Abstract
Context
Sex steroid hormone receptor (SHR) dynamics are well-documented in human endometrium but have not been comprehensively studied in Fallopian tube (FT).
Objective
To compare expression patterns and hormonal regulation of SHR in FT with that described in endometrium, and determine whether SHR expression is altered in FT of women with ectopic pregnancy (EP).
Design
Tissue analysis and culture.
Patients or Other Participants
Women undergoing surgery for benign gynaecological conditions (n=14) and EP (n=6).
Interventions
Q-RT-PCR and immunohistochemistry were used to determine SHR mRNA expression and protein localization, respectively. SHR levels were measured in tubal explant cultures stimulated with estrogen and progestogen.
Results
ERα and ERβ mRNAs were constitutively expressed in FT during the menstrual cycle. PR-AB and PR-B mRNAs were decreased in mid-luteal compared to follicular phase. ERα, PR-AB and PR-B mRNAs were downregulated in human FT in vitro by treatment with progestogen. ERα, ERβ1, ERβ2, PR and AR proteins localised to cell nuclei of epithelium, stroma and smooth muscle of non-pregnant FT. In FT from women with EP, PR-B mRNA was decreased when compared to mid-luteal FT, and ERα protein was not detected.
Conclusions
SHR expression in FT is different from that observed in endometrium recovered at similar stages of the menstrual cycle and expression in FT from women with EP is also altered compared with normal FT. These data are an important benchmark for furthering understanding of normal human FT physiology, transcriptional changes in FT in response to progesterone, and disorders of FT function, such as EP.
Keywords: Estrogen receptor, progesterone receptor, androgen receptor, allopian tube, ectopic pregnancy
Introduction
The female reproductive system is exposed to fluctuating levels of sex steroids, including estrogen and progesterone during the normal menstrual cycle. A number of studies have investigated the expression patterns of the estrogen (ER), progesterone (PR) and androgen (AR) receptors in the human endometrium and documented both cell-specific patterns of expression as well as hormone dependent changes during the menstrual cycle (reviewed in (1)). However, the expression of sex steroid hormone receptors has yet to be comprehensively studied in the normal human Fallopian tube or in the context of tubal pathologies, such as ectopic pregnancy.
Sex steroid-regulated changes in Fallopian tube gene expression and function likely contribute to successful embryo tubal transport and implantation. The aim of this study was to compare expression patterns and regulation of sex steroid hormone receptors in the Fallopian tube with that described in endometrium. We also hypothesised that their expression pattern would be altered in Fallopian tube of women with ectopic pregnancy.
Sex steroid hormone receptors belong to a superfamily of genes that function as ligand-activated transcription factors (2). They have a conserved arrangement of functional domains, the most important of which are the DNA-binding domain containing two zinc fingers and a ligand binding domain found towards the C-terminus of the protein.
Two ER genes have been identified: ERα (ESR1; (3)) and ERβ (ESR2; (4)). Splice variant isoforms of the latter are expressed in a variety of human tissues (5, 6). ERα and ERβ exhibit different functional properties in vitro and show distinct patterns of gene regulation (7, 8). At present, although expression of ERβ1 and ERβ2 has been detected in normal endometrium, their roles are still unclear (1). However, it has been suggested that both ERβ1 and ERβ2 may have an impact on ERα-mediated gene expression (9).
Expression of ER in the human Fallopian tube was first described prior to the discovery of ERβ (10, 11). ER was immunolocalised to epithelial cells in the ampullary and fimbrial sections of the Fallopian tube and was reputed to increase throughout the follicular phase before reaching a plateau in the luteal phase (11). The antibody used in the studies was a mouse monoclonal antibody raised against purified calf uterine estrogen receptor. Expression of ERβ was recently documented in the human Fallopian tube using a rabbit polyclonal antibody raised against the amino-terminus of ERβ but levels were not assessed at different phases of the menstrual cycle (12). Expression of ERα or ERβ has not been examined in Fallopian tube from women with ectopic pregnancy.
Human PR is expressed as two isoforms, PR-A (94 kDa) and PR-B (116 kDa) both encoded by a single gene (13). PR-A is a truncated form of PR-B and lacks 164 amino acids from the N-terminus. Although both PR-A and PR-B bind progesterone, selective physiological roles for the two isoforms of PR have been documented (14). Generally, PR-B is transcriptionally the more active of the two isoforms (15). Furthermore, PR-A can act as a dominant repressor of PR-B-dependent activation of progestin-sensitive reporter genes, and likewise inhibits the transcriptional activity of receptors for androgens, glucocorticoids and mineralocorticoids (16).
Expression of PR in the Fallopian tube was first characterised in the early 1990s (10, 11). Using antibodies that crossreact with both PR-A and PR-B, these studies showed that immunoexpression of epithelial PR expression was most intense in the follicular phase but that it was not detected in the late luteal phase (11). A general reduction in PR protein expression has also been reported in Fallopian tube from women with ectopic pregnancy using immunoscoring and a mouse monoclonal antibody (IgG1 clone PR88) raised against purified human progesterone receptor (17). More recently, expression of mRNAs specific for PR-A and B have been studied in non-pregnant Fallopian tube. Regional tubal expression of PR-A and PR-B mRNA was shown to vary, but again this was not studied in relation to cycle phase (18).
Human AR is encoded by a single copy gene on the X chromosome and in vivo it binds either testosterone or 5α-dihydrotestosterone (19). AR expression has been documented in the non-pregnant human Fallopian tube by Western blot analysis using rabbit monoclonal antibodies which recognise full length AR proteins (20), but no quantitative or topographical studies have, to our knowledge, been performed.
