Abstract
The endogenous purine nucleoside adenosine is an important antiinflammatory mediator that contributes to the control of CD4+ T cell responses. While adenosine clearly has direct effects on CD4+ T cells, it remains to be determined whether actions on APC such as dendritic cells (DC) are also important. In this report we characterize DC maturation and function in BMDC stimulated with LPS in the presence or absence of the nonselective adenosine receptor agonist NECA (5′-N-ethylcarboxamidoadenosine). We found that NECA inhibited TNF-α and IL-12 in a concentration-dependent manner, whereas IL-10 production was increased. NECA-treated BMDC also expressed reduced levels of MHC class II and CD86 and were less effective at stimulating CD4+ T cell proliferation and IL-2 production compared with BMDC exposed to vehicle control. Based on real-time RT-PCR, the A2A adenosine receptor (A2AAR) and A2BAR were the predominant adenosine receptors expressed in BMDC. Using adenosine receptor subtype selective antagonists and BMDC derived from A2AAR−/− and A2BAR−/− mice, it was shown that NECA modulates TNF-α, IL-12, IL-10, and CD86 responses predominantly via A2BAR. These data indicate that engagement of A2BAR modifies murine BMDC maturation and suggest that adenosine regulates CD4+ T cell responses by selecting for DC with impaired immunogencity.
Adenosine is an endogenous purine nucleoside that exerts myriad antiinflammatory activities on host cells via signaling through the G protein-coupled P1 purinergic receptors (1). Postulated to act in an autoinhibitory fashion to restrain local inflammation, adenosine accumulates at hypoxic or inflammatory sites and binds to adenosine receptors that are up-regulated on cells following activation (1–3). Of the four characterized P1 receptors, which include the A1 adenosine receptor (A1AR)3, A2AAR, A2BAR, and A3AR, the Gs-coupled A2AAR has been regarded to mediate most antiinflammatory responses (4, 5) However, other adenosine receptors may also be important inflammatory regulators (6–10).
Adenosine analogs have proven to be protective in a number of inflammatory disease models, including ischemia-reperfusion, colitis, autoimmune pneumonitis, and type I diabetes (11–15). Actions on CD4+ T cells are suggested to underlie many of the beneficial effects described in these models (11, 12, 16, 17). Cytokine production, expression of activation markers, and proliferation are impaired by direct engagement of A2AAR on stimulated CD4+ cells in vitro (11, 17, 18). In a T cell transfer model of colitis, both effector and regulatory T cells were shown to be defective when lacking A2AAR (11). Furthermore, regulatory T cell-generated adenosine may suppress T effectors, as expression of CD39 and CD73 on regulatory T cells converts nucleotide precursors into adenosine (19–21). Finally, adenosine has been reported to promote peripheral tolerance by favoring the development of adaptive LAG-3+ regulatory T cells (12).
In addition to direct effects, adenosine may also indirectly modify CD4+ T cell responses via regulation of accessory cells such as dendritic cells (DC). DC are potent APC that play an important role in controlling the quantity and quality of immune responses through recognition of both foreign and host molecules (22–24). Previous studies have shown that adenosine receptors are expressed on human and murine DC and have been shown to have effects on cytokine production, migration, and costimulatory marker expression (25–29). The role of specific adenosine receptors in controlling DC, and in turn CD4+ T cell responses, is still unclear. The aim of this study was to characterize adenosine receptor expression and function in murine DC maturation using a combined genetic and pharmacologic approach. We found that the nonselective adenosine agonist 5′-N-ethylcarboximadoadenosine (NECA) modulates TNF-α, IL-12, and IL-10 production, as well as CD86 and MHC-II expression in BMDC stimulated with LPS, predominantly via A2BAR. Moreover, as these BMDC did not efficiently support CD4+ T cell activation, these data suggest that adenosine impairs immunogenicity through actions on the DC.
Materials and Methods
Mice
C57BL/6, BALB/c, and OT-II mice were purchased from The Jackson Laboratory. A2AAR−/−, A2BAR−/− (kindly provided by Dr. K. Ravid), A3AR−/−, and A2AAR−/−/A2BAR−/− mice on a C57BL/6 background were bred and maintained at the University of Virginia (7, 17). All mice were housed and handled in accordance with the Institutional Animal Care and Use Committee of the University of Virginia.
Reagents
NECA was purchased from Sigma-Aldrich; 4-(2-[7-amino-2-(2-furyl)-[1,2,4]triazolo[2,3-α][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) and 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c] pyrimidin-5-amine (SCH58261) were purchased from Tocris Bioscience. A novel 1,3-dialkyl-8-hetero substituted xanthine (ATL692) was kindly provided by Adenosine Therapeutics. Anti-mouse CD11c-FITC (HL3), I-A/I-E-PE (M5/114.15.2), CD40-PE (3/23), CD80-PE (16-10A1), and CD86-PE (GL1) Abs were purchased from BD Biosciences. Anti-mouse IL-10 (JES5-16E3) and anti-TGFβ (MAB1835) were purchased from eBioscience and R&D Systems, respectively.
