Abstract
Exposure to pro-oxidants and defects in the repair of oxidative base damage are associated with disease and ageing and also contribute to the development of anaemia, bone marrow failure and haematopoietic malignancies. This Review assesses emerging data indicative of a specific role for the RB tumour suppressor pathway in the response of the haematopoietic system to oxidative stress. This is mediated through signalling pathways that involve DNA damage sensors, forkhead box O (Foxo) transcription factors and p38 mitogen-activated protein kinases and has downstream consequences for cell cycle progression, antioxidant capacity, mitochondrial mass and cellular metabolism.
Mammalian cell cycle checkpoints are activated in response to intracellular and extracellular stresses, such as growth factor deprivation, nucleotide depletion and DNA damage, and the retinoblastoma (RB) tumour suppressor is central in enforcing these checkpoints1–3. This stress response function of RB is the key to understanding the importance of the BMI1–INK4A–cyclin D–RB–E2f axis in preventing aberrant proliferation and tumour growth4–6 (FIG. 1). The signalling pathways that regulate the dephosphorylation and activation of RB in response to serum deprivation and DNA damage and result in cell cycle arrest are well-described6–8 (BOX 1). In particular, growth factor stimulation of Ras activity promotes activator protein 1-dependent transcriptional induction of cyclin D1, whereas serum-dependent induction of MYC has a similar role for transactivation of cyclin D2 (REF. 9). D-type cyclin expression promotes the activity of cyclin dependent kinase 4 (CDK4) and CDK6 in phosphorylating RB, leading to its inactivation and de-repression of E2f transcription factors6,10. Conversely, DNA damage shuts down E2f target gene expression by promoting RB dephosphorylation, mediated in part through induction of p53-dependent expression of p21, which binds to and represses CDK2 activity11, and also through the activation of protein phosphatase 2A (PP2A)12,13. Recent reports have suggested a link between the accumulation of reactive oxygen species (ROS) and cell cycle progression14, but a mechanistic link between increased levels of ROS and cell cycle control has not been established. New data links increased ROS to activation of the RB pathway in the haematopoietic system and this Review focuses on our current understanding of the role of the BMI1–INK4A–cyclin D–RB–E2f axis in responding to oxidative stress through crosstalk with other regulatory pathways to determine rates of cellular proliferation, stem cell self-renewal and terminal differentiation in the blood system.
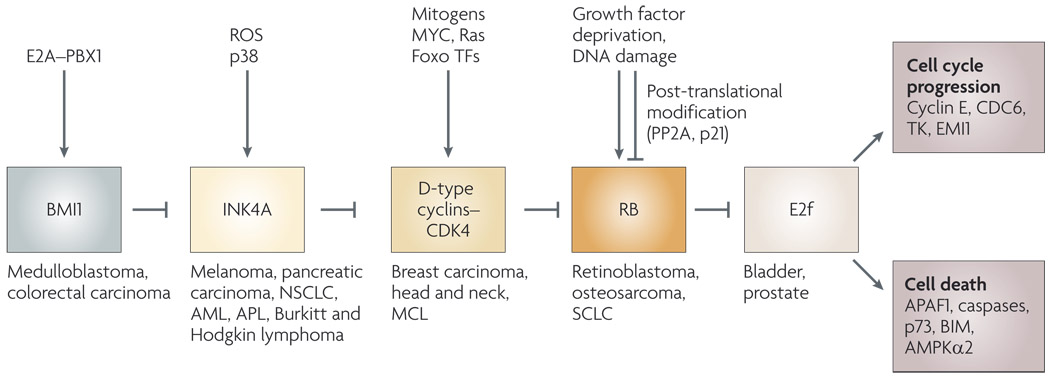
Figure 1. The retinoblastoma (RB) pathway in stress responses and cancer.
As originally proposed by Weinberg138, the RB tumour suppressor is functionally inactivated in most human cancers, either directly by RB1 deletion or mutation, or indirectly by deregulation of upstream components of the RB pathway, such as the D-type cyclins, the cyclin-dependent kinase (Cdk) inhibitor INK4A or the polycomb protein BMI1 (involved in repression of the CDKN2A locus, which encodes INK4A and ARF)4,132. Human tumour types in which components of the RB pathway are deregulated are indicated. The selective pressure to inactivate RB is removed in cancers that have thus disrupted upstream components of the RB pathway, such as INK4A158 or cyclin D1 (REF. 159), or indeed downstream events such as the amplification of E2F3 in human bladder and prostate cancers160,161. Signalling through the RB pathway determines changes in expression of E2f target genes that regulate cell cycle progression, including E-type cyclins, cell division cycle 6 (CDC6), thymidine kinase (TK) and EMI1 (REF. 10). In the absence of pro-survival signals, E2f can transactivate pro-apoptotic target genes, including apoptotic protease-activating factor (APAF1) (REF. 162), caspases163, AMPKα2 (REF. 164) and key BH3-only proteins, such as BIM165. Extrinsic signals and stresses feed directly into the RB pathway to regulate the cell cycle through various mechanisms, including transcriptional activation of D-type cyclins in response to serum stimulation signalling through Ras and MYC9 as well as their transcriptional repression by Foxo transcription factors (TFs). Dephosphorylation and activation of RB is mediated by protein phosphatase 2A (PP2A) and Cdk inhibition in response to DNA damage12,166, whereas induction of INK4A occurs in response to reactive oxygen species (signalling through p38 mitogen-activated protein kinase54 or induction of BMI1 by the protein products of chromosomal translocations, such as E2A–PBX1 (REF. 167)). AML, acute myeloid leukaemia; APL, acute promyelomonocytic leukaemia; MCL, mantle cell lymphoma; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung carcinoma.
Box 1 Summary of the properties and functions of RB
The retinoblastoma (RB) tumour suppressor is functionally inactivated in the majority of human cancers, either directly through deletion or mutation of RB1, or indirectly as a consequence of deregulated activity of upstream factors (such as the cyclin-dependent kinase (Cdk) inhibitor INK4A or the D-type cyclins)4,138.
RB1 encodes a nuclear phosphoprotein (RB) of 110–115 kDa that is characterized by a hydrophobic ‘pocket domain’ at its carboxyl terminus through which most of its protein–protein interactions are mediated, including its interactions with E2f transcription factors, viral oncoproteins and other proteins that contain the RB-interacting LxCxE motif. The pocket domain is conserved in the two other Rb family members, p107 and p130, although neither deletion nor mutation of these factors is known to predispose to human cancer139.
RB acts to repress the activity of E2F1, E2F2 and E2F3 through recruitment of chromatin remodelling enzymes, including histone deacetylases and BRG1–BRM1 (subunits of SWI–SNF chromatin remodelling complexes) during cellular quiescence, and the histone methyltransferase SUV39H1 during cellular senescence. This activity of RB is referred to as ‘active repression’140–142. E2f target genes include those involved in cell cycle progression and components of the mitotic checkpoint, such as MAD2 (REFS 10,143).
The interaction of RB with E2fs is disrupted by phosphorylation of RB on 13 serine and threonine residues, mediated by CDK4 and CDK6 when they are associated with their cognate D-type cyclin partners, and by CDK2 when it is bound to E-type cyclins144. RB is required for cell cycle arrest associated with cellular quiescence, senescence and terminal differentiation in different tissues. Inactivation of RB leads to aberrant cell cycle of post-mitotic cells in vivo and in vitro129,145.
RB also promotes cellular survival, as shown by the cell death observed in the developing nervous system, liver and ocular lens of Rb1-null mice, by the effects of overexpressing E2fs or inactivating RB (as well as p107 and p130) with DNA tumour virus-encoded oncoproteins (such as SV40 T antigen, human papillomavirus E7 and adenovirus E1A) and by the increased sensitivity of RB-deficient cells to death induced by DNA-damaging agents146,147.
ROS and the cell cycle
ROS are highly reactive molecules produced by the one-electron reduction of oxygen. They include oxygen anions, such as superoxide and the hydroxyl radical, as well as hydrogen peroxide and peroxynitrite, which arises from the reaction of superoxide with nitric oxide15,16. They are primarily generated following release of electrons from complex I and complex III of the electron transport chain at the mitochondria17,18, and to a lesser extent by peroxisomes and by the activity of NADPH oxidases (Nox) at the plasma membrane19 (BOX 2). They have important functions in cell signalling in general but their role in regulating cell cycle progression is poorly understood. ROS levels increase significantly as cells pass from G1 into S phase of cell cycle14,20 and ROS are required for S phase entry, as demonstrated by the cell cycle arrest induced by quenching ROS14,21. However, cell cycle checkpoints are also activated by increased ROS22, indicating that cellular proliferation relies on maintaining ROS levels within a functional range. ROS have been proposed to modulate the cell cycle in a variety of ways, including through effects on the activity of enzymes with redox-sensitive amino acids at their catalytic sites20 that are required for cell cycle progression. These include ribonucleotide reductase, which is crucial for nucleotide biosynthesis23. ROS have also been proposed to inhibit the turnover of key cell cycle progression factors by as yet unknown mechanisms. For example, ROS stabilize levels of F-box only protein 5 (FBXO5, also known as EMI1)14, an E2f target gene that inhibits the anaphase-promoting complex (APC) in S and G2 phases of cell cycle by acting as a pseudo-substrate, thereby preventing premature entry into mitosis24.