In the current study, we performed a detailed analysis of expression of ERα, ERβ1, ERβ2, PR-A, PR-B and AR in the human Fallopian tube across the menstrual cycle and compared this with expression in Fallopian tubes of women with ectopic pregnancy. These in vivo observations were complemented and extended using an in vitro model.
Methods
Tissue collection
Ethical approval for this study was obtained from Lothian Research Ethics Committee (04/S1103/20). All women were aged 18-45 years. Written and informed consent was obtained from all patients before sample collection. Fallopian tube biopsies (all from ampullary region unless stated), endometrial biopsies (for histological dating) and sera (for measurement of circulating estradiol and progesterone concentrations for endocrine staging) were collected from women with regular menstrual cycles (21-35 days) undergoing gynecological procedures for benign conditions who had no previous history of ectopic pregnancy and had not taken any hormonal preparations in the three months prior to surgery (n=14; see Table 1). None of the gynaecological conditions listed have been reported to date to affect sex steroid hormone receptor expression in the endometrium so it was assumed that this would also be the case in the Fallopian tube. Fallopian tube was also obtained from women undergoing surgical management of tubal ectopic pregnancy (n=6; see Table 2). None of the women undergoing surgical management of ectopic pregnancy presented acutely with haemodynamic shock, and all required serial serum beta-HCG and ultrasound monitoring prior to diagnosis. Part of the Fallopian tube was (a) immersed in RNAlater™ (Ambion, Texas, USA) at 4°C overnight then flash frozen at −70°C; part of the Fallopian tube and the endometrial biopsies were (b) fixed in 10% neutral buffered formalin overnight at 4°C, stored in 70% ethanol, and wax embedded; or (c) collected into PBS for tissue culture. The endometrial biopsies underwent haematoxylin and eosin staining and dating by an expert histopathologist.
Table 1.
Demographics for Fallopian tube and endometrial biopsies from women undergoing surgery for benign gynaecological conditions.
| Age | Para | Cycle phase |
Serum E2 (pmol/L) |
Serum Prog (nmol/L) |
Endometrial histology |
Surgery | Reason for surgery |
Uterine pathology |
|
|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 2 | Follicular | 1022.87 | 0.81 | Proliferative | TAH | HMB | Adenomyosis |
| 2 | 37 | 2 | Follicular | 940.44 | 3.82 | Proliferative | TAH | HMB, pelvic pain |
Adenomyosis |
| 3 | 44 | 3 | Follicular | 829.42 | 4.19 | Proliferative | TAH | HMB, pelvic pain |
Adenomyosis, fibroid |
| 4 | 36 | 3 | Follicular | 770.63 | 5.16 | Proliferative | TAH | HMB | No abnormality |
| 5 | 44 | 2 | Follicular | 116.3 | 2.88 | Proliferative | STAH | HMB, dysmenorrhoea |
No abnormality |
| 6 | 34 | 2 | Mid luteal |
471 | 62.65 | Mid secretory |
LAVH | HMB, dysmenorrhoea |
Adenomyosis, fibroid |
| 7 | 40 | 2 | Mid luteal |
242 | 53.1 | Mid secretory |
TAH | HMB, dysmenorrhoea |
No abnormality |
| 8 | 35 | 2 | Mid luteal |
424 | 76.9 | Mid secretory |
TAH | pelvic pain | No abnormality |
| 9 | 38 | 3 | Mid luteal |
266 | 37.1 | Mid secretory |
TAH | HMB | No abnormality |
| 10 | 32 | 1 | Mid luteal |
549.91 | 88 | Mid secretory |
TAH | Dysmenorrhoea | No abnormality |
| 11 | 43 | 2 | Mid luteal |
201 | 24.55 | Mid secretory |
TAH | HMB, pelvic pain |
Fibroid |
| 12 | 40 | 1 | Mid luteal |
1633.0 | 54.38 | Mid secretory |
TAH | HMB, dysmenorrhoea |
No abnormality |
| 13 | 35 | 4 | Menstrual | 73 | 2.67 | Menstrual | TAH | HMB, dysmenorrhoea |
Adenomyosis |
| 14 | 42 | 2 | Menstrual | 55 | 15.05 | Menstrual | TAH | HMB | No abnormality |
TAH = total abdominal hysterectomy
STAH = sub-total hysterectomy
LAVH = laparoscopically-assisted vaginal hysterectomy
HMB = heavy menstrual bleeding
Table 2.
Demographics of Fallopian tube biopsies for women undergoing surgery for ectopic pregnancy.
| Gestation (days) | hCG (IU/L) | Prog (nmol/L) | |
|---|---|---|---|
| 1 | 58 | 15956 | 67.43 |
| 2 | 50 | 487 | 39.21 |
| 3 | 59 | 2056 | 24.58 |
| 4 | 53 | 1854 | 63.07 |
| 5 | 52 | 2425 | 61.68 |
| 6 | 56 | 225 | 20.36 |
Quantitative RT-PCR
RNA was extracted from cells/tissues as detailed in the manufacturer's protocol (Qiagen, RNeasy mini kits). All samples were treated with DNase I (Qiagen) in order to remove any contaminating genomic DNA. Complementary DNA was synthesised from 400ng of total RNA in 20μl reaction volumes containing: 1×RT buffer, magnesium chloride, dNTPs, random hexamers, RNase inhibitor and Multiscribe reverse transcriptase (Applied Biosystems, Cheshire, UK). Reactions were incubated at 25°C for 20 min, 42°C for 60 min then 95°C for 5 min. Negative controls contained either template RNA but no reverse transcriptase (RT negative) or no template RNA (RT water). PCR reaction mixtures contained Taqman 2x Master-mix (1x; Applied Biosystems), forward and reverse primers (300nM; Eurogentec) and probe (200nM; Eurogentec) for ERα, ERβ, PR-AB, PR-B or AR, and forward and reverse primers and probe for ribosomal 18S (all 50nM; Applied Biosystems). ERα, ERβ, PR-AB, PR-B and AR primers and probes were designed using Primer Express software and their sequences are shown in Table 3. Ribosomal 18S was used as a housekeeping gene. Negative control (water in place of cDNA) samples were included in each PCR run along with the RT negative and RT H2O control samples described above. All samples were analysed in triplicate using the 2−ΔΔCt method. PCR reactions were run on an ABI 7900 Sequence Detection System (Perkin-Elmer Applied Biosystems, USA).