Generation of BMDC
Donor mice were euthanized and then bone marrow was flushed from femurs and tibias, RBC were lysed with ACK buffer, and cells were suspended to 1 × 106/ml in RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-Me, 25 mM HEPES, 2 mM sodium pyruvate, and 20 ng/ml GM-CSF (PeproTech). Cells were cultured in T-75 flasks at 37°C/5% CO2, and then fresh media was supplemented with GM-CSF added on day 3. In some experiments 3 ng/ml GM-CSF and 3 mg/ml IL-4 (PeproTech) were used in lieu of 20 ng/ml GM-CSF. After 6 days, nonadherent cells were harvested and enriched with CD11c MACS kit and LS columns (Miltenyi Biotec) according to the manufacturer’s instructions. Experiments with BMDC were conducted in complete media supplemented with 5 ng/ml GM-CSF.
Isolation of lamina propria DC
CD11c+-enriched cells from small intestine lamina propria were isolated as described previously (30). Briefly, small intestines were removed from euthanized donor C57BL/6 mice, opened longitudinally to flush out feces, cut into 15-mm pieces, and then incubated for 20 min at 37°C on a shaker in HBSS supplemented with 5% heat-inactivated FBS and 2 mM EDTA. After passing the preparation through a metal filter, intestinal fragments were collected and the step was repeated. Then, intestinal fragments were minced and incubated for 20 min at 37°C on a shaker in HBSS supplemented with 5% heat-inactivated FBS and 1 mg/ml type VIII collagenase (Sigma-Aldrich). The cell suspension was passed through a cell strainer to remove debris, washed once with complete BMDC media, and then CD11c+ cells were enriched with the CD11c MACS kit.
Cytokines
Supernatant was collected from BMDC treated in presence or absence of 500 ng/ml LPS (Escherichia coli O127:B8, phenol purified (31); Sigma-Aldrich) and NECA for 18 h. In some experiments cells were pretreated for 15 min with the adenosine receptor selective antagonists ZM241385, SCH58261, or ATL692. TNF-α, IL-12p70, and IL-10 protein were measured using ELISA kits (BD Pharmingen).
Flow cytometry
BMDC were treated in presence or absence of LPS and NECA for 24 h and nonadherent/semiadherent cells were harvested by washing twice with PBS. BMDC were then resuspended into PBS/2% FBS, stained with FITC-or PE-conjugated Abs on ice for 30 min, washed twice with PBS/2% FBS, and fixed in PBS/2% paraformaldehyde. Samples were assayed with a FACSCalibur flow cytometer and data were analyzed with CellQuest (BD Biosciences) and FlowJo (Tree Star) software. For some coculture experiments, BMDC treated with NECA and LPS for 16 h were harvested and stained with anti-CD86 Ab. CD86low and CD86high BMDC were sorted using a FACSVantage cell sorter (BD Biosciences).
Real-time RT-PCR
BMDC were lysed and total RNA was isolated with Qiagen Miniprep kits and then reverse transcribed into cDNA with SuperScript II (Invitrogen) according to the manufacturer’s recommendations. Adenosine receptor and cytokine transcripts were measured by real-time RT-PCR with primer and dual-labeled probes (Applied Biosystems) detected in a SmartCycler (Cepheid) and normalized against 18S rRNA. The fold change in expression of a given gene of interest was determined using the ΔΔCT method (32). The ability of all primers and dual-labeled probes to detect transcripts using mRNA from the appropriate positive control tissue or cell line was confirmed.
cAMP
BMDC were treated with 1 μM rolipram (Sigma-Aldrich) in presence or absence of 250 nM NECA for 15 min and then lysed with 0.1M HCl/0.1% Triton X-100. In some experiments cells were pretreated overnight with 500 ng/ml LPS. Lysate was stored at −70°C until assayed by cAMP kit (Assay Designs) according to the manufacturer’s instructions.
Cocultures
BMDC from different treatment groups, including in some cases BMDC sorted into CD86low and CD86high populations, were added to wells of 96-well plates in graded concentrations. For proliferation experiments BMDC were irradiated with 3000 rad before addition to cocultures. CD4+ T cells were isolated from OT-II, C57BL/6, or BALB/c splenocytes by positive selection using CD4 MACS bead enrichment (Miltenyi Biotec) and 1 × 105 CD4+ T cells were added to the cultures. For cocultures with OT-II CD4+ T cells, BMDC were first pulsed overnight with 5 μg/ml OVA and then washed. For polyclonal syngeneic cocultures, 500 ng/ml soluble anti-CD3 Ab (145-2C11; BD Biosciences) was added. Cell proliferation was assessed after 72–96 h by measuring the incorporation of [3H]thymidine (MP Biomedicals) added during the last 16 h of culture using a TriLux liquid scintillation counter (Perkin-Elmer). Supernatant was collected after 24 – 48 h and IL-2 was assayed by ELISA kit (BD Biosciences).
Radioligand binding assays
Our binding methodology has been described previously (33). In brief, all four subtypes of recombinant murine adenosine receptors were stably expressed in HEK293 cells (A2AAR, A2BAR, and A3AR) or CHO-K1 cells (A1AR). Crude membrane preparations from these transfected cells were diluted in HE buffer (50 mM HEPES, 1 mM EDTA (pH 7.4)) at concentrations between 2 and 35 μg/tube in a volume of 150 μl. Dilutions of antagonist compounds were prepared in HE plus adenosine deaminase (2 U/ml), MgCl2 (10 mM), and an appropriate radioligand (125I-ABA for A1AR and A3AR, 125I-ZM241385 for A2AAR, and 125I-ABOPX for A2BAR). Diluted antagonists (25 μl) were added to each membrane sample (150 μl), and the tubes were incubated for 2 h at room temperature and then filtered through glass-fiber filters and counted. Samples were assayed in triplicate. Nonspecific binding was measured in the presence of NECA (100 μM).