Box 2 Reactive oxygen species and the ‘free radical theory of ageing’
Originally synonymous with the ‘rate of living’ hypothesis, which argued that the metabolic rate of animals determined life expectancy, the ‘free radical theory of ageing’ postulated that endogenous oxygen radicals induced cumulative oxidative damage to DNA, proteins and lipids that explained cellular degeneration and loss of function over the lifetime of the animal15.
Diatomic oxygen is a potent acceptor of electrons, and highly reactive superoxide and hydrogen peroxide radicals generated by one-electron reduction of oxygen at the mitochondria are the major forms of intracellular oxygen radicals, despite the scavenging activity of mitochondrial superoxide dismutase (mSOD) and cytochrome c in the inter-membrane space16. Factors limiting the escape of electrons from the mitochondrial respiratory chain include the availability of ADP and, ironically, oxygen, which is required in sufficient but not excess quantities to accept four electrons per molecule to form water, rather than free radicals148.
Loss of mitochondrial integrity induced by peroxidation of mitochondrial lipids or damage to mitochondrial proteins that are in close proximity to the source of reactive oxygen species (ROS) leads to more ROS production that feeds back in a vicious cycle to cause more damage.
Telomere attrition, which increases with age, explains aspects of cellular senescence and failed tissue regeneration149,150. Additionally, the age-dependent increase in INK4A expression may be directly attributed to the generation of ROS that activate p38 mitogen-activated protein kinase to promote INK4A transcription, thereby explaining aspects of the role of ROS in cellular senescence, reduced stem cell self-renewal and ageing18,25.
At a glance
Reactive oxygen species (ROS) produced at the mitochondrial respiratory chain and to a lesser extent by peroxisomes and plasma membrane-bound NADPH oxidases have a crucial role in signalling pathways in the cell, including a requirement for ROS to promote S phase entry.
Excess ROS can lead to cell cycle arrest, senescence or cell death and thus proper management of ROS is required to prevent premature ageing and disease.
Increased ROS levels in the haematopoietic system lead to stem cell depletion and bone marrow failure and are associated with activation of the retinoblastoma (RB) pathway through induction of the cyclin-dependent kinase inhibitor INK4A.
RB is required to maintain the haematopoietic stem cell (HSC) pool and prevent aberrant S phase entry of HSCs in response to proliferative stress.
Defects in Rb1-deficient haematopoiesis are due in part to non-cell-autonomous events, including the influence of the bone marrow niche on the development of myeloproliferative disease and the sensitivity of stem cells and erythroid cells to proliferative stress. These observations are indicative of a role for RB in regulating the stromal microenvironment.
Disruption of ataxia telangiectasia mutated (ATM) or the Forkhead box O (Foxo) transcription factors in mice leads to increased ROS levels in HSCs that induce p38 mitogen-activated protein kinase activity, INK4A expression, downregulation of N-cadherin and increased stem cell recruitment into the cell cycle, probably due to reduced expression of the cyclin-dependent kinase inhibitors p21 and p27. Thus, ATM and Foxo act upstream of RB in the response of the cell to ROS.
Proliferative stress also induces erythroid maturation defects in the Rb1-null mouse that include failure to exit the cell cycle during terminal differentiation, increased ROS levels, increased DNA damage and altered management of mitochondrial mass.
Acetylation of RB modulates its rate of turnover in response to oxidative stress and protein phosphatase 2A-dependent dephosphorylation modulates its activity.
The role of the RB pathway in responding to oxidative stress in the haematopoietic system has implications for how other cell types respond to ROS, including human tumours that lack a functional RB pathway. Such cancers exhibit defective stress responses and increased levels of ROS, making them more susceptible to chemotherapy-induced death.
Cell cycle control and the haematopoietic system
The importance of appropriate cell cycle control for haematopoietic homeostasis in general and for stem cell self-renewal in particular has been highlighted by phenotypical analysis of genetically engineered mice lacking the expression of key cell cycle regulatory proteins such as INK4A, p21, D-type cyclins and RB25–32 (TABLE 1). The ability of the organism to respond to physiological stresses such as anaemia, infection and natural attrition of short-lived cells relies on the regulated recruitment of haematopoietic stem cells (HSCs) from their predominantly quiescent state and into the cell cycle to replenish the stem cell pool and also to provide progenitor cells that expand and differentiate producing functional end-stage blood cells32,33. Loss of cell cycle-inhibitory molecules, such as p21, results in stem cell depletion as a consequence of increased cell cycle entry and differentiation at the expense of self-renewal26,32. Conversely, inactivation of all three D-type cyclins resulted in haematopoietic insufficiency due to the failure of stem cells to proliferate27. The role of the cyclin-dependent kinase inhibitor INK4A in HSC self-renewal is age-dependent, which is consistent with increased INK4A expression over time in mouse stem cells25, not only in the haematopoietic system but also in the pancreas and in neural stem cells34,35. INK4A-deficient HSC cells from ageing mice showed increased cell cycle entry and self-renewal when injected into lethally irradiated recipient mice, but corresponding cells from younger mice displayed reduced self-renewal capacity, perhaps reflecting a requirement for INK4A in the response to proliferative stress associated with the transplant of donor bone marrow cells into the ablated host blood system25. The mechanism that promotes INK4A expression in an age-dependent manner in HSCs is not understood but, as will be discussed below, probably involves signalling pathways induced by oxidative stress that accumulates with age, consistent with the ‘free radical theory of ageing’15 (BOX 2). For example, the activity of the polycomb protein BMI1, which negatively regulates expression of INK4A and ARF, may be modulated by oxidative stress as further discussed below (FIG. 2), and targeted deletion of BMI1 resulted in increased expression of both INK4A and ARF in bone marrow HSCs and a marked loss of stem cell self-renewal capacity36,37.
Table 1.
Summary of haematopoietic phenotypes associated with Rb1 deletion in mice
| Rb1 mutant allele | Genotype | Tissues with Rb1 deletion | Haematopoietic phenotype | Refs |
|---|---|---|---|---|
| Rbx3t/x3t | Rb1-null | All tissues | Defective erythroid maturation (failure to exit cell cycle, reduced representation of TER119+ erythroblasts and defective enucleation) leading to anaemia, erythroblast islands disrupted due to hypoxia and cell death in developing fetal liver. Embryos die around E13.5 | 28, 81, 156, 157 |
| Rbx3t/x3t | Transplanted Rb1-null fetal liver | Host bone marrow, spleen and blood | Anaemia (reduced red cell numbers, reticulocytosis), increased representation of CD71+ erythroblasts, myeloid hyperplasia, extramedullary hematopoiesis to the spleen and liver, bone marrow failure, premature demise 2–6 months post-transplant | 28 |
| Rbx3t/x3t | wt;Rb1−/− chimaeras | All tissues, except the placenta | Peripheral blood shows mild increase in immature, nucleated red cells at E13.5 but recovers by E15.5; adult haematopoieisis appears normal under unstressed conditions; haemolytic anaemia induces erythroid defects in Rb1-null cells despite presence of wild-type cells, including macrophages | 28, 38, 39 |
| Rbx3t/x3t | Rag2;Rb1−/− chimaeras | B and T cells | Phenotypically normal, mature lymphocytes | 159 |
| Rbx3t/x3t | Rb1−/−;E2f2−/− | All tissues | Loss of E2f2 rescues red cell maturation defects in Rb1-null erythroblasts, including promoting cell cycle arrest, up-regulation of TER119 and enucleation. Loss of E2f2 did not improve the placental defects observed in Rb1-null mice. Embryos die around E16.5 | 83 |
| Rbx3t/x3t | Rb1−/−;Id2−/− | All tissues | Loss of Id2 rescued red cell maturation defects examined at E17.5. Embryos die around birth. The effect of Id2 loss on developmental defects in the Rb1-null placenta was not examined | 160 |
| Rbflox/flox Δexon 19 | Meox2–Cre | All embryonic tissues, but not placenta | Mild increase in numbers of nucleated immature red cells (similar to Wt;Rb1−/− chimaeras). Mice are viable to end of gestation | 28, 152, 161 |
| Rbflox/flox Δexon 19 | Cyp19–Cre | Placenta | Mild increase in numbers of nucleated immature red cells (similar to Wt;Rb1−/− chimaeras). Embryos die around E15.5 | 153 |
| Rbflox/flox Δexon 19 | Vav–Cre | Definitive HSCs and their progeny; endothelial cells | Increased numbers of nucleated red cells in peripheral blood of adult mouse, anaemia, extramedullary haematopoiesis to the spleen, increased neutrophils in bone marrow. Increased numbers of HSCs in the peripheral blood. Reduced self-renewal and repopulating capacity of HSCs. Increased S phase entry of HSCs in response to treatment of mice with 5-FU | 29 |
| Rbflox/flox Δexon 19 | Mx1–Cre (+pIpC injection) | Haematopoietic system, liver, kidney, others (bone?) | Anaemia, increased platelets and white blood cells in peripheral blood. Increased numbers of HSCs and progenitors in peripheral blood and spleen. Reduced bone marrow HSCs and reduced repopulating capacity. Increased bone marrow neutrophils and macrophages — myeloproliferative disease. Increased bone marrow cellularity initially, followed by bone marrow failure and reduced bone density | 30, 41 |
| Rbflox/flox Δexon 19 | LysM–Cre | Myeloid cells (progenitors and mature cells); earlier haematopoietic progenitors (?) | Myeloproliferative disease upon transplant of LysM–Cre bone marrow into lethally irradiated Rb1flox/flox;Mx1–Cre host mice | 30, 162 |
| Rbflox/flox Δexon 19 | EpoR–Cre | Embryonic and adult erythroblasts | Normocytic anaemia. Extramedullary erythropoiesis to the spleen. Red cells show cell intrinsic defects in maturation, including failure to exit cell cycle, reduced mitochondrial mass and increased representation of CD71+ erythroblasts. Embryos and adult mice are viable | 91 |
5-FU, 5-fluorouracil; E, embryonic day; HSC, haematopoietic stem cell; pIpC, poly(I)poly(C); wt, wild-type.