Table 3.
Sex steroid receptor primer/probe sequences used for Taqman RT-PCR.
| Primer / Probe |
Sequence | Position | Accession No |
|---|---|---|---|
| ERα forward | TGATTGGTCTCGTCTGGCG | 1523–1541 | NM_000125 |
| ERα reverse | CATGCCCTCTACACATTTTCCC | 1602–1624 (r) | NM_000125 |
| ERα probe | TGCTCCTAACTTGCTCTTGGACAGGAACC | 1572–1600 | NM_000125 |
| ERß1 forward | CCTGGCTAACCTCCTGATGCT | 1459–1480 | AB006590 |
| ERß1 reverse | CCACATTTTTGCACTTCATGTTG | 1529–1552 (r) | AB006590 |
| ERß1 probe | AGATGTTCCATGCCCTTGTTACTCGCA | 1499–1525 (r) | AB006590 |
| ERß2 forward | ATCCATGCGCCTGGCTAAC | 2628–2647 | AB006589 |
| ERß2 reverse | GAGTGTTTGAGAGGCCTTTTCTG | 2684–2707 (r) |
AB006589 |
| ERß2 probe | TCCTGATGCTCCTGTCCCACGTCA | 2648–2671 | AB006589 |
| PR-AB forward |
CAGTGGGCGTTCCAAATGA | 2151–2170 | NM_000926 |
| PR-AB reverse |
TGGTGGAATCAACTGTATGTCTTGA | 2209–2233(r) | NM_000926 |
| PR-AB probe | AGCCAAGCCCTAAGCCAGAGATTCACTTT | 2170–2199 | NM_000926 |
| PR-B forward | CGGACACCTTGCCTGAATT | 1579-1595 | NM_000926 |
| PR-B reverse | CAGGGCCGAGGGAAGAGTAG | 1626-1645 | NM_000926 |
| PR-B probe | CGGCCATACCT ATCTCCCTGG ACGG | 1600-1624 | NM_000926 |
| AR forward | GTACCCTGGCGGCATGGT | 951–1016 | L29496 |
| AR reverse | CCCATTTCGCTTTTGACACA | 951–1016 | L29496 |
| AR probe | AGCAGAGTGCCCTATCCCAGTCCCA | 951–1016 | L29496 |
Immunohistochemistry
Immunohistochemical localisation of ERα, ERβ1, ERβ2, PR and AR was performed on Fallopian tube sections using biotinylated secondary antibodies and peroxidase conjugated detection systems. Immunoreactivity was detected using the chromagen 3,3′-diaminobenzidine (DAB). In brief, tissue sections were dewaxed in xylene and rehydrated in descending grades of alcohol. Sections were subjected to antigen retrieval as described in Table 4 and then non-specific activity was blocked sequentially with 3% hydrogen peroxide (Sigma-Aldrich), avidin and biotin, and protein blocks. Sections were incubated overnight at 4°C with antibodies specific to either: ERα, ERβ1, ERβ2, PR and AR (see Table 4). Sections were subsequently incubated with biotinylated secondary antibodies (Vector Laboratories, Peterborough, UK) and HRP complex (ABC-Elite, Vector Laboratories) or Streptavidin (Dako, Cambridge, UK), then immunoreactivity detected using DAB (Vector Laboratories). Counterstaining was then performed with Harris' haemotoxylin and mounted in Pertex (Cellpath Technologies, Hemel Hempstead, UK).
Table 4.
Antibodies used for immunohistochemistry.
| Receptor | Antigen retrieval |
Antibody diluent |
Primary antibody |
Secondary antibody |
Negative control |
|---|---|---|---|---|---|
| ERα | Pressure cook in 0.01M sodium citrate pH6 |
Normal goat serum and 5% BSA |
Mouse monoclonal antibody M7407 (Dako) |
Biotinylated goat-anti- mouse |
MIgG |
| ERβ1 | Pressure cook in 0.01M sodium citrate pH6 |
Normal goat serum and 5% BSA |
Mouse monoclonal IgG2a (Serotec) |
Biotinylated goat-anti- mouse |
Pre-absorbed peptide |
| ERβ2 | Pressure cook in 0.01M sodium citrate pH6 |
Normal goat serum and 5% BSA |
Mouse monoclonal (Serotec) |
Biotinylated goat anti- mouse |
Pre-absorbed peptide |
| PR (recognises both isoforms) |
Pressure cook in 0.01M sodium citrate pH6 |
Normal goat serum and 5% BSA |
Mouse monoclonal (Novatec) |
Biotinylated goat-anti- mouse |
MIgG |
| AR | Pressure cook in 0.05M glycine EDTA pH8 |
Tris Buffered Saline |
Rabbit-anti- human AR (Santa Cruz) |
Biotinylated goat-anti- rabbit |
RIgG |
Tissue culture
Tubal explant cultures were performed as previously described (21) using surgical tissue from the ampullary region (unless indicated otherwise) of non-pregnant Fallopian tubes collected across the menstrual cycle. Fallopian tube biopsies were cut into small pieces (2-3 mm), placed in a 12-well dish with the luminal epithelial surface facing upwards and cultured in RPMI containing 10% charcoal-stripped fetal-bovine serum at 37°C in 5% CO2. After 24 hours in culture, the tissues (n=5) were exposed to ethanol (as a control), and either estradiol (E2) (10nM), medroxyprogesterone acetate (MPA) (1μM), or a combination of 10nM E2 and 1μM MPA, for a further 24 hours. Following treatments quantitative RT-PCR for ERα, ERβ1, ERβ2, PR-AB, PR-B and AR was performed as above.