Statistical analysis
Percentage maximal cytokine production was determined by: % Max = 100 – [(vehicle control treated with LPS – untreated) – (NECA with LPS – untreated)]/(vehicle control treated with LPS – untreated) × 100. Data are reported as means ± SEM. The two-tailed Student’s t test assuming unequal variance was performed using Microsoft Excel software. A p value of <0.05 was considered significant and is denoted by an asterisk.
Results
LPS-induced maturation of BMDC is modulated by an adenosine analog
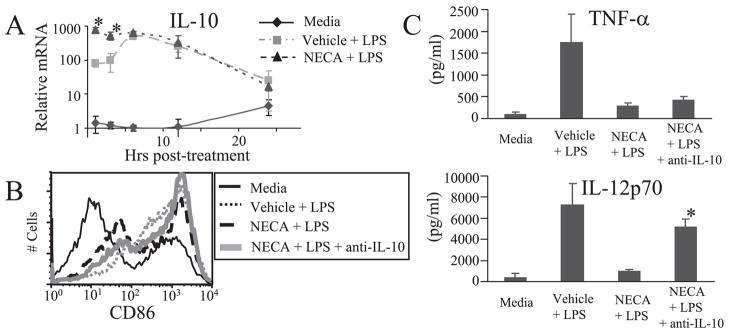
The stable, nonselective adenosine analog NECA was used to determine the role of adenosine receptors in regulating DC maturation and function in response to an inflammatory stimulus. BMDC were stimulated with LPS in the presence of NECA or DMSO vehicle control and assessed for cytokine production, costimulatory marker, and MHC-II expression, as well as the capacity to promote CD4+ T cell activation. Because previous studies have shown that TNF-α, IL-12, and IL-10 expression are regulated by adenosine receptors in some APC (26, 34), we determined whether these cytokines are modulated in murine BMDC. We found that NECA significantly inhibited TNF-α and IL-12 in a concentration-dependent manner, whereas the antiinflammatory cytokine IL-10 was increased (Fig. 1A). IL-10 was further investigated by real-time RT-PCR and found to be rapidly up-regulated by NECA (Fig. 2A). In the absence of inflammatory stimulus, NECA had little effect on cytokine production. As MHC-II and costimulatory molecules are important in CD4+ T cell activation by DC, we characterized the effect of NECA on BMDC expression of MHC-II, CD40, CD80, and CD86. NECA significantly impaired expression of MHC-II and CD86 in LPS-stimulated BMDC as compared with vehicle control, but had little effect on CD40 or CD80 (Fig. 1B and Table I). Interestingly, the MHC-II and CD86 inhibition was consistently observed in a subset of BMDC but not in the entire population. As an additional measure of DC maturation and function, LPS-stimulated BMDC were treated with NECA or vehicle control for 18 h and then washed thoroughly and cocultured with CD4+ T cells. An OVA323–339-specific coculture with CD4+ T cells derived from OT-II mice, as well as a one-way MLR with C57BL/6 BMDC and BALB/c CD4+ T cells, was employed. After 24 – 48 h supernatant was collected and assayed for IL-2 as a measure of T cell activation. Significantly less IL-2 was produced with NECA-treated BMDC in both the OVA323–339-specific coculture and the one-way MLR, as compared with vehicle control-treated BMDC (Fig. 1C). To determine the role that antiinflammatory molecules such as IL-10 or TGFβ may play in mediating the observed effects of NECA, studies were conducted using neutralizing Abs. NECA impairment of IL-12 and CD86, but not TNF-α, was largely reversed by a neutralizing IL-10 Ab (Fig. 2, B and C). In contrast, a neutralizing Ab to TGFβ had little effect (data not shown).
FIGURE 1.
The nonselective adenosine analog NECA modulates LPS-induced BMDC maturation. A, MACS CD11c+ BMDC were treated with 1 μM NECA or DMSO vehicle control in the presence or absence of 500 ng/ml LPS, and after 18 h supernatant was collected. TNF-α, IL-12p70, and IL-10 were assayed by ELISA. Data represent the mean ± SEM from three or more experiments B, Representative histogram of CD86 expression 24 h after BMDC treated in the presence or absence of LPS and 250 nM NECA or vehicle control. C, IL-2 from cocultures with CD4+ T cells. Before coculture, BMDC were treated in the presence or absence of LPS and NECA overnight and then washed thoroughly. In the syngeneic coculture BMDC were pulsed with OVA during overnight incubation and then cultured with OT-II CD4+ T cells for 24 h. Data represent the mean ± SEM from three experiments. In the allogeneic study BMDC from C57BL/6 mice were cultured with CD4+ T cells from BALB/cJ mice for 48 h. Data represent the mean ± SEM from two experiments conducted in duplicate. *, p < 0.05.
FIGURE 2.