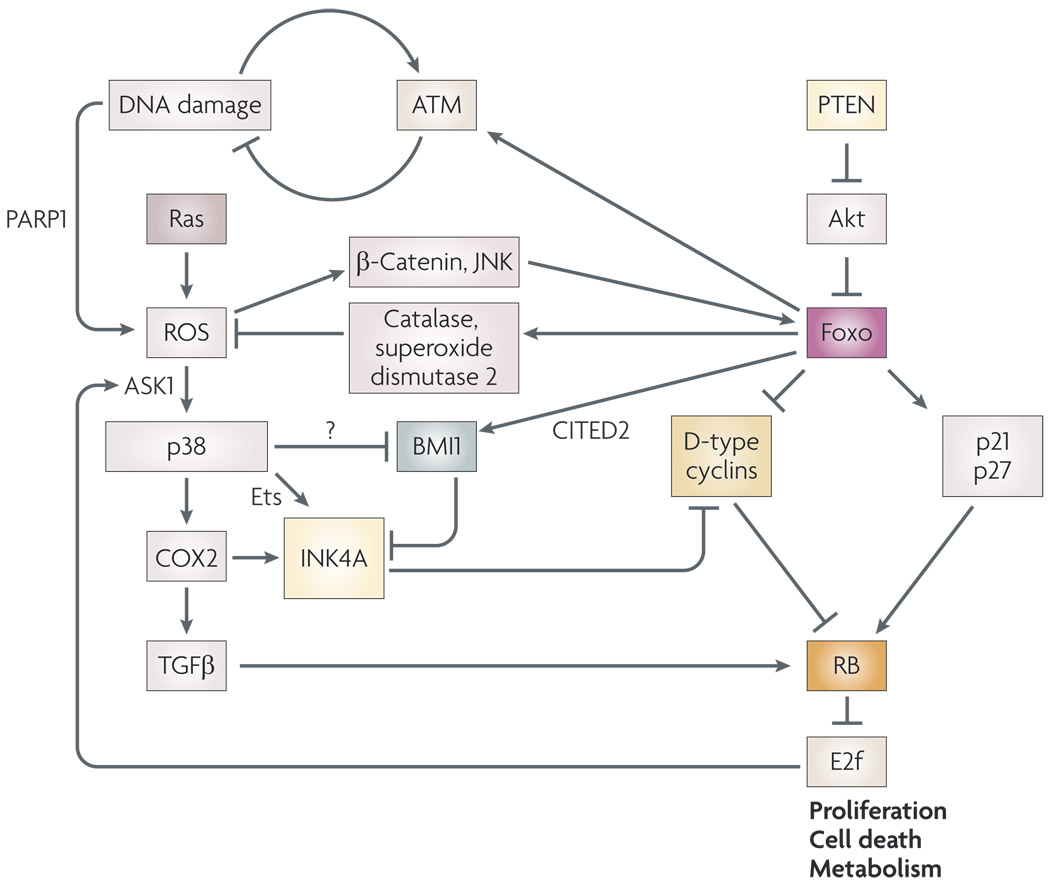
Figure 2. Oxidative stress responses link the retinoblastoma (RB) pathway and Forkhead box O (Foxo) transcription factors.
Activation of Foxos in response to oxidative stress is mediated by reactive oxygen species (ROS)-activated kinases, such as JUN N-terminal kinase (JNK) and by the ROS-induced interaction of β-catenin with Foxos. Active Foxos suppress cellular ROS levels through transcriptional induction of antioxidant genes, such as catalase and superoxide dismutase 2 (REF. 49). In addition to regulation of ROS levels, Foxos modulate both proliferation and cell death49,168 in response to incoming signals, such as those mediated through PTEN and Akt49. Among key cell cycle regulatory targets of Foxos are the cyclin-dependent kinase (Cdk) inhibitors p21 and p27, which are positively regulated by Foxos49, and D-type cyclins, which are negatively regulated49. RB is a downstream effector of both these sets of Foxo target genes. Foxo transcription factors also regulate the expression of the transcriptional co-activator CITED2 (REF. 122), which modulates BMI1 levels169, feeding into regulation of the RB pathway through repression of INK4A. Foxos may also influence RB activity through effects on ROS-dependent activation of p38 mitogen-activated protein kinase, which induces transcription of INK4A through Ets transcription factors. In addition, p38 promotes cyclooxygenase 2 (COX2) expression, which induces RB-dependent cell cycle arrest mediated through transforming growth factor β (TGFβ) or INK4A. Interestingly, E2f activity feeds back to induce expression of ASK1, which modulates p38MAPK activity by ROS170. Direct interaction of FOXO3A with ataxia telangiectasia mutated (ATM) has been reported to promote the cellular response to DNA double-strand breaks, and loss of Foxo or ATM leads to increased DNA damage and ROS in cells58. The mechanism by which DNA damage induces ROS is not clear but may be mediated by increased activity of poly(ADP)-ribose polymerase 1 (PARP1), which depletes NAD+ and inhibits the pentose phosphate pathway72,74.
A role for RB in haematopoietic homeostasis
Understanding the function of the RB tumour suppressor itself, as opposed to its upstream regulators, in haematopoiesis has been complicated by the apparent role of the microenvironment in haematopoietic phenotypes associated with loss of Rb1 in the mouse (TABLE 1). From the first study of Rb1-null chimaeric mice demonstrating that the presence of wild-type cells could rescue observed phenotypic defects in the Rb1-null embryo38,39, our appreciation of the role of RB in haematopoiesis has been permeated by the debate over whether defects are cell-autonomous or non-cell-autonomous40 (BOX 3). Despite progress in understanding RB function in various developmental contexts, the role of RB in HSC homeostasis remains controversial28–30,41. Early evidence pointed to decreased potential of long-term repopulating cells when Rb1-null fetal liver was used as a source of donor stem cells28 but, given that these cells had developed in an abnormal environment, many factors could have explained these observations. More recent work has taken advantage of conditional targeting to examine the role of RB in HSC function in the adult mouse29,30,41 and, although inconsistencies persist, a few salient features of defective HSC function emerge (TABLE 1). Consistent with previous repopulation studies28, adult Rb1flox/flox mice deleted for RB in the haematopoietic system (using either Vav–Cre or poly(I)poly(C)-induced Mx1–Cre) were anaemic and developed myeloproliferative disease with age. This was initially associated with increased bone marrow cellularity but was followed by bone marrow insufficiency and extramedullary haematopoiesis in the spleen29,30. Intriguingly, both studies demonstrated increased HSC activity in the peripheral blood and spleen and reduced numbers in the bone marrow of mice with an Rb1-deficient haematopoietic system29,30, suggesting a role for RB in retaining HSCs within the bone marrow niche (BOX 4). Cell cycle analysis of HSCs from Rb1flox/flox;Vav–Cre mice indicated that an increased proportion of Rb1-deleted HSCs were in the S phase of cell cycle when challenged with agents that induce replicative stress, such as 5-fluorouracil (5-FU)29. By inhibiting thymidylate synthase and depleting nucleotides required for DNA replication, 5-FU induces DNA damage and death of proliferating cells42. However, perhaps as a consequence of the death of cycling progenitors, 5-FU has been shown to induce cell cycle re-entry of Rb1-null HSCs43 and is associated with increased production of ROS, reduced expression of N-cadherin and the detachment of HSCs from the bone marrow niche43. The demonstration that Rb1-null cells are more susceptible to cell cycle re-entry in response to treatment with 5-FU implicates RB in HSC quiescence but also in mediating responses to DNA damage and ROS production, as Rb1-deficient HSCs showed similar cell cycle phase distribution to wild-type cells in the absence of replicative stress29. Indeed, recent work has demonstrated that the RB-related proteins p107 (also known as RB-like 1 (RBL1)) and p130 (RBL2) probably compensate for loss of Rb1 in HSCs under steady-state conditions44.
