Dominance and fertility among female primates. Zool Soc Lond. 1987;213:471–487. doi:10.1111/j.1469-7998.1987.tb03721.x. [Google Scholar]
- Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. doi:10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes RW, Alexander L, Duffy SW, Leong J, Chen LH, Lee WH. Dietary intake of soybean protein and menstrual cycle length in pre-menopausal Singapore Chinese women. Public Health Nutr. 2001;4:191–196. doi: 10.1079/phn200063. [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Rankin SE, Weaver DS, Carlson CS, Clarkson TB. Accuracy and precision of lumbar bone mineral content by dual-energy X-ray absorptiometry in live female monkeys. Calcif Tissue Int. 1991;49:438–440. doi: 10.1007/BF02555858. doi:10.1007/BF02555858. [DOI] [PubMed] [Google Scholar]
- Jones GES. Some newer aspects of the management of infertility. J Am Med Assoc. 1949;141:1123–1129. doi: 10.1001/jama.1949.02910160013004. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci. 1998;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. doi:10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause. 2008;15:768–776. doi: 10.1097/gme.0b013e31815eb18e. doi:10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. doi:10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15:2094–2100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71:732–741. doi: 10.1002/ajp.20707. doi:10.1016/S0029-7844(01)01659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002a;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. doi:10.1016/S0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002b;26:431–443. doi: 10.1016/S0893-133X(01)00344-X. doi:10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of ovulation. Hum Reprod. 1988;3:469–472. doi: 10.1093/oxfordjournals.humrep.a136730. [DOI] [PubMed] [Google Scholar]
- Kurzer MS. Hormonal effects of soy in premenopausal women and men. J Nutr. 2002;132:570S–573S. doi: 10.1093/jn/132.3.570S. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanksi A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. doi: 10.1210/jc.2009-1046. doi:10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees CJ, Register TC, Turner CH, Wang T, Stancill M, Jerome CP. Effects of raloxifene on bone density, biomarkers, and histomorphometric and biomechanical measures in ovariectomized cynomolgus monkeys. Menopause. 2002;9:320–328. doi: 10.1097/00042192-200209000-00004. doi:10.1097/00042192-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88:297–311. doi: 10.1210/jc.2002-020369. doi:10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- Lu L-JW, Anderson KE, Grady JJ, Nagamani M. Effects of Soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996;5:63–70. [PubMed] [Google Scholar]
- Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt1):1739–1744. [PubMed] [Google Scholar]
- Merz CN, Johnson BD, Berga S, Braunstein G, Reis SE, Bittner V WISE Study Group. Past oral contraceptive use and angiographic coronary artery disease in postmenopausal women: data from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Fertil Steril. 2006;85:1425–1431. doi: 10.1016/j.fertnstert.2006.01.009. doi:10.1016/j.fertnstert.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. doi:10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek RJ. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994;49:153–160. doi: 10.1016/0960-0760(94)90005-1. doi:10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Misra M, Klibanski A. Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord. 2006;7:91–99. doi: 10.1007/s11154-006-9005-1. doi:10.1007/s11154-006-9005-1. [DOI] [PubMed] [Google Scholar]
- Nagata C, Kabuto M, Kurisu Y, Shimizu H. Decreased serum estradiol concentration associated with high dietary intake of soy products in premenopausal Japanese women. Nutr Cancer. 1997;29:228–233. doi: 10.1080/01635589709514629. doi:10.1080/01635589709514629. [DOI] [PubMed] [Google Scholar]
- Newton TL, Philhower CL. Socioemotional correlates of self-reported menstrual cycle irregularity in premenopausal women. Psychosom Med. 2003;65:1065–1069. doi: 10.1097/01.psy.0000097346.39776.59. doi:10.1097/01.PSY.0000097346.39776.59. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J, editors. Cancer Incidence in Five Continents. VI. Lyon: International Agency for Research on Cancer; 1992. pp. 145–173. IARC Scientific Publication no. 120. [Google Scholar]
- Pazol K, Kaplan JR, Abbott D, Appt SE, Wilson ME. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta. 2004;340:117–126. doi: 10.1016/j.cccn.2003.10.010. doi:10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Reifenstein EC., Jr Psychogenic or ‘hypothalamic' amenorrhea. Med Clin North Am. 1946;30:110–1114. doi: 10.1016/s0025-7125(16)35908-9. [DOI] [PubMed] [Google Scholar]