IL-10 is an important mediator in the regulation of BMDC by NECA. A, BMDC were treated in presence or absence of LPS and 250 nM NECA. At various time points, cells were lysed, RNA was isolated, and real-time RT-PCR was conducted to determine IL-10 mRNA levels. Transcripts were normalized to the 1-h media control treatment group. Data represent the mean ± SEM from three or more experiments. B, Representative histogram of CD86 expression 24 h after BMDC treated in the presence or absence of LPS, 250 nM NECA, or 10 μg/ml anti-IL-10 neutralizing Ab. C, BMDC were treated with 1 μM NECA or DMSO vehicle control in the presence or absence of 500 ng/ml LPS, and after 18 h supernatant was collected. Anti-IL-10 neutralizing Ab (10 μg/ml) was added where indicated. TNF-α and IL-12p70 were assayed by ELISA. Data represent the mean ± SEM from two experiments conducted in duplicate. *, p < 0.05.
Table I.
MHC-II, CD40, CD80, and CD86 expression on BMDC: cells treated with NECA and LPS are compared to relevant controlsa
| Mean Fluorescence Intensity |
||||
|---|---|---|---|---|
| MHC-II | CD40 | CD80 | CD86 | |
| Media | 993 ± 81 | 5.6 ± 0.3 | 343 ± 65 | 317 ± 47 |
| Vehicle + LPS | 1060 ± 60 | 11.8 ± 1.1 | 1111 ± 323 | 927 ± 107 |
| NECA + LPS | 781 ± 52* | 8.9 ± 0.4 | 1131 ± 304 | 616 ± 87* |
Averaged mean fluorescence intensity ± SEM from three or more experiments.
p < 0.05.
Adenosine receptor expression in DC
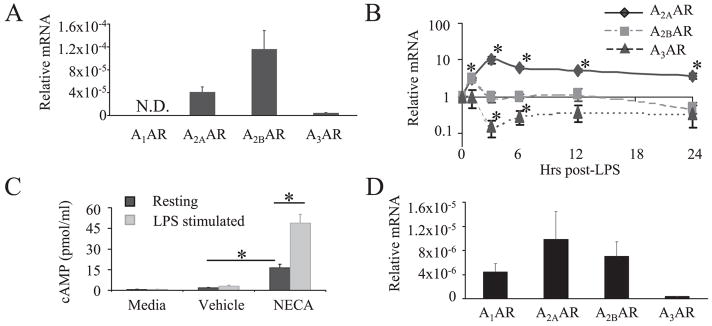
To determine which adenosine receptors modulate BMDC maturation, we first determined which receptors were expressed in BMDC using real-time RT-PCR. A2AAR, A2BAR, and A3AR, but not A1AR, transcripts were detected to varying degrees in resting BMDC (Fig. 3A), with A2BAR being the predominant transcript in resting cells. Since previous studies have shown that A2AAR is substantially up-regulated in other immune cells in response to activation, we characterized adenosine receptor transcripts in LPS-stimulated BMDC (2, 17). As shown in Fig. 3B, A2AAR transcripts were significantly induced in BMDC, peaking at 3 h poststimulation with a 10.1-fold increase over resting levels. A2BAR transcripts increased less dramatically, showing a significant elevation of 2.9-fold at 1 h and then returning to baseline. In contrast to A2AAR and A2BAR, A3AR levels decreased upon LPS stimulation and the A1AR transcript was still not detected. Accumulation of intracellular cAMP can be used as a measure of functional A2AAR and A2BAR activity because both receptors are Gs-coupled. Thus, to validate that adenosine receptor mRNA correlates with the expression of functional receptors, cAMP assays were conducted. As shown in Fig. 3C, BMDC treated with NECA had significantly elevated cAMP levels as compared with vehicle control-treated cells. Moreover, BMDC that had been stimulated overnight with LPS produced significantly more cAMP than did resting cells in response to NECA. To further characterize adenosine receptor expression in an important in vivo DC population, we enriched for CD11c+ cells from small intestine lamina propria. Although these cells expressed a different adenosine receptor profile than our BMDC, A2BAR was still expressed at relatively high levels (Fig. 3D).
FIGURE 3.
DC expression of adenosine receptors as measured by real-time RT-PCR and cAMP functional studies. A, Adenosine receptor mRNA in resting BMDC. Data represent the mean ± SEM from 10 or more experiments B, Kinetics of adenosine receptor mRNA expression in LPS-stimulated BMDC. Each gene transcript was compared with resting transcript levels. Data represent the mean ± SEM from three experiments. C, cAMP produced by BMDC treated with 250 nM NECA or DMSO vehicle control for 15 min in presence of 1 μM rolipram. In some cases cells had been treated overnight with 500 ng/ml LPS. Data represent the mean ± SEM from three or more experiments. D, MACS CD11c-enriched cells were isolated from intestinal lamina propria as described in Materials and Methods, and adenosine receptor mRNA was evaluated. Data represent the mean ± SEM from three experiments.*, p < 0.05.