Box 3 Cell-intrinsic versus non-cell-autonomous effects in development
The major factor determining the lethality of Rb1-null embryos came to light with seminal work by Leone and colleagues showing that RB is crucial for normal placental development151 owing to its promotion of cell cycle arrest in cycling trophoblast stem cells and/or progenitors. Loss of Rb1 led to over-expansion of trophoblasts and aberrant placental architecture152. However, in chimaeric embryos, the placenta is derived from the extra-embryonic layer of wild-type blastocytes, thus explaining the reduced incidence of cell death in Rb1-null chimaeric embryos that have a wild-type placenta153 and highlighting the role of nutrient deprivation and hypoxia arising from placental malfunction in determining the phenotype of the Rb1-null embryo. Indeed, subsequent work has demonstrated upregulation of hypoxia-inducible genes in tissues of the Rb1-null embryo86, and identified a role for hypoxia in disrupting erythroblast islands (made up of a central macrophage providing developmental cues to closely associated differentiating erythroblasts) in the fetal liver of Rb1-null mice154,155, thereby explaining previous data that postulated a role for macrophages in the non-cell-autonomous aspect of defective erythropoiesis in these mice156. However, the question remained whether, in addition to the non-cell-autonomous defects observed in Rb1-null embryos, cell-intrinsic defects also contributed to the differentiation and viability defects in various Rb1-null tissues. Indeed, the failed cell cycle exit in differentiating neurons153, together with age- and stress-dependent defects in red blood cells28 observed in Rb1-null chimaeric mice, confirmed cell-intrinsic consequences of Rb1 loss. Further, a variety of different cell types (primary and tumour cells) deficient for RB exhibited increased susceptibility to cell death induced by hypoxia and ischaemia compared with wild-type cells under the same conditions86, pointing to a cell-intrinsic defect in the response to nutrient stress. Thus, the cell-intrinsic cell-cycle defects associated with Rb1 loss sensitize cells to non-cell-autonomous stresses such as nutrient deprivation and hypoxia, which are themselves consequences of Rb1-dependent defects in tissue development.
Box 4 Functions of the bone marrow niche in haematopoietic homeostasis
The bone marrow microenvironment provides cytokines, extracellular matrix, cell–cell contacts and bone minerals to haematopoietic stem cells (HSCs) that reside primarily at the endosteal surface. This environment is frequently referred to as the bone marrow niche. The niche has an instructive role in modulating stem cell renewal, expansion and differentiation46,47.
Mesenchyme-derived osteoblasts that give rise to bone have a key role within the bone marrow niche in supporting HSCs through direct contact (mediated by molecules including osteopontin, N-cadherin, angiopoietin and TIE2, as well as various chemokines) and through the release of important regulatory factors, such as Notch ligand46.
Extracellular calcium is required for homing of HSCs to the bone marrow and for their interaction with the endosteal surface157. Hypoxia promotes the expansion of HSCs but it is not completely clear whether oxygen gradients within the bone marrow are consistent with the locations of stem cells123,124. Increased levels of reactive oxygen species in the niche may have a role in sensing the metabolic state of the animal and acting to limit HSC expansion through activation of INK4A and other mechanisms.
The extent to which non-cell-autonomous factors and the bone marrow microenvironment contribute to haematopoietic defects in adult mice carrying targeted deletion of Rb1 is unclear, as some of the Cre-deleter strains used (notably Mx1–Cre) are not haematopoietic-cell-specific in their expression30. Based on competitive repopulation experiments in lethally irradiated wild-type host mice using bone marrow from Rbflox/flox;Mx1–Cre mice mixed with bone marrow from control Rb1flox/flox mice, Walkley and colleagues initially reported that Rb1 was not required for the self-renewal and long-term multi-lineage repopulating capacity of HSCs41. These data contradicted previous work using Rb1-null fetal liver28 and more recent studies using bone marrow from Rb1flox/flox;Vav–Cre mice29. However, Walkley and colleagues subsequently reported that they did in fact observe myeloproliferative disease, extramedullary haematopoiesis and loss of HSCs from the bone marrow of Rb1flox/flox;Mx1–Cre mice30. Notably, loss of p107 had previously been linked to the development of myeloproliferative disease in BALB/C mice45 and expansion of mature myeloid elements was described in the bone marrow of mice reconstituted with Rb1-null fetal liver28. Intriguingly, the same extent of myeloproliferation detected in Rb1flox/flox;Mx1–Cre mice was not observed in Rb1flox/flox;LysM–Cre mice in which the lysozyme M promoter drives Cre expression in the myeloid compartment (consisting of both progenitors and end-stage cells)30. Indeed, interferon-induced, Mx1-driven Cre expression in HSCs results in haematopoietic progeny and lineages deleted for Rb1, including myeloid-derived osteoclasts and macrophages that make up the bone marrow microenvironment30. Thus, although Walkley and colleagues did not observe haematopoietic defects when transplanting bone marrow from Rb1flox/flox;Mx1–Cre mice into lethally irradiated wild-type hosts (with wild-type bone marrow microenvironment) or vice versa, they did observe myeloproliferative disease when they transplanted bone marrow from Rb1flox/flox;LysM–Cre mice into lethally irradiated Rb1flox/flox;Mx1–Cre mice30. These mice developed a completely penetrant myeloproliferative disorder similar to that observed in Rb1flox/flox;Mx1–Cre mice, and the authors proposed that this was indicative of an interaction between myeloid progenitors and the bone marrow niche. However, given that osteoclasts and macrophages in the bone marrow niche are themselves myeloid in origin, this did not explain why Rb1flox/flox;LysM–Cre mice did not also show this fully penetrant phenotype, unless there is an additional crucial cell type in the bone marrow niche that is targeted by Mx1–Cre but not by LysM–Cre, an issue that remains to be addressed. Given the importance of mesenchyme-derived osteoblasts in the bone marrow niche46,47 (BOX 4), and the role of RB in osteoblast differentiation48, an obvious line of enquiry is to examine the effect of osteoblast-specific deletion of Rb1 for both HSC function and myeloproliferation. Furthermore, because Rb1 is not deleted from HSCs in Rb1flox/flox;LysM–Cre mice, studies to date have not addressed whether loss of Rb1 in the bone marrow niche specifically affects HSC behaviour, as opposed to myeloproliferation. Such a role is, however, supported by the presence of a HSC phenotype in Rb1flox/flox;Mx1–Cre mice30 but not in wild-type host mice transplanted with Rb1flox/flox;Mx1–Cre bone marrow41. The previously discussed dependence on inducers of replicative stress to unmask the cell cycle defect in Rb1flox/flox;Vav–Cre HSCs points to a potential role for the niche in safe-guarding the HSC against oxidative stress.
Oxidative stress in the haematopoietic system
Among the key modulators of oxidative stress in the haematopoietic system are the forkhead box O (Foxo) transcription factors that regulate the expression of antioxidants, such as superoxide dismutase 2 (SOD2) and catalase49. Deletion of Foxo3a in mice reduced expression of SOD2 and catalase, increased ROS in HSCs, and reduced HSC self-renewal potential in an age-dependent manner that was associated with increased cell cycle entry50. Mice with PTEN-deficient stem cells showed a similar phenotype, consistent with PTEN acting upstream of Foxo49 (FIG. 2). In addition to regulation of antioxidants, Foxos are implicated in transcriptional upregulation of cell cycle inhibitors, including p21, p27 and p130, and downregulation of D-type cyclins.49 Accordingly, these genes were deregulated in HSCs deleted for Foxo1, Foxo3a and Foxo4, a result consistent with increased numbers of HSCs in the S/G2 and M phases of the cell cycle, increased differentiation and reduced self-renewal capacity51. Interestingly, although deletion of Foxo3a alone induced a HSC phenotype and anaemia51,52, concomitant deletion of Foxo1, Foxo3a and Foxo4 was also associated with a myeloproliferative disorder akin to that observed in mice with an Rb1-deficient haematopoietic system28–30. These parallels in haematopoietic phenotypes might be explained by the fact that key Foxo target genes mediate their cell cycle regulatory functions through effects on the phosphorylation and subsequent inactivation of RB8.
Additional links between Foxo transcription factors and the RB pathway can be made by considering the role of ROS in the haematopoietic system (FIG. 2). Foxo transcription factors function in HSCs in a cell-autonomous manner to regulate ROS levels51. Treatment of Foxo3a−/− mice or compound Foxo-deleted mutant mice (engineered through targeted deletion of Foxo1, Foxo3 and Foxo4 in the haematopoietic system) with the glutathione donor and antioxidant agent N-acetyl cysteine (NAC) restored HSC pool size and rescued HSC function50,51. Thus, these studies implicate increased ROS in the observed HSC defects in Foxo mutant mice.