Statistical analysis
Data were logarithmically transformed prior to statistical analysis. Significant difference was determined by one-way ANOVA and Tukey's posthoc analysis.
Results
Expression of PR-AB and PR-B mRNAs is significantly diminished in the mid-luteal phase of the menstrual cycle
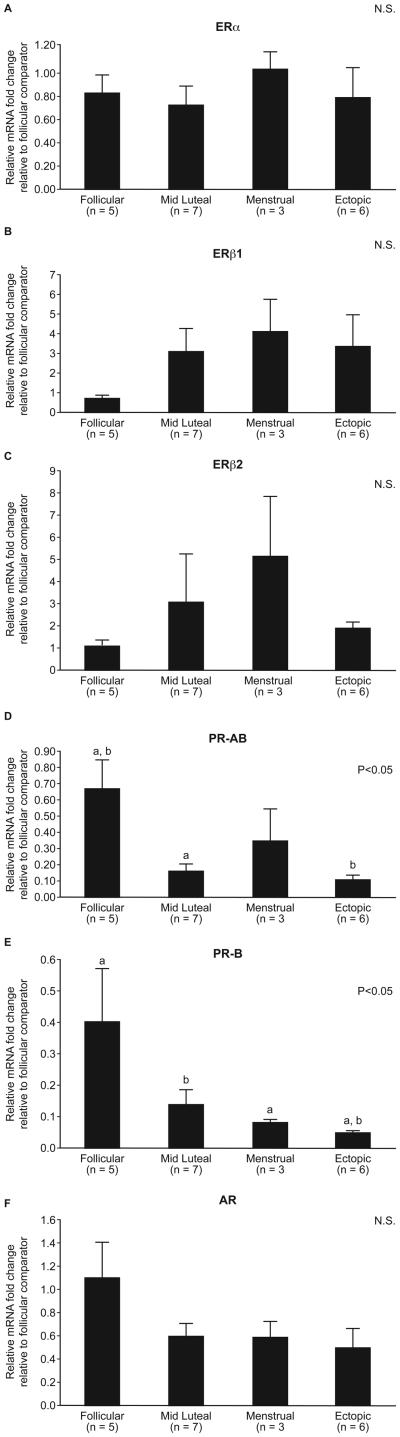
Expression of ERα and ERβ2 mRNAs did not change according to phase of menstrual cycle (Fig. 1A and C). There was a trend for expression of ERβ1 mRNA to be higher in the mid-luteal compared to the follicular phase of the menstrual cycle although this was not statistically significant (Fig. 1B). Expression of PR-AB and PR-B mRNAs was significantly reduced in the mid-luteal phase compared to the follicular phase of the menstrual cycle (p<0.05, Fig. 1D and E). Tubal AR mRNA expression showed a similar, but non-significant trend (Fig. 1D).
Fig. 1. Sex steroid hormone receptor mRNA expression in the human Fallopian tube across the menstrual cycle (follicular, mid-luteal and menstrual phases).
Expression of ERα (A), ERβ1 (B), ERβ2 (C), and AR (F) did not change according to phase of menstrual cycle. PR-AB (D) and PR-B (E) mRNA expression were significantly diminished in the luteal phase compared to expression in the follicular phase of the cycle (p<0.05).
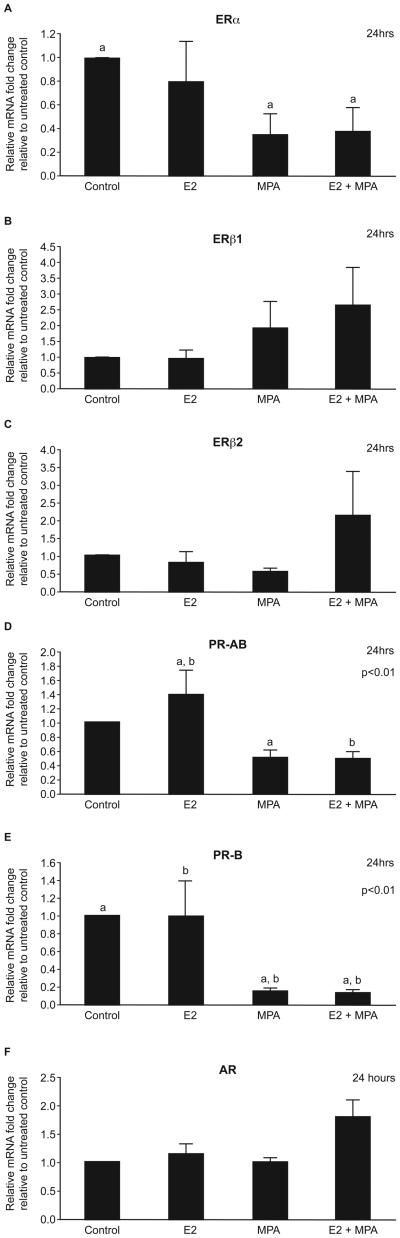
Progestogen downregulates expression of ERα, PR-AB and PR-B mRNAs in the human Fallopian tube in vitro
Incubation of tubal explants for 24h in the presence of E2 alone had no impact on total concentrations of any of the SHR mRNAs (Fig. 2). Likewise there was no change in expression of ERβ1, ERβ2 or AR mRNAs after incubation with MPA alone or E2 plus MPA (Fig. 2B, C and F, respectively). In contrast expression of ERα, PR-AB and PR-B mRNAs was significantly downregulated after incubation with MPA alone or MPA plus E2 for 24 hours (p<0.01) (Fig. 2A, D and E, respectively).