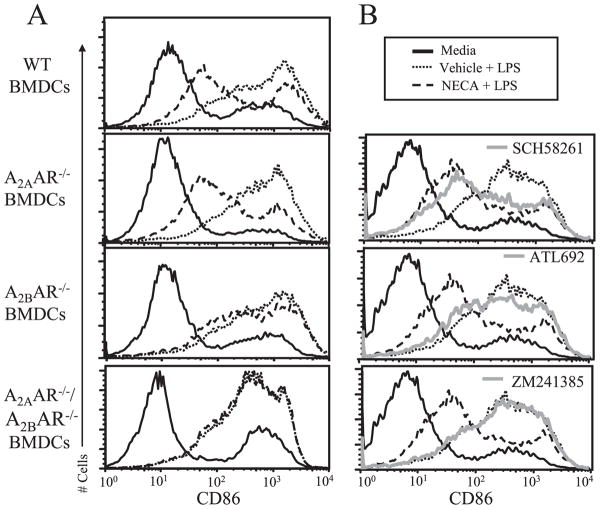
NECA impairment of BMDC maturation requires the A2B adenosine receptor
Having determined that A2AAR and A2BAR are the predominant adenosine receptors expressed in BMDC, additional experiments were conducted to define the relative contribution of these receptors in modulating BMDC maturation. Responses to NECA were compared in BMDC from wild-type mice or from mice deficient for various adenosine receptors. As a complementary approach, adenosine receptor antagonists selective for A2AAR and/or A2BAR (Table II) were also used. NECA modulation of TNF-α, IL-12, and IL-10 was significantly ablated in A2BAR−/− as compared with wild-type BMDC (Fig. 4A). In contrast, cytokine production in A2AAR−/− BMDC treated with NECA differed little from wild-type cells. Importantly, neither baseline nor LPS-induced cytokine levels differed substantially between wild-type and knockout mice (supplemental Table I).4 To further probe adenosine receptor regulation of cytokine responses, experiments were conducted with BMDC deficient for A3AR or both A2AAR and A2BAR. Whereas NECA modulated A3AR−/− BMDC comparable to wild-type, it had essentially no effect on A2AAR−/−/A2BAR−/− BMDC (supplemental Fig. 1). Preincubation of wild-type BMDC with the mixed A2AAR/A2BAR antagonist ZM241385 or the A2BAR selective antagonist ATL692 right-shifted NECA dose-response curves (Fig. 4B), whereas the A2AAR selective antagonist SCH58261 had a less substantial effect. Taken together, both genetic and pharmacologic approaches suggest that NECA modulation of TNF-α, IL-12, and IL-10 in LPS-stimulated BMDC is mediated predominantly by activation of the A2BAR, with a small contribution from A2AAR. Because a recent study using BMDC generated with both GM-CSF and IL-4 implicated A1AR in regulating DC maturation (29), we also assessed the expression and function of A2BAR in BMDC cultivated with 3 ng/ml GM-CSF and 3 ng/ml IL-4. BMDC prepared in this manner still did not express A1AR and expressed comparable levels of A2BAR and A3AR. Interestingly, A2AAR was elevated ~5-fold in BMDC cultivated with both GM-CSF and IL-4 as compared with GM-CSF alone (supplemental Fig. 2A). Using the pharmacologic approach it was determined that A2AAR plays a more substantial role in regulating LPS-induced cytokine responses in these cells; however, A2BAR is still important (supplemental Fig. 2B).
Table II.
Antagonist binding affinities for mouse adenosine receptorsa
| Mean Ki (nM ± SEM) |
||||
|---|---|---|---|---|
| A1AR | A2AAR | A2BAR | A3AR | |
| ZM241385 | 3,743 ± 2,154 | 1.5 ± 1.0 | 41 ± 7 | >10,000 |
| SCH58261 | 172 ± 72 | 0.8 ± 0.08 | 321 ± 57 | >10,000 |
| ATL692 | 6,856 ± 2,396 | 8,809 ± 4,073 | 3.3 ± 0.8 | >10,000 |
Ki values are mean ± SEM from three or more independent experiments.
FIGURE 4.
NECA modulates LPS-induced TNF-α, IL-12, and IL-10 in an A2BAR-dependent manner. BMDC were treated with LPS in the presence or absence of varying concentrations of NECA. Supernatants were collected after 18 h and cytokines were measured by ELISA. A, BMDC derived from wild-type, A2AAR−/−, or A2BAR−/− mice. B, Wild-type BMDC were pretreated for 15 min with 100 nM ZM241385, SCH58261, or ATL692 before addition of NECA and LPS. Data represent the mean ± SEM from three or more experiments. *, p < 0.05.
The relative contribution of A2AAR and A2BAR in mediating NECA inhibition of CD86 was also investigated. Whereas NECA impaired CD86 expression in wild-type and A2AAR−/− BMDC, this impairment was largely lost in A2BAR−/− BMDC (Fig. 5A and supplemental Fig. 3A). Similar experiments were also conducted with BMDC lacking A3AR or both A2AAR and A2BAR. NECA inhibited CD86 in A3AR−/− BMDC comparable to wild-type, whereas the effect was completely abolished in the A2AAR−/−/A2BAR−/− BMDC (Fig. 5A and supplemental Fig. 1B). Preincubation of wild-type BMDC with the mixed A2AAR/A2BAR antagonist ZM241385 blocked NECA impairment of CD86, confirming that NECA acts through some combination of A2AAR and A2BAR to achieve its effect. The A2AAR selective antagonist SCH58261 had little effect on the NECA response, whereas the A2BAR selective antagonist ATL692 largely deterred NECA actions on CD86 (Fig. 5B and supplemental Fig. 3B). Collectively, these data suggest that NECA impairment of CD86 is mediated predominantly through A2BAR, and they can be accounted for entirely by the combined effects of A2AAR and A2BAR.