ROS was previously identified as causative in the HSC defects observed in ataxia telangiectasia mutated (Atm)-null mice53, in which age-dependent loss of HSC and multilineage bone marrow failure and anaemia were observed. Again, treatment with NAC rescued the observed defects in these mice. Expression of human papillomavirus protein E7 (which inactivates the Rb family of pocket proteins, including RB, p107 and p130, see BOX 1) prevented stem cell depletion and restored repopulation capacity to Atm-null HSCs53, highlighting the importance of the RB pathway in preventing HSC depletion downstream of Atm and ROS generation. Interestingly, INK4A levels were shown to be increased in both Atm-null and in Foxo3a-null HSCs, and in both cases this was dependent on ROS and on the activity of p38 mitogen-activated protein kinase (MAPK)50,54. JUN N-terminal kinases (JNKs) and p38 are activated in response to various cellular stresses that alter the redox state of the cell, such as endoplasmic reticulum stress, DNA damage, inflammatory cytokines and calcium flux55, and their activation is dependent on apoptosis signal-regulating kinase 1 (ASK1, also known as MAP3K5)56. ASK1 is maintained in an inactive state through interaction with reduced thioredoxin, which contains two redox-sensitive cysteine residues that are oxidized in response to redox stress. This releases ASK1 from thioredoxin, resulting in downstream activation of mitogen-activated protein kinase kinase 3 (MAP2K3) and MAP2K6, which subsequently activate p38 and JNKs56. Of note, JNK requires signalling through MAP2K4, which is activated independently of ASK1, to be fully active in response to ROS56. In the haematopoietic system, ROS induced by loss of either ATM or the Foxo proteins led to activation of p38, and inhibition of p38 (or ASK1) prevented both INK4A induction and downregulation of N-cadherin in HSCs, resulting in restored HSC self-renewal43,50,54. This work implicates p38 activity downstream of ROS and Foxo transcription factors in activating the RB pathway through induction of INK4A (FIG. 2). The mechanism by which p38 induces INK4A is not completely understood, although proposed models include p38-mediated phosphorylation and inactivation of BMI1 (involved in repressing the CDKN2A locus, which encodes both INK4A and ARF) or through p38-induced activation of Ets transcription factors that positively regulate INK4A transcription57.
DNA damage and oxidative stress
These studies assign a key role to both ATM and Foxos upstream of p38 in managing cellular ROS levels in HSCs (FIG. 2) and, intriguingly, recent work has shown a direct interaction between ATM and FOXO3A at DNA damage foci and invoked a role for FOXO3A in promoting autophosphorylation and activation of ATM in response to damage58. Defects in sensing DNA damage or in homologous recombination involved in repair of double-strand breaks have been previously shown to contribute to diseases in the haematopoietic system59,60 and RB and E2f have been implicated in the transcriptional regulation of several key DNA repair proteins, including FANCD2, MSH2 and RAD51 (REFS 61–63). Furthermore, deficiency for the Fanconi anaemia protein FANCD2 or ataxia telangiectasia and Rad3-related (ATR), both of which are required for cell cycle arrest and repair in response to DNA crosslinking agents, is associated with anaemia and bone marrow failure64,65. Similarly, haematopoietic malignancies, immunodeficiency and myelofibrosis are observed in individuals with ataxia telangiectasia, Nijmegen breakage syndrome (NBS) and hereditary breast and ovarian cancer syndrome, which are associated with mutations in ATM, NBN and BRCA2, respectively64,66,67. Of particular interest, the Fanconi anaemia pathway lies downstream of ATM kinase in the response to DNA damage and, similarly to ataxia telangiectasia cells, Fanconi anaemia cells show increased levels of ROS and oxidative DNA base damage68. Intriguingly, cells from the FANCC complementation group were only sensitive to the crosslinking effects of the chemotherapeutic agent mitomycin C at high oxygen concentration and behaved like normal cells at low oxygen levels, implicating ROS directly in the observed defects69. Oxidative stress induces multimerization of FANCA and FANCG, suggesting that the Fanconi anaemia pathway may have a role in sensing cellular levels of ROS independent of DNA damage70 and perhaps explaining the unique sensitivity of Fanconi anaemia cells to oxygen tension71.
Debate still surrounds how and why DNA damage leads to ROS production. As mentioned above, FOXO3A may have a role in activating ATM and, conversely, ATM may contribute towards antioxidant gene expression by inducing checkpoint kinase 2 (CHK2)-dependent phosphorylation of Foxos49. An additional source of ROS in ATM-deficient cells may be attributed to the activity of the DNA-damage-sensing enzyme poly(ADP-ribose) polymerase 1 (PARP1). This hydrolyses cytosolic NAD+ to generate poly(ADP-ribose), which is conjugated to target proteins including histones and PARP1 itself 72. Defects in DNA double-strand break repair due to ATM deficiency lead to persistent PARP1 activity, and treatment of cells from ataxia telangiectasia patients with inhibitors of PARP1 activity reduced ROS levels73. Depletion of NAD+ has been proposed to contribute to cellular ROS as a consequence of limiting the activity of the pentose phosphate pathway, which uses NADPH to make glutathione, a key cellular antioxidant, particularly in red blood cells74.
Erythropoiesis and oxidative stress
Analysis of the mouse models discussed above has revealed how the interplay between oxidative stress sensing pathways and the cell cycle machinery in HSCs is important for the long-term survival of the animal. However, a cursory glance at the phenotypes of mice with defects in ROS management75,76 or of humans with mutations in ROS regulatory enzymes, such as glutathione S-transferase-θ or NADPH quinone oxidoreductase, reveals that survival in response to ageing or acute stresses such as blood loss75–77 or occupational exposure to chemicals such as benzene78,79 is a function of red blood cell performance. The red blood cell is important for mammalian viability, not just in terms of its capacity to transport oxygen in the form of oxyhaemoglobin to peripheral tissues, but in its antioxidant capacity. Peroxynitrite and superoxide taken up from the environment through red blood cell-specific membrane anion channels are reduced by glutathione and other antioxidants, which are expressed at particularly high levels in red blood cells74. In addition to handling extracellular ROS, the red blood cell is equipped to reduce the endogenous ROS that is generated at high levels owing to routine tasks including oxygen and iron uptake, haem biosynthesis and globin chain imbalance management. In particular, the oxidation of ferrous (Fe2+) haemoglobin to ferric (Fe3+) haemoglobin produces superoxide and hydroxyl radicals that need to be quenched on a scale unparalleled in other mammalian cell types. Failure to manage ROS production owing to inherited genetic defects, such as mutations in glucose-6-phosphate dehydrogenase, which converts NADP to NADPH (required for glutathione reduction), results in reduced red blood cell lifespan and life-threatening anaemia under stressed conditions80. Red blood cells also express high levels of antioxidants, including catalase, peroxyredoxins and SODs. Interestingly, FOXO3A becomes increasingly nuclear in localization and transcriptionally active during erythroid maturation52. The significance of FOXO3A regulation of SOD2 and catalase for red blood cell production is manifested by increased ROS in Foxo3a-null erythroblasts and the reduced numbers and lifespan of mature red blood cells in Foxo3a-null mice52. Given the tight regulation of antioxidant expression during erythroid maturation, it is also tempting to speculate that ROS has an instructive role in erythroid differentiation, perhaps by promoting cell cycle arrest.
New roles of RB–E2F in oxidative stress responses
Loss of Rb1 in the erythroid system resulted in increased ROS that may be explained by the failure of Rb1-null erythroblasts to downregulate the transferrin receptor (CD71)31, resulting in increased labile iron that could generate increased ROS levels through the Fenton reaction. However, the defining feature of Rb1-null erythropoiesis is the failure of differentiating erythroblasts to properly exit the cell cycle28,81. During erythroid maturation, RB is activated by dephosphorylation and E2F2 is specifically upregulated82,83. RB was found predominantly in complexes with E2F2 in end-stage erythroblasts at the promoters of cell cycle regulatory genes, and loss of E2F2 restored cell cycle exit and maturation capability to Rb1-null erythroblasts83. These results suggested that Rb1-null erythroblasts are defective for terminal maturation owing to deregulated activity of E2F2, which, when complexed with active RB, represses E2f target gene expression and promotes cell cycle exit, but functions as a cell cycle activator in the absence of RB. These studies revealed a new tissue-restricted role for a member of the E2f family of transcription factors and suggested a unique role for E2F2 in erythroid maturation beyond simple regulation of the classical set of E2f target genes involved in cell cycle. However, the nature of this erythroid-specific function is not clear.