Fig. 2. Tubal explant culture with sex steroids.
Stimulation of tubal explants (n=5) with estradiol (E2) (10nM) and medroxyprogesterone acetate (MPA) (1μM) showed no change in ERβ1 (B), ERβ2 (C) and AR (F) mRNA expression after 24 hours. ERα (A), PRA-AB (D) and PR-B (E) mRNA expression was significantly downregulated after treatment with medroxyprogesterone acetate, and in combination with estradiol (p<0.01).
Expression of PR-B mRNA expression is significantly diminished in the Fallopian tube of women with ectopic pregnancy
Total concentrations of ERα, ERβ1, ERβ2, PR-AB, and AR mRNAs were not statistically different in the Fallopian tube of women with ectopic pregnancy and those recovered during the luteal phase of the cycle (Fig. 3A, B, C and D, respectively). However, expression of PR-B mRNA was significantly reduced in the Fallopian tubes of women with ectopic pregnancy (p<0.05) (Fig. 3E) compared to those from the mid-luteal phase.
Fig. 3. Sex steroid hormone receptor mRNA expression in the human Fallopian of women with ectopic pregnancy.
ERα (A), ERβ1 (B), ERβ2 (C), PR-AB (D) and AR (F). PR-B mRNA expression was significantly lower in the Fallopian tube of women with ectopic pregnancy compared to expression in the non-pregnant Fallopian tube in the mid-luteal phase of the cycle (p<0.05).
ERα protein was not detected in the Fallopian tube of women with ectopic pregnancy
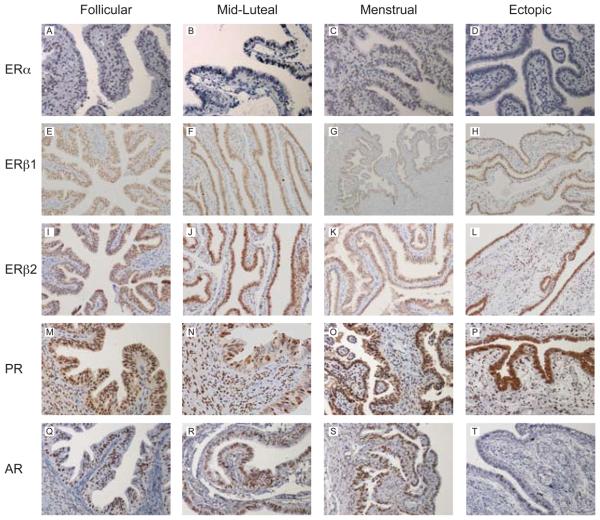
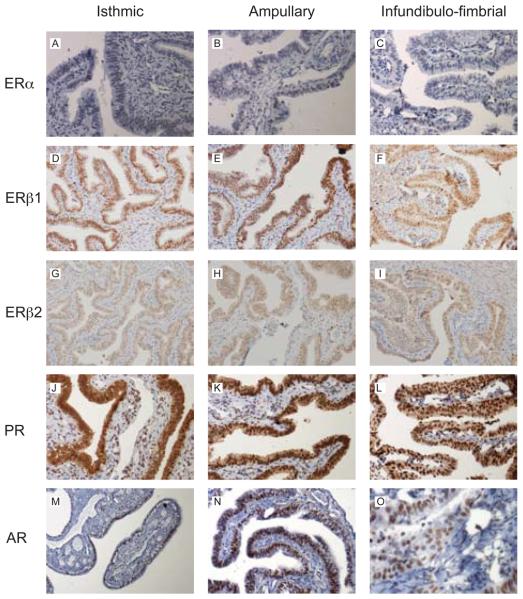
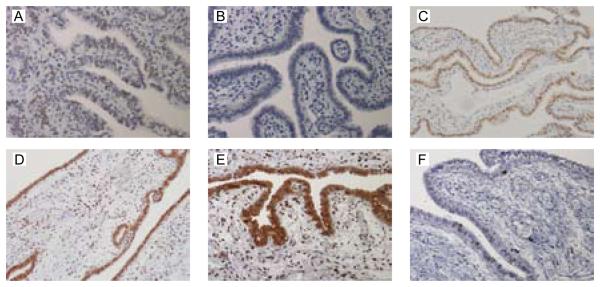
ERα protein was immunolocalised to the nuclei of the epithelial cells, stroma and smooth muscle of non-pregnant Fallopian tube from all phases of the menstrual cycle (representative images in Figs. 4A and 5A) but expression was not detected in women with ectopic pregnancy (Fig. 5B). ERβ1, ERβ2, PR and AR were all detected in all the nuclei within the epithelial layer and within approximately 50% of those within the stromal and smooth muscle layers in all the Fallopian tube biopsies (representative images in Fig. 4B-F and Fig. 5C-F). One full-length Fallopian tube from the mid-luteal phase was examined for regional expression of SHR proteins. Similar patterns of nuclear staining were detected in epithelial, stromal and smooth muscle cells using antibodies specific for ERα, ERβ1, ERβ2, PR and AR in sections from the isthmic, ampullary and infundibulo-fimbrial regions.