FIGURE 5.
NECA inhibits LPS-induced CD86 expression in an A2BAR-dependent manner. BMDC were treated with LPS in the presence or absence of 250 nM NECA. Cells were harvested after 24 h, stained with anti-CD86-PE or isotype control, and assayed by flow cytometry. A, Representative histograms of BMDC derived from wild-type, A2AAR−/−, A2BAR−/−, or A2AAR−/−/A2BAR−/− mice. B, Representative histograms of wild-type BMDC pretreated for 15 min with 1 μM ZM241385, SCH58261, or ATL692 before addition of NECA and LPS. All histograms are representative of at least three independent experiments.
NECA-treated CD86low BMDC do not support CD4+ T cell activation
NECA clearly impairs CD86 expression in LPS-stimulated BMDC (Fig. 1B and Table I); however, in all experiments conducted this response was incomplete and a subset of BMDC maintained elevated CD86 expression. This was the case even when higher concentrations of NECA were used, indicating that it is not simply a dose effect (data not shown). We sought to determine whether the NECA-responsive and nonresponsive populations differed in their expression of adenosine receptors and whether they had different functional capacities. BMDC stimulated with LPS in the presence of NECA for 16 h were harvested, stained with anti-CD86 Ab, and then sorted into CD86low and CD86high subsets (Fig. 6A). The CD86low population expressed 2.6 ± 0.2-fold more A2BAR mRNA than did the CD86high population, whereas for A2AAR the opposite was observed and CD86low cells expressed 3.6 ± 0.8-fold less than did CD86high cells (Fig. 6B).
FIGURE 6.
NECA-treated CD86low BMDC express more A2BAR and altered cytokine response as compared with NECA-treated CD86high or vehicle control-treated BMDC. NECA-treated, LPS-stimulated BMDC were harvested after 16 h and sorted into CD86low and CD86high populations. These sorted cells were compared with unsorted BMDC that were untreated or with LPS-stimulated cells in the presence of the vehicle control. A, Representative histograms showing NECA-treated, LPS-stimulated BMDC sorted for CD86 expression. B, A2AAR and A2BAR mRNA in BMDC. Data represent the mean ± SEM from three independent experiments. C, Cytokine mRNA in BMDC. Transcripts were standardized to the media-treated control group and represent the mean ± SEM from three independent experiments. *, p < 0.05.
Expression of TNF-α, IL-12p40, and IL-10 mRNA was also evaluated in the NECA-treated CD86low and CD86high populations, as well as unsorted control populations (Fig. 6C). Compared with unsorted BMDC treated with vehicle control, both the CD86low and CD86high NECA-treated populations had significantly reduced TNF-α mRNA. IL-12p40 mRNA was significantly reduced in NECA-treated CD86low cells as compared with both unsorted vehicle-treated BMDC and NECA-treated CD86high cells. NECA-treated CD86low cells expressed 2.9 ± 0.4 more IL-10 mRNA than did comparably treated CD86high cells.
Because the NECA-treated CD86low and CD86high populations had unique adenosine receptor and cytokine profiles, we further investigated the two populations by flow cytometry for DC lineage and maturation markers. Compared with each other, or to vehicle control-treated cells, there was no evidence that NECA-treated CD86low or CD86high populations differentially expressed CD4, CD8, GR-1, CD11b, or CD103, markers associated with different DC subsets (30, 35) (data not shown). Cell surface maturation markers such as MHC-II, CD40, and CD80 were also compared in the CD86low and CD86high BMDC to determine whether these markers co-associate with CD86 expression. Only MHC-II was found to segregate with CD86, as CD86low cells expressed substantially less MHC-II than did CD86high or vehicle control cells (data not shown).
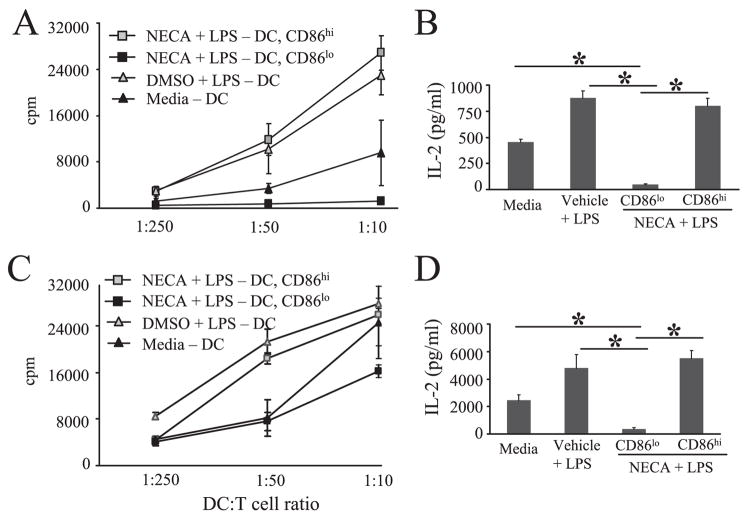
We previously demonstrated that NECA treatment of LPS-stimulated BMDC inhibited CD4+ T cell activation as measured by IL-2 production (Fig. 1C). Because CD86 costimulation is important for T cell activation, we hypothesized that the CD86low subset of NECA-treated BMDC was primarily responsible for this effect. Thus, sorted CD86low and CD86high BMDC that had been treated with NECA and LPS were compared for capacity to support CD4+ T cell activation and proliferation in syngeneic and allogeneic co-culture experiments. As shown in Fig. 7, both IL-2 production and T cell proliferation were impaired in cocultures with the CD86low BMDC, as compared with the CD86high BMDC or compared with unsorted control BMDC.