An emerging theme from microarray analyses of E2f target gene expression is the overlap with other transcription factors in regulating both known and new subsets of target genes (FIG. 3). This has perhaps been best illustrated by analyses showing a striking overlap in target genes between E2F4 and the nuclear respiratory factor 1 (NRF1), wherein there were 96 gene promoters regulated in common among 465 that were regulated by E2F4 and 691 that were regulated by NRF1 (REF. 84). NRF1 is a key regulator of nuclear encoded genes involved in mitochondrial biogenesis and the response to oxidative stress, including respiratory chain enzymes and transcription factor A, mitochondrial (TFAM)85. TFAM regulates the expression of genes encoded by the mitochondrial genome that are required for mitochondrial function, including certain subunits of cytochrome c oxidase and ATP synthase85. Similarly, there are several reports identifying genes that are co-regulated by E2F1 and hypoxia-inducible factors (HIFs), including BNIP3, BRCA1, haem oxygenase and the transcription factor DEC1 (REFS 61,86,87). Thus, the unique role of E2F2 in erythropoiesis could be explained by its interaction with as yet unidentified cooperating transcription factors involved in red blood cell differentiation, similar to the restricted interactions of E2F1 and E2F3 with the transcription factors YY1 and TFE3 in other settings88–90. The type of target gene regulated by E2F2 in differentiating red blood cells awaits further characterization, but recent work showing that Rb1-null erythroblasts had lower mitochondrial mass than control erythroblasts91 suggested a role for RB in regulating genes involved in mitochondrial biogenesis, such as peroxisome proliferator-activated receptor γ (PPARγ) co-activator 1β (PGC-1β, also known as PPARGC1β), NRF1 and NRF2. However, given that erythroblasts destroy their mitochondria during terminal differentiation through a process known as mitophagy, which requires the function of a BH3-only protein known as NIX92,93, alternative explanations for the observed reduction in mitochondrial mass may be defined. Reduced mitochondrial mass at a specific stage of Rb1-null erythroid differentiation might simply reflect uncoupling of cell cycle exit from mitophagy such that mitophagy proceeds normally (in a similar fashion to the uncoupling of cell cycle exit from other aspects of differentiation, such as globin gene expression), even though other aspects of maturation are arrested. Alternatively, increased mitochondrial mass observed in CCND1-null cells94 points to a more direct role for the RB pathway in the regulation of mitochondrial biogenesis, either through coordinate regulation of target genes by E2fs and NRF1 as described previously84, or through regulation of NRF1 activity by Cdks94 and other ill-defined mechanisms.
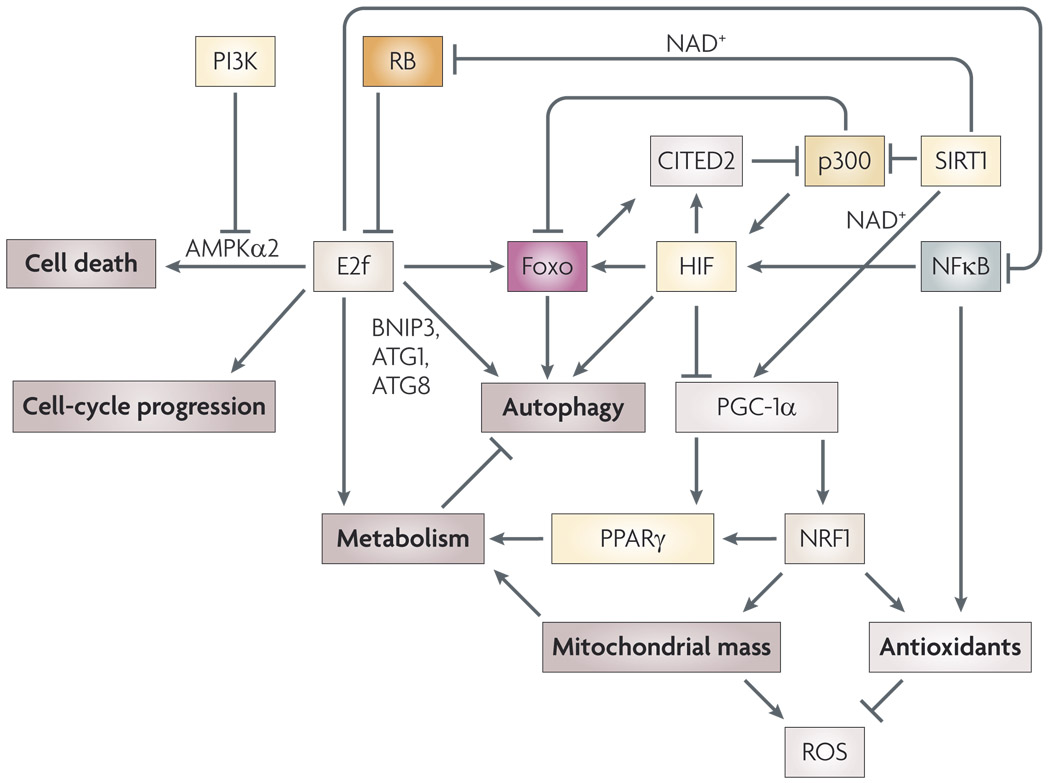
Figure 3. Functional interactions of E2f transcription factors with other transcription factors in oxidative stress responses.
In addition to conventional cell cycle regulatory target genes, E2fs have been reported to interact functionally with other transcription factors, such as Foxos171, hypoxia-inducible factor 1α (HIF-1α)86, nuclear factor κB (NFκB)172, nuclear respiratory factor 1 (NRF1)84 and peroxisome proliferator-activated receptor γ (PPARγ)101, to modulate effects on autophagy86, metabolism84, mitochondrial mass91 and the redox response of cells84. These activities are distinct from its role in regulating pro-apoptotic genes, but may indirectly impinge upon the viability of cells by determining the overall levels of nutrients, ATP, NAD+ and reactive oxygen species (ROS). For example, E2F1 cooperates with HIF to induce BNIP3 expression86, which promotes mitochondrial autophagy in response to hypoxia, thereby limiting ROS and necrosis96. E2F1 has also been shown to regulate the expression of other autophagy regulators, such as ATG1 and ATG8 (REF. 100). E2F1 modulates cellular ROS levels through inhibition of NFκB activity mediated by direct protein–protein interactions between E2F1 and the NFκB subunit RELA (REF. 172). Multiple feedback loops operate at the transcriptional level, with NFκB feeding back to induce HIF173, and E2Fs regulating Foxo expression171, which in turn negatively modulates HIF activity through induction of the transcriptional co-activator CITED2 (REF. 122). Upstream of HIF and Foxos, the SIRT1 deacetylase counters the activity of p300 and is dependent on available NAD+ for its ability to activate Foxos, its ability to promote RB turnover114 and its downstream interaction with and activation of peroxisome proliferator-activated receptor γ, coactivator 1α (PGC-1α)117,118.
Regulation of mitochondrial mass is emerging as a key response to hypoxia and oxidative stress95. As mentioned, the mitochondria are the major source of ROS in cells, and reducing the size of the mitochondrial reticulum in cells under conditions in which oxidative phosphorylation and electron transport is disrupted has been proposed to limit the generation of ROS96,97, and thereby promote cellular integrity and survival. In particular, hypoxia has been shown to induce uptake of mitochondria by autophagosomes that are then turned over following fusion with lysosomes96. It has been suggested that, in addition to limiting ROS generation under hypoxic conditions, this process might selectively rid the cell of ‘damaged’ mitochondria (for example, those with oxidized lipid membranes) 97, although the basis of such selectivity is not clear. BNIP3 is a HIF target gene implicated in hypoxia-induced autophagy86,98 and reportedly functions to promote autophagy by titrating the inhibitory activity of BCL2 away from the autophagy regulator beclin 1 (REF. 96). As already discussed, BNIP3 is transcriptionally regulated by RB, E2F and HIF such that E2F1 synergizes with HIF1α to activate BNIP3, whereas RB represses induction of BNIP3 by HIF86. BNIP3 is also directly regulated by FOXO3A99 and this may explain the hypoxia-independent expression of BNIP3 observed in skeletal and cardiac muscle98,99. E2F1 has also been implicated in the transcriptional regulation of ATG1 and ATG8, which encode key components of the basal autophagy machinery100. In addition to induction of mitophagy, oxidative stress represses mitochondrial biogenesis95. HIF-dependent repression of MYC activity leads to inhibition of PGC-1β, a MYC target gene85. Effects of PGC-1α (PPARGC1α) and PGC-1β on mitochondrial mass are mediated in part through co-activation, with NRF1 and NRF2, of genes such as TFAM, as well as respiratory chain subunits85. Intriguingly, RB–E2F activity has been shown to modulate the expression and activity of both PGC-1α and its cofactor, PPARγ, in adipose tissue101–103, although the significance of this interaction for mitochondrial biogenesis has not been explored. Thus, through cooperative interactions with other transcription factors, RB–E2f appears to indirectly modulate mitochondrial mass of cells and, by extension, levels of ROS (FIG. 3). This may have significance for understanding how mitochondrial numbers are coordinated with the cell cycle, but also, as highlighted here, for how the cell responds to oxidative stress in terms of cell cycle progression and metabolism.