Fig. 4. Representative images of the immunolocalisation of sex steroid hormone receptor protein in the human Fallopian tube across the menstrual cycle.
A) ERα expression in the follicular phase. B) ERβ1 expression in the mid-luteal phase. C) ERβ2 expression in the follicular phase. D) PR expression in the follicular phase. E) PR expression in the mid-luteal phase. F) AR expression in the follicular phase. Sex steroid hormone receptor protein was localised to the nuclei of all the epithelial cells, approximately 50% of the stromal cells and the smooth muscle in all biopsies of non-pregnant Fallopian tube at all stages of the menstrual cycle.
Fig. 5. Representative images of the immunolocalisation of sex steroid hormone receptor protein in Fallopian tube from women with ectopic pregnancy.
A) ERα expression in the menstrual phase (control). B) ERα expression in women with ectopic pregnancy. ERβ1 (C), ERβ2 (D), PR (E), and AR (F) expression in women with ectopic pregnancy. There was no evidence of ERα protein expression in Fallopian tube obtained from women with ectopic pregnancy (compare A with B). However, ERβ1, ERβ2, PR, and AR immunolocalisation in Fallopian tube from women with ectopic pregnancy was similar to that observed for non-pregnant Fallopian tube.
Discussion
To our knowledge, this is the first comprehensive description of the patterns of expression of ERα, ERβ1, ERβ2, PR-A, PR-B and AR in the human Fallopian tube at different stages of the menstrual cycle. We have also used an in vitro model system to investigate the impact of acute exposure to E2 and/or MPA on expression of sex steroid hormone receptors in this tissue. In addition, we report on differences in sex steroid hormone receptor expression in Fallopian tube from women with ectopic pregnancy compared to non-pregnant Fallopian tube.
We demonstrate that total expression of PR-AB and PR-B mRNAs are significantly reduced in non-pregnant Fallopian tubes recovered during the mid-luteal phase of the cycle as compared with those obtained during during the follicular phase. Surprisingly, expression of ERα mRNA in the Fallopian tube remains constant across the menstrual cycle i.e. ERα is not downregulated in the mid luteal phase, when the tissue is exposed to peak levels of circulating progesterone.
Our data extend the findings of a study that examined expression of PR-B mRNA in the Fallopian tube during the mid-luteal phase but did not compare expression to other phases of the menstrual cycle (22). In the context of Fallopian tube function, particularly the ciliary and tubal smooth muscle activity essential for successful embryo-tubal transport and subsequent implantation in the uterus, the downregulation of PR is important (23). Progesterone is reported to have an inhibitory action on ciliary and tubal smooth muscle activity because high progesterone levels in the luteal phase coincide with a reduced frequency of contractions and ciliary activity in vitro (24, 25). Information on the regulation of specific genes by progesterone in the oviduct is limited, largely due to limited access to human Fallopian tube cells. This contrasts with the data on the endometrium where as many as 571 genes (representing 131 biochemical pathways) have been shown to be progesterone-regulated (26).
However, our data suggest that expression of SHR in the Fallopian tube do not simply mirror the changes seen in the endometrium. For example, in vivo expression of PR, and ERα, is reduced in endometrium in the mid-secretory phase of the menstrual cycle under the influence of progesterone and seems to be closely connected to the onset of endometrial receptivity (27). We therefore used an in vitro system to confirm and extend the findings that we observed in the non-pregnant Fallopian tube during the menstrual cycle by examining the impact of E2 and MPA on sex steroid hormone receptor expression. In these studies, we demonstrated downregulation of expression of PR-AB, PR-B and ERα mRNAs after treatment of Fallopian tube explants with MPA alone, and in combination with E2.
There are many in vivo and in vitro studies which report results consistent with the downregulation in expression of PR-A, PR-B and ERα mRNAs after incubation with progestagens (reviewed in (1)). For example, several studies have shown that administration of the PR antagonist, mifepristone, to patients in the early secretory phase of the menstrual cycle prevents downregulation of PR and ERα expression in the mid secretory phase (28, 29). Progestogens attenuate the actions of E2 in endometrium (30) and both PR-A and PR-B have been shown to inhibit ER transcriptional activity via a ligand-dependent mechanism in rat uterine cells (31). A more recent study has reported that PR-B overexpression in breast cancer cell lines reduces expression of ERα (32). This effect is dependent on recruitment of a corepressor transcriptional complex to a progesterone response element half site in the ERα promoter (32). In vitro studies have also shown that PR is downregulated by progestogen although this effect is cell-type dependent. In the T47D breast cancer cell line and in endometrial epithelial cells expression of both PR-A and PR-B is suppressed by progestogen treatment (10nM ORG 2058 and 0.2μm MPA, respectively) (33, 34). In contrast, PR-A and PR-B are upregulated in endometrial stromal cells by progestogen treatment (0.2μm MPA) (34). The differences seen in our in vivo compared to our in vitro findings in the Fallopian tube suggest that additional local factors may be involved in the regulation of ERα expression.