FIGURE 7.
The CD86low subset of NECA-treated, LPS-stimulated BMDC have impaired immunogenicity. A, MLR with BMDC derived from C57BL/6 mice and CD4+ T cells from BALB/c mice. Sorted CD86low and CD86high NECA-treated BMDC and unsorted control BMDC were irradiated and then cocultured with CD4+ T cells for 96 h at indicated ratios, and 1 μCi of [3H]thymidine was added for the final 16 h. This is representative of three experiments conducted. B, MLR using 1:10 ratio of BMDC to CD4+ T cells. Supernatant was harvested at 48 h and assayed for IL-2. Data represent the mean ± SEM from three independent experiments. C, Syngeneic coculture with sorted CD86low and CD86high ADO-BMDC and unsorted control cells. BMDC were irradiated and then cocultured with CD4+ T cells for 72 h with 500 ng/ml soluble anti-CD3 Ab at indicated ratios with 1 μCi of [3H]thymidine added for the final 16 h. This is representative of three experiments conducted. D, Syngeneic coculture using nonirradiated BMDC and conducted at 1:10 ratio of BMDC to CD4+ T cells. Supernatants were harvested at 24 h and assayed for IL-2. Data represent the mean ± SEM from three independent experiments. *, p < 0.05.
Discussion
Adenosine is an important regulator of inflammatory and immune responses, and recent studies have clearly shown that this includes regulation of T cell responses (11, 12, 16, 17). Genetic ablation or pharmacologic blockade of A2AAR or A2BAR leads to overexuberant immune responses and pathology (4, 7, 36). Conversely, adenosine analogs are protective in many models of inflammatory disease (11–15). While the direct effects of adenosine on T cells have been studied in some detail, substantially less is known about the indirect effects that occur due to the effects of adenosine on the function of DC. The present study demonstrates that A2BAR modulates murine BMDC maturation such that their immunogenicity is altered.
Although previous efforts have been made to examine adenosine receptor expression and function on DC, these studies have proved incomplete, and in some cases conflicting. Panther et al. reported that adenosine impairs TNF-α and IL-12 responses to LPS in human monocyte-derived DC, while enhancing IL-10 production (26). However, adenosine did not affect LPS-induced CD86 or MHC-II expression, nor did adenosine treatment impair their capacity to promote CD4+ T cell proliferation in an MLR. The authors suggest that the observed responses were mediated via the A2AAR, whereas A2BAR was not even expressed (25). In contrast, Hofer et al. reported that adenosine slows the migration of human monocyte-derived DC, but has no effect on cytokine production or surface marker expression in cells stimulated with a cocktail of TNF-α, IL-1β, IL-6, and prostaglandin E2 (27). A2BAR mRNA was detected in their cells, but at very low levels compared with A2AAR or A3AR. Differences in the stimuli used to mature the DC and/or in the protocols used to harvest and generate the DC may explain the conflicting results.
To investigate the role of adenosine receptors in regulating murine DC, we elected to study BMDC. These cells can be generated in large numbers by in vitro culture and are widely used as a model myeloid DC (37). We found that the nonselective adenosine analog NECA had little measurable effect on TNF-α, IL-12, IL-10, CD86, or MHC-II responses on resting, nonstimulated BMDC. However, in the context of an LPS stimulus, NECA significantly modulated these markers. Our data indicate that IL-10, but not TGFβ, is an important mediator in the regulation of IL-12 and CD86 responses by NECA. Interestingly, TNF-α does not seem to be under the control of IL-10 in this system, suggesting that adenosine differentially regulates these responses. Our combined approach of using adenosine receptor knockout mice and pharmacologic inhibitors strongly suggests that A2BAR is the predominant immunoregulatory receptor in BMDC. This observation is consistent with the report by Yang et al. indicating a prominent regulatory role for A2BAR in another APC, the peritoneal macrophage (7). This finding is also consistent with two recent reports demonstrating that A2BAR modulates DC development and function (38, 39). However, the A2BAR finding does conflict with a previous study with DC. For instance, Desrosiers et al. concluded that adenosine regulates BMDC cytokine and CD86 expression via A1AR (29). Although we did not directly address A1AR in our studies with A1AR−/− mice or selective antagonists to the A1AR, A1AR mRNA was not detectable in our BMDC, and moreover the observed effects of NECA seem to be entirely accounted for by the combined effects of A2AAR and A2BAR. One explanation for this discrepancy may be in the protocols used to generate or manipulate BMDC. For instance, our BMDC were cultivated with 20 ng/ml GM-CSF, in contrast to 3 ng/ml GM-CSF and 3 ng/ml IL-4 in the previous report. To address this we directly compared adenosine receptor expression in BMDC generated with both GM-CSF and IL-4 to our typical BMDC preparation. A2BAR expression was found to be similar in both BMDC preparations, and the receptor continued to have a substantial functional role. However, it was interesting that both the expression and function of A2AAR was enhanced in BMDC cultivated with GM-CSF and IL-4. Thus, while the data presented herein show that A2BAR was predominantly responsible for immunoregulatory effects in the BMDC population we studied, we cannot exclude a role for other adenosine receptors in regulating DC prepared by other means or isolated from distinct tissues. Accordingly, while preliminary studies indicate that DC enriched from intestinal lamina propria express A2BAR, it will be important in future studies to examine the functional role of A2BAR in this DC population.