- Ripley HS, Papanicolaou GN. The menstrual cycle with vaginal smear studies in schizophrenia, depression, and elation. Am J Psychiatry. 1942;98:567–574. [Google Scholar]
- Rocca WA, Grossardt BR, Andrade de M, Malkasian GD, Melton LJ. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. doi:10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1073. doi: 10.1212/01.wnl.0000276984.19542.e6. doi:10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Sade DS. An ethogram for Rhesus monkeys. I. Antithetical contrasts in posture and movement. Am J Phys Anthropol. 1973;38:534–542. doi: 10.1002/ajpa.1330380263. [DOI] [PubMed] [Google Scholar]
- Sade DS, Cushing K, Cushing P, Dunaif J, Figueroa A, Kaplan JR, Lauer C, Rhodes D, Schneider J. Population dynamics in relation to social structure on Cayo Santiago. Yearbook Phys Anthropol. 1976;20:253–262. [Google Scholar]
- Shively CA, Clarkson TB. Social status and coronary artery atherosclerosis in female monkeys. Arterioscler Thromb. 1994;14:721–726. doi: 10.1161/01.atv.14.5.721. [DOI] [PubMed] [Google Scholar]
- Silk JB. Practice random acts of aggression and senseless acts of intimidation: the logic of status contests in social groups. Evol Anthropol. 2002;11:221–225. doi:10.1002/evan.10038. [Google Scholar]
- Snell-Bergeon JK, Dabelea D, Ogden LG, Hokanson JE, Kinney GL, Ehrlich J, Rewers M. Reproductive history and hormonal birth control use are associated with coronary calcium progression in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2142–2148. doi: 10.1210/jc.2007-2025. doi:10.1210/jc.2007-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, Jannausch M. Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998;13:1134–1140. doi: 10.1359/jbmr.1998.13.7.1134. doi:10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- Speroff L. Gonads are the heart of the matter. Menopause. 2007;14:342–344. doi: 10.1097/GME.0b013e31803816ec. [DOI] [PubMed] [Google Scholar]
- Stute P, Wood CE, Kaplan JR, Cline JM. Cyclic changes in the mammary gland of cynomolgus macaques. Fertil Steril. 2004;82(Suppl 3):1160–1170. doi: 10.1016/j.fertnstert.2004.04.035. doi:10.1016/j.fertnstert.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Ursin G, Bernstein L, Pike MC. Breast cancer. In: Doll R, Fraumeni JF, Muir CS, editors. Trends in Cancer Incidence and Mortality. vol 19/20. Plainview, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 241–264. [Google Scholar]
- Vitolins MZ, Anthony MS, Burke GL. Phytoestrogens and Health. 1st edn. Champaign, IL: AOCS Press; 2002. p. 260. [Google Scholar]
- Walker SE, Register TC, Appt SE, Adams M, Clarkson TB, Chen H, Isom S, Franke AA, Kaplan JR. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15:950–957. doi: 10.1097/gme.0b013e3181612cef. doi:10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. doi:10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- Wilks JW, Hodgen GD, Ross GT. Luteal phase defects in the rhesus monkey: The significance of serum FSH:LH ratios. J Clin Endocrinol Metab. 1976;43:1261–1267. doi: 10.1210/jcem-43-6-1261. doi:10.1210/jcem-43-6-1261. [DOI] [PubMed] [Google Scholar]
- Williams RF, Hodgen GD. The reproductive cycle in female macaques. Am J Primatol. 1982;3(Suppl 1):181–192. doi:10.1002/ajp.1350030524. [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–E276. doi: 10.1152/ajpendo.00108.2007. doi:10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol. 1998;158:247–257. doi: 10.1677/joe.0.1580247. doi:10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. doi:10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Wood CE, Kaplan JR, Stute P, Cline JM. Effects of soy on the mammary glands of premenopausal female monkeys. Fertil Steril. 2006;85(Suppl 1):1179–1186. doi: 10.1016/j.fertnstert.2005.08.059. doi:10.1016/j.fertnstert.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 2000;82:1879–1886. doi: 10.1054/bjoc.1999.1218. doi:10.1054/bjoc.1999.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M. Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the rhesus monkey. J Clin Endocrinol Metab. 2002;87:2232–2237. doi: 10.1210/jcem.87.5.8500. doi:10.1210/jc.87.5.2232. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. The menstrual cycle of the primates. I. General nature and homology. Proc Zool Soc Lond. 1930;100:691–754. [Google Scholar]
- Zuckerman S, Parks MA. The menstrual cycle of primates. V. The cycle of the baboon. Proc Zool Soc Lond. 1932;102:138–191. [Google Scholar]