We also report reduced expression of PR-B mRNA in Fallopian tube from women with ectopic pregnancy compared to non-pregnant Fallopian tube from the mid-luteal phase of the menstrual cycle. It is not possible to collect Fallopian tube from women with intra-uterine pregnancies and so Fallopian tube collected from the mid luteal phase, when circulating progesterone levels are raised, provides the most appropriate control. Our findings support those of an earlier immunohistochemical study that could not detect PR protein expression in the Fallopian tube of women with ectopic pregnancy (17). It may also offer an explanation for the absence of adequate tubal decidualization observed with this condition (35). Mice deficient in PR fail to mount a decidual response and a recent study in humans has shown that PRs regulate distinct gene networks and cellular functions in decidualizing endometrium (36, 37).
In addition, we were unable to detect expression of ERα protein by immunohistochemistry in the Fallopian tube from women with ectopic pregnancy which was surprising as there was no difference in the total amount of ERα mRNA. Although this may reflect the limitations of our immunohistochemical approach, receptor protein was readily detected in tubes obtained during the normal cycle. Notably, ERα gene polymorphisms have been associated with female infertility (38), and ERα has been shown to serve as a dominant regulator in Fallopian tube development in the rat (39). Furthermore, a recent study in mice has identified a molecular mechanism for ERα-mediated tubal protein synthesis and secretion that appears to be important for successful embryonic development (40). Further studies are required to determine whether ERα plays a critical role in Fallopian tube physiology and to establish whether reduced translation or enhanced degradation may contribute to reduced expression of protein.
In summary, we report that there are variations in sex steroid hormone receptor expression in the non-pregnant human Fallopian tube during the menstrual cycle, that there is in vitro regulation of some SHRs in the Fallopian tube by E2 and MPA, and identified differences in expression in Fallopian tubes from women with ectopic pregnancy compared to the non-pregnant Fallopian tube. Sex steroid receptor dynamics and responsiveness to estrogen and progestogen have been demonstrated in many studies in the endometrium (reviewed in (1)). However, studies in the human Fallopian tube have been limited to date. Our data is therefore an important benchmark for furthering understanding of normal human Fallopian tube physiology, transcriptional changes in the Fallopian tube in response to progesterone, and the aetiology of disorders of Fallopian tube function, such as ectopic pregnancy.
Acknowledgements
The authors would like to thank Paula Lourenco for technical support; Catherine Cairns, Sharon McPherson and Catherine Murray for patient recruitment; and Ronnie Grant for graphical assistance and Sheila Milne for secretarial support.
Funding
This work was supported by an MRC Programme Grant (G0500047) (HODC), Wellbeing of Women (R40608) (AWH/HODC) and the Caledonian Research Foundation (AEK).
Footnotes
Disclosure summary
The authors have nothing to disclose.
References
- 1.Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009;16:191–199. doi: 10.1177/1933719108331121. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Critchley HO, Henderson TA, Kelly RW, Scobie GS, Evans LR, Groome NP, Saunders PT. Wild-type estrogen receptor (ERbeta1) and the splice variant (ERbetacx/beta2) are both expressed within the human endometrium throughout the normal menstrual cycle. J Clin Endocrinol Metab. 2002;87:5265–5273. doi: 10.1210/jc.2002-020502. [DOI] [PubMed] [Google Scholar]
- 6.Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002;87:2706–2715. doi: 10.1210/jcem.87.6.8619. [DOI] [PubMed] [Google Scholar]
- 7.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 8.Tena-Sempere M, Navarro J, Pinilla L, Gonzalez LC, Huhtaniemi I, Aguilar E. Neonatal exposure to estrogen differentially alters estrogen receptor alpha and beta mRNA expression in rat testis during postnatal development. J Endocrinol. 2000;165:345–357. doi: 10.1677/joe.0.1650345. [DOI] [PubMed] [Google Scholar]
- 9.Vasudevan N, Koibuchi N, Chin WW, Pfaff DW. Differential crosstalk between estrogen receptor (ER)alpha and ERbeta and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Brain Res Mol Brain Res. 2001;95:9–17. doi: 10.1016/s0169-328x(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 10.Coppens MT, de Boever JG, Dhont MA, Serreyn RF, Vandekerckhove DA, Roels HJ. Topographical distribution of oestrogen and progesterone receptors in the human endometrium and fallopian tube. An immunocytochemical study. Histochemistry. 1993;99:127–131. doi: 10.1007/BF00571873. [DOI] [PubMed] [Google Scholar]
- 11.Amso NN, Crow J, Shaw RW. Comparative immunohistochemical study of oestrogen and progesterone receptors in the fallopian tube and uterus at different stages of the menstrual cycle and the menopause. Hum Reprod. 1994;9:1027–1037. doi: 10.1093/oxfordjournals.humrep.a138628. [DOI] [PubMed] [Google Scholar]
- 12.Christow A, Sun X, Gemzell-Danielsson K. Effect of mifepristone and levonorgestrel on expression of steroid receptors in the human Fallopian tube. Mol Hum Reprod. 2002;8:333–340. doi: 10.1093/molehr/8.4.333. [DOI] [PubMed] [Google Scholar]
- 13.Savouret JF, Misrahi M, Milgrom E. Molecular action of progesterone. Int J Biochem. 1990;22:579–594. doi: 10.1016/0020-711x(90)90033-y. [DOI] [PubMed] [Google Scholar]