The data reported herein also suggest that BMDC are a heterogeneous population with varying capacity to respond to NECA. CD86 expression was clearly impaired in a subset of BMDC that were stimulated with LPS in the presence of NECA, but some BMDC still expressed elevated CD86. This heterogeneity may reflect the presence of unique DC subsets, or alternatively may indicate that BMDC are at different maturation stages before being treated with NECA. Although it is not evident why two different populations emerge, it is clear that the NECA-responsive CD86low population expresses a unique adenosine receptor and cytokine expression “signature” and has reduced capacity to stimulate CD4+ T cells, as compared with the comparably treated CD86high cells. The NECA responsive CD86low population, characterized as IL-12p40low, IL-10+, CD86low, MHC-IIlow, and with poor capacity to stimulate CD4+ T cells, has features descriptive of a tolerogenic DC (40, 41). It is not clear whether enhanced A2BAR expression facilitates NECA impairment of CD86 in a subset of cells and/or whether A2BAR is elevated as a result of NECA treatment. It also remains to be seen whether NECA confers a unique phenotype on stimulated BMDC, or whether NECA treatment selects for a preexisting population of BMDC with reduced immunogenicity. Another outstanding question is whether NECA responsive CD86low BMDC favor regulatory T cells and thus active suppression, or whether deficient costimulation leads to anergic CD4+ T cells. Future studies will characterize the role that adenosine receptors play in regulating other DC populations and determine whether adenosine favors tolerance in vivo through action on DC. Illumination of adenosine’s role in controlling host responses will have implications for the therapeutic use of adenosine agonists and antagonists to treat inflammatory disorders.
Acknowledgments
We thank Joanne Lannigan, Mike Solga, and Paul Brewer for technical assistance.
Footnotes
This work was supported by the Crohn’s and Colitis Foundation of America (CCFA); by National Institutes of Health Grants DK50980, AI069880, AI070491 (to P.B.E.), and AI075526 (to Richard Guerrant for ATL compound development); and by the Immunology and Cell Isolation Core as well as the Molecular Biology Core of the University of Virginia Digestive Health Research Center (DK67629).
Abbreviations used in this paper: AR, adenosine receptor; ATL692, 1,3-dialkyl-8-hetero substituted xanthine; DC, dendritic cell; NECA, 5′-N-ethylcarboxamidoadenosine; SCH58261, 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; ZM241385, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-α][1,3,5] triazin-5-ylamino]ethyl)phenol.
The online version of this article contains supplemental material.
Disclosures
Drs. R. Figler, J. Linden, and J. Rieger own shares in Adenosine Therapeutics.
References
- 1.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NFκB in A2A adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 4.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 5.Lukashev DE, Smith PT, Caldwell CC, Ohta A, Apasov SG, Sitkovsky MV. Analysis of A2a receptor-deficient mice reveals no significant compensatory increases in the expression of A2b, A1, and A3 adenosine receptors in lymphoid organs. Biochem Pharmacol. 2003;65:2081–2090. doi: 10.1016/s0006-2952(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, Hilaire CS, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 9.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-γ production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358:261–268. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- 10.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 11.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting Edge: critical role for adenosine A2A receptors in the T cell mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 12.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okusa MD, Linden J, MacDonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 14.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth ZH, Bleich D, Csoka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabo C, Cronstein BN, Hasko G. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 19.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine mono-phosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 21.Borsellino G, Kleinewietfeld M, Di MD, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 22.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 23.Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host-pathogen interface. Nat Immunol. 2006;7:117–120. doi: 10.1038/ni0206-117. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 25.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 26.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 27.Hofer S, Ivarsson L, Stoitzner P, Auffinger M, Rainer C, Romani N, Heufler C. Adenosine slows migration of dendritic cells but does not affect other aspects of dendritic cell maturation. J Invest Dermatol. 2003;121:300–307. doi: 10.1046/j.1523-1747.2003.12369.x. [DOI] [PubMed] [Google Scholar]
- 28.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–1397. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 29.Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007;179:1884–1892. doi: 10.4049/jimmunol.179.3.1884. [DOI] [PubMed] [Google Scholar]
- 30.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 31.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(− ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 35.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 36.Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 37.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 38.Ben AA, Lefort A, Hua X, Libert F, Communi D, Ledent C, Macours P, Tilley SL, Boeynaems JM, Robaye B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A2B receptor. Eur J Immunol. 2008;38:1610–1620. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- 39.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 41.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]