Regulation of RB levels and activity by ROS
In addition to modulating RB activity by inducing INK4A50,54, ROS activates PP2A, which promotes dephosphorylation of RB in response to oxidative stress12,104,105. The activation of PP2A by ROS requires Ca2+ mobilization for the activity of the PR70 subunit of PP2A105. Thus, ROS induces dephosphorylation and activation of RB through two mechanisms: inhibition of Cdks and activation of PP2A.
RB levels have also been linked to the redox state of the cell106, although the mechanistic basis of this link is not understood. Regulation of protein degradation is a major mechanism used by cells to modulate cell cycle checkpoints under different growth conditions107. For example, the p130 pocket protein, which is structurally and functionally related to RB, is regulated by proteolysis during normal cell cycle progression108. Degradation of p130 is regulated by interaction with SKP2, a substrate recognition component of the SKP1–cullin–F box (SCF) ubiquitin ligase complex, in a similar manner to the SCF-regulated degradation of p27 (REF. 109). Interestingly, degradation of p27 by SCF is negatively modulated by RB109 such that interaction between active RB and the CDH1 subunit of the APC promotes APC-mediated degradation of SKP2 (REF. 110). In contrast to p130 and p27, RB is not itself degraded during normal cell cycle108, although, as described below, redox stress during differentiation and senescence has emerged as a likely modulator of RB levels.
Acetylation of RB appears to be crucial to regulating its turnover. RB is acetylated by p300 on lysines 873 and 874 and this blocks its phosphorylation by Cdks and inhibits RB degradation112,113. Conversely, SIRT1 (formerly known as SIR2α) has also been reported to be a potent deacetylase for RB114. SIRT1 is an NAD+-dependent deacetylase known to regulate p53 levels115,116 and to also regulate the activity of Foxos and PGC-1α49,117,118 (FIG. 3). Notably, SIRT1 interacts with the p300/CBP-associated factor (PCAF)–MYOD1 complex to inhibit muscle differentiation119, whereas RB acts to promote muscle differentiation through interaction with PCAF120. Furthermore, hypoxia blocked the induction of RB levels during muscle differentiation121 suggesting that SIRT1 inhibits muscle differentiation in response to redox stress by promoting the turnover of RB. Alternatively, hypoxia may inhibit acetylation of RB through induction of the transcriptional co-activator CITED2, a FOXO3A and HIF target gene that binds directly to p300 to inhibit its activity122. Hypoxia has been shown to regulate the location of HSCs within the bone marrow niche123 and to promote expansion of HSCs and neural stem cells124,125 and, although this has been linked to a requirement for HIF1α to fully activate Notch target genes that are required for stem cell expansion and renewal125, it will also be interesting to assess how hypoxia and ROS affect RB levels in stem cells.
Finally, seladin is a ROS-induced modulator of cellular p53 protein levels that interacts directly with p53 to prevent its targeting by the E3 ubiquitin ligase MDM2 for degradation111, and it has been suggested that seladin may also act to stabilize RB levels in response to ROS induced by Ras activation111.
RB and ROS in other systems
This Review has focused on the role of ROS in modulating the RB pathway in the haematopoietic system, but there are various studies that support a role for the RB pathway in the response to ROS in other cell systems. For example, induction of cellular senescence by activated Ras oncogenes is dependent on ROS126, on DNA-damage responses127,128 and on functional RB to induce cell cycle arrest129. Ras-induced ROS production was blocked by inhibitors of Nox, and both NOX1 and NOX4 were upregulated in response to HRAS activation130. Similar to the haematopoietic system54, p38 is also a key mediator of Ras-induced ROS signalling in tumour cells130 but, in contrast to primary HSCs, many tumour cells are deleted or mutated for INK4A or RB, so p38 activation in response to Ras-induced ROS frequently results in apoptosis130, possibly mediated by phosphorylation and inactivation of BCL2 (REF. 55).
The integrity of the RB pathway has also been identified as a key factor in stress responses that predict the progression of primary human breast cancer131,132. High INK4A levels in combination with high Ki67 at the ductal carcinoma in situ stage of breast cancer was a strong indicator of tumour progression to invasive cancer131. Similarly, high levels of cyclooxygenase 2 (COX2, PTGS2) in combination with high Ki67 staining predicted breast cancer progression. Notably, transcriptional activation of COX2 in response to pro-inflammatory cytokines and bacterial lipopolysaccharides is mediated by ROS-induced p38 activity133–135. COX2 expression induced INK4A expression and the ability of COX2 to induce cell cycle arrest was dependent on functional RB131. Thus, these studies suggest that ROS generation, p38 activation, COX2 and INK4A induction are linked to RB-dependent cell cycle arrest (FIG. 2) by a positive feedback loop that is disrupted in cancer, leading to increased COX2 and INK4A expression but continued proliferation.
Inactivation of the RB pathway is a common event in human cancers4,132 and RB1-deficient cells are preferentially sensitive to DNA-damaging agents, such as cisplatin and etoposide, compared with control cells1,3. The sensitivity of RB1-deficient cells to genotoxic agents is associated with the increased ROS, increased PARP1 activity and NAD+ depletion that lead to necrotic cell death (H. Liu, J. R. Knabb, B. T. Spike & K.F.M, unpublished data). Cancer cells generally have higher levels of ROS than normal cells, in large part owing to their increased proliferation and metabolic rate, as well as the activity of oncogenes, such as Ras136. Genotoxic agents used to treat cancer induce ROS as part of their modality, and inhibition of antioxidant activity in tumour cells can further sensitize them to drug-induced necrotic cell death137. Thus, although ROS may have a role in the genesis of cancer through oxidative base damage of DNA and disruption of signalling pathways, they also present new avenues through which to specifically target tumour cells.
Summary
Cancer is largely a disease of post-reproductive years in susceptible animals and it is thus likely that tumour suppressor genes evolved as mediators of stress response mechanisms, as opposed to tumour suppressors per se. Given its central function in cell cycle checkpoint control, it was initially surprising that loss of Rb1 had such limited consequences for embryonic development but this may be explained if, similar to p53, RB function only becomes crucial under stressed conditions. This Review has highlighted the role of RB in the response to oxidative stress, based largely on work in the haematopoietic system but bringing in relevant analyses from other systems. What has emerged is a growing realization of the extent to which regulation of cell cycle by the RB pathway is coupled to rates of cellular metabolism, ROS levels and mitochondrial mass. Under normal circumstances, this may allow the cell to integrate mitochondrial biogenesis with cell cycle and to link proliferation to favourable levels of metabolites, with ROS acting as key signalling intermediates in the process. Given the accepted role of serum growth factors and DNA damage in modulating the RB pathway and cell cycle, it should not be surprising that the cell would also have evolved mechanisms to couple proliferation to sensors of metabolites and ROS levels. Sensitivity of the RB pathway to the oxidative state of the intracellular and extracellular milieu through ROS-induced INK4A expression and altered RB protein stability and activity is a new and physiologically significant perspective on the function of this important tumour suppressor. Importantly, these findings suggest that tumour cells lacking an intact RB pathway might be susceptible to killing by drugs that target metabolism or induce ROS.
Acknowledgements
The author is grateful to the J. P. McCarthy Foundation, the Aplastic Anaemia and MDS International Foundation and the National Heart Lung & Blood Institute (RO1 HL080262) for funding of work in her laboratory relating to oxidative stress, erythropoiesis and haematopoietic diseases. The author also gratefully acknowledges the experimental skills and intellectual input to work in the laboratory in the related research area by current laboratory members K. Tracy, J. Knabb and D. Glick and by former graduate students in the laboratory B. T. Spike and A. Dirlam.
Glossary
- Haematopoietic stem cell
A cell residing at the apex of the cellular hierarchy in the haematopoietic system that gives rise to all other lineages and cell types in the blood system.
- Non-cell-autonomous
Effects in tissues that are the consequence of a defect or altered phenotype in a different tissue or cell type.
- Vav–Cre
Expression of the Cre recombinase under the control of the Vav promoter. which is restricted in its expression to haemangioblast-derived cell types. Thus Vav–Cre drives deletion of floxed alleles in embryonic and adult haematopoietic stem cells and their progeny, as well as in endothelial cells.
- Poly(I)poly(C)
A form of double-stranded RNA that is a potent inducer of interferon and is used to activate the Mx1 promoter driving Cre recombinase expression.
- Mx1–Cre
Expression of the Cre recombinase under the control of the silent Mx1 gene promoter: Cre is not expressed unless the transgenic mouse is challenged with agents that induce the interferon response, such as poly(I)poly(C). Expression is high in the haematopoietic system, liver and kidneys and lower in other cell types tested.
- N-cadherin
A member of the cadherin family of cell–cell adhesion proteins bearing conserved structural motifs known as ‘cadherin repeats’. N-cadherin is highly expressed in mature neurons.
- Repopulating capacity
The ability of haematopoietic tissue and cells to regenerate the blood system when transplanted into host animals that have had their bone marrow ablated through exposure to lethal or sublethal doses of ionizing irradiation or to cytotoxic drugs.
- Myeloproliferation
Expansion of myeloid elements within the blood system.
- Osteoclasts
Myeloid-derived cells within the bone marrow niche that interact with bone matrix and osteoblasts to influence stem cell development.
- Antioxidants
Compounds and enzymes that neutralize ROS by accepting electrons from free radicals.
- Fanconi anaemia
A cancer susceptibility syndrome in which genetically predisposed individuals are sensitized to DNA crosslinking agents, experience bone marrow failure and anaemia and show varying degrees of developmental abnormalities.
- Poly(ADP-ribose)
A polymer generated by and conjugated to target proteins by members of the poly(ADP)-ribose polymerase resulting in increased negative charge and altered activities of modified proteins.
- Pentose phosphate pathway
A series of enzymatic reactions in which NADPH is produced in cells by conversion of glucose-6-phsophate to ribulose-5-phosphate.
- Fenton reaction
The oxidation of Fe2+ to generate Fe3+ and highly reactive hydroxyl radicals.
- Ischaemia
Deprivation or insufficiency of blood supply to tissues associated with hypoxia and nutrient deprivation, frequently with necrotic cell death.
- Endosteal surface
The inner surface of bone bordering the bone marrow cavity.
- Ki67
Ki67 is a marker of proliferation that may have a role in ribosome biogenesis. Immunohistochemical staining for Ki67 on tumour sections and tissues is commonly used to mark out proliferating cells in situ.
- Cyclooxygenase 2
(COX2). An enzyme with both peroxidase and dioxygenase activity involved in synthesis of prostaglandins from arachadonic acid, COX2 is activated by inflammation and upregulated in colorectal, breast and other cancers. COX2 inhibitors are used as anti-inflammatory drugs.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
Atm | BNIP3 | BRCA1 | BRCA2 | DEC1 | Foxo1 | FOXO3 | Foxo3a | Foxo4 | NBN
National Cancer Institute: http://www.cancer.gov/bladder cancer | prostate cancer
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary/
5-fluorouracil | cisplatin | etoposide | mitomycin C
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
ataxia telangiectasia | Nijmegen breakage syndrome
UniProtKB: http://www.uniprot.org
ATR | beclin 1 | BMI1 | catalase | CD71 | CDH1 | CDK2 | CDK4 | CDK6 | CHK2 | CITED2 | cyclin D1 | cyclin D2 | E2F1 | E2F2 | E2F3 | E2F4 | FANCA | FANCC | FANCD2 | FANCG | FBXO5 | HRAS | INK4A | MAP2K3 | MAP2K4 | MAP2K6 | MAP3K5 | MDM2 | MSH2 | MYC | MYOD1 | N-cadherin | NIX | NOX1 | NOX4 | NRF1 | NRF2 | osteopontin | p130 | p21 | p27 | p300 | p53 | PARP1 | PCAF | PPARγ | PPARGC1α | PPARGC1β | PTEN | PTGS2 | RAD51 | RB | RBL1 | RBL2 | RELA | seladin | SIRT1 | SOD2 | TFAM | TFE3 | TIE2 | YY1
FURTHER INFORMATION
K. F. Macleod’s homepage: http://ben-may.bsd.uchicago.edu/bmi2/faculty/macleod/macleod.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Almasan A, et al. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes and apoptosis. Proc. Natl Acad. Sci. USA. 1995;92:5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington EA, Bruce JL, Harlow E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc. Natl Acad. Sci. USA. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen KE, et al. RB-dependent S-phase response to DNA damage. Mol. Cell. Biol. 2000;20:7751–7763. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim WY, Sharpless NE. The regulation of INK4/ARF in Cancer and Aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nature Rev. Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cam H, Dynlacht BD. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell. 2003;3:311–316. doi: 10.1016/s1535-6108(03)00080-1. [DOI] [PubMed] [Google Scholar]
- 8.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 9.Yu Q, Ciermeych MA, Sicinski P. Ras and Myc can drive oncogenic cell proliferation through individual D-cyclins. Oncogene. 2005;24:7114–7119. doi: 10.1038/sj.onc.1208853. [DOI] [PubMed] [Google Scholar]
- 10.Trimarchi J, Lees JA. Sibling rivalry in the E2F family. Nature Rev. Mol. Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- 11.El-Deiry WS, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 12.Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F. Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107 and p130. J. Biol. Chem. 2003;278:19509–19517. doi: 10.1074/jbc.M300511200. [DOI] [PubMed] [Google Scholar]
- 13.Avni D, et al. Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol. Cell. 2003;12:735–746. doi: 10.1016/s1097-2765(03)00355-1. [DOI] [PubMed] [Google Scholar]
- 14. Havens CG, Ho H, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APCCdh1 by reactive oxygen. Mol. Cell. Biol. 2006;26:4701–4711. doi: 10.1128/MCB.00303-06. Quenching ROS induced a G1 arrest suggesting that endogenous ROS were required for S phase entry. Cell cycle arrest was associated with failure to stabilize cyclin A1, SKP2 or EMI1 and persistent activity of the APC–CDH1 ubiquitin ligase complex.
- 15.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of aging. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 18.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Rev. Cancer. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 20.Conour JE, Graham WV, Gaskins HR. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol. Genomics. 2004;18:196–205. doi: 10.1152/physiolgenomics.00058.2004. [DOI] [PubMed] [Google Scholar]
- 21.Menon SG, et al. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63:2109–2117. [PubMed] [Google Scholar]
- 22.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2006;24:1–9. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 23.Stubbe J, Riggs-Gelasco P. Harnessing free radicals: formation and function of the tyrosyl radical in ribonucleotide reductase. Trends Biochem. Sci. 1998;23:438–443. doi: 10.1016/s0968-0004(98)01296-1. [DOI] [PubMed] [Google Scholar]
- 24.Miller JJ, et al. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4A. Nature. 2006;443:421–426. doi: 10.1038/nature05159. Showed that INK4A levels increased with age in HSCs and such cells also showed reduced self-renewal and were less effective in homing to and repopulating host bone marrow. Targeted deletion of INK4A in mice improved the transplant potential of ageing stem cells and restored tolerance of cytotoxic agents.
- 26.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 27.Kozar K, et al. Mouse development and cell proliferation in the absence of D-type cyclins. Cell. 2003;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 28. Spike BT, et al. The Rb tumor suppressor is required for stress erythropoiesis. EMBO J. 2004;23:4319–4329. doi: 10.1038/sj.emboj.7600432. Shows that red cell maturation defects can be induced by acute deletion of Rb1 and haemolytic anaemia in vivo. Rb1-null haematopoietic tissue is also defective for long-term repopulation resulting in myeloproliferation and bone marrow failure.
- 29. Daria D, et al. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood. 2008;111:1894–1902. doi: 10.1182/blood-2007-02-071746. This paper shows that Rb1-deleted adult HSCs have reduced repopulating potential and increased activity in peripheral tissues. Following challenge with 5-fluorouracil, HSCs from these animals are more often in S phase of cell cycle and there is increased extramedullary haematopoiesis in the spleen.
- 30. Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. The authors showed that myeloproliferative disease in Rb1flox/flox;Mx1–Cre mice resulted from a failure in synergistic interactions between myeloid cells and the BM niche and was independent of the HSC defect.
- 31.Spike BT, Macleod KF. The Rb tumor suppressor in stress responses and hematopoietic homeostasis. Cell Cycle. 2005;4:e181–e184. doi: 10.4161/cc.4.1.1337. [DOI] [PubMed] [Google Scholar]
- 32.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nature Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 33.Fleming WH, et al. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J. Cell Biol. 1993;122:897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamurthy J, et al. p16INK4A induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 35.Molofsky AV, et al. Increasing p16INK4A expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park I, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 37.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 38.Williams BO, et al. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robanus-Maandag EC, et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whyatt D, Grosveld F. Cell-autonomous function of the retinoblastoma tumor suppressor protein: new interpretations of old phenotypes. EMBO Rep. 2002;3:130–135. doi: 10.1093/embo-reports/kvf033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walkley CR. Rb is dispensable for self-renewal and multilineage differentiation of adult hematopoietic stem cells. Proc. Natl Acad. Sci. USA. 2006;103:9057–9062. doi: 10.1073/pnas.0603389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 43.Hosokawa K, et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem. Biophys. Res. Comm. 2007;363:578–583. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Viatour P, et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. doi: 10.1016/j.stem.2008.07.009. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lecouter JE, et al. Strain-dependent myeloid hyperplasia, growth deficiency, and accelerated cell cycle in mice lacking the Rb-related p107 gene. Mol. Cell. Biol. 1998;18:7455–7465. doi: 10.1128/mcb.18.12.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nature Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 47.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 48.Thomas DM, et al. Retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol. Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J. Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 50. Miyamoto K, et al. FoxO3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. Knockout of