- 14.Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giangrande PH, Pollio G, McDonnell DP. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem. 1997;272:32889–32900. doi: 10.1074/jbc.272.52.32889. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48:425–432. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 17.Land JA, Arends JW. Immunohistochemical analysis of estrogen and progesterone receptors in fallopian tubes during ectopic pregnancy. Fertil Steril. 1992;58:335–337. doi: 10.1016/s0015-0282(16)55208-x. [DOI] [PubMed] [Google Scholar]
- 18.Briton-Jones C, Lok IH, Cheung CK, Po AL, Chiu TT, Haines C. Ratio of mRNA expression of progesterone receptor isoforms AB is to B in human oviduct mucosal cells during the ovulatory cycle. J Assist Reprod Genet. 2005;22:429–435. doi: 10.1007/s10815-005-7203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeap BB, Wilce JA, Leedman PJ. The androgen receptor mRNA. Bioessays. 2004;26:672–682. doi: 10.1002/bies.20051. [DOI] [PubMed] [Google Scholar]
- 20.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg JM, Friedman CI. Effect of hormonal manipulation on human fallopian tubal epithelium in vitro. J Assist Reprod Genet. 1995;12:132–135. doi: 10.1007/BF02211382. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Christow A, Marions L, Gemzell-Danielsson K. Progesterone receptor isoform B in the human fallopian tube and endometrium following mifepristone. Contraception. 2003;67:319–326. doi: 10.1016/s0010-7824(02)00513-9. [DOI] [PubMed] [Google Scholar]
- 23.Croxatto HB. Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online. 2002;4:160–169. doi: 10.1016/s1472-6483(10)61935-9. [DOI] [PubMed] [Google Scholar]
- 24.Paltieli Y, Eibschitz I, Ziskind G, Ohel G, Silbermann M, Weichselbaum A. High progesterone levels and ciliary dysfunction--a possible cause of ectopic pregnancy. J Assist Reprod Genet. 2000;17:103–106. doi: 10.1023/A:1009465900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanggren K, Stavreus-Evers A, Olsson C, Andersson E, Gemzell-Danielsson K. Regulation of muscular contractions in the human Fallopian tube through prostaglandins and progestagens. Hum Reprod. 2008;23:2359–2368. doi: 10.1093/humrep/den260. [DOI] [PubMed] [Google Scholar]
- 26.Catalano RD, Critchley HO, Heikinheimo O, Baird DT, Hapangama D, Sherwin JR, Charnock-Jones DS, Smith SK, Sharkey AM. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod. 2007;13:641–654. doi: 10.1093/molehr/gam021. [DOI] [PubMed] [Google Scholar]
- 27.Stavreus-Evers A, Nikas G, Sahlin L, Eriksson H, Landgren BM. Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil Steril. 2001;76:782–791. doi: 10.1016/s0015-0282(01)01993-8. [DOI] [PubMed] [Google Scholar]
- 28.Cameron ST, Critchley HO, Thong KJ, Buckley CH, Williams AR, Baird DT. Effects of daily low dose mifepristone on endometrial maturation and proliferation. Hum Reprod. 1996;11:2518–2526. doi: 10.1093/oxfordjournals.humrep.a019151. [DOI] [PubMed] [Google Scholar]
- 29.Maentausta O, Svalander P, Danielsson KG, Bygdeman M, Vihko R. The effects of an antiprogestin, mifepristone, and an antiestrogen, tamoxifen, on endometrial 17 beta-hydroxysteroid dehydrogenase and progestin and estrogen receptors during the luteal phase of the menstrual cycle: an immunohistochemical study. J Clin Endocrinol Metab. 1993;77:913–918. doi: 10.1210/jcem.77.4.8408465. [DOI] [PubMed] [Google Scholar]
- 30.Kirkland JL, Murthy L, Stancel GM. Progesterone inhibits the estrogen-induced expression of c-fos messenger ribonucleic acid in the uterus. Endocrinology. 1992;130:3223–3230. doi: 10.1210/endo.130.6.1375896. [DOI] [PubMed] [Google Scholar]
- 31.Kraus WL, Weis KE, Katzenellenbogen BS. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol. 1995;15:1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Amicis F, Zupo S, Panno ML, Malivindi R, Giordano F, Barone I, Mauro L, Fuqua SA, Ando S. Progesterone receptor B recruits a repressor complex to a half-PRE site of the estrogen receptor alpha gene promoter. Mol Endocrinol. 2009;23:454–465. doi: 10.1210/me.2008-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL. Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. J Biol Chem. 1995;270:30693–30700. doi: 10.1074/jbc.270.51.30693. [DOI] [PubMed] [Google Scholar]
- 34.Tseng L, Zhu HH. Regulation of progesterone receptor messenger ribonucleic acid by progestin in human endometrial stromal cells. Biol Reprod. 1997;57:1360–1366. doi: 10.1095/biolreprod57.6.1360. [DOI] [PubMed] [Google Scholar]
- 35.Randall S, Buckley CH, Fox H. Placentation in the fallopian tube. Int J Gynecol Pathol. 1987;6:132–139. doi: 10.1097/00004347-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr., Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 37.Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbo RM, Ulizzi L, Piombo L, Martinez-Labarga C, De Stefano GF, Scacchi R. Estrogen receptor alpha polymorphisms and fertility in populations with different reproductive patterns. Mol Hum Reprod. 2007;13:537–540. doi: 10.1093/molehr/gam041. [DOI] [PubMed] [Google Scholar]
- 39.Mowa CN, Iwanaga T. Developmental changes of the oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of the rat--an analysis by in situ hybridization. J Endocrinol. 2000;167:363–369. doi: 10.1677/joe.0.1670363. [DOI] [PubMed] [Google Scholar]
- 40.Shao R, Egecioglu E, Weijdegard B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007;293:E1430–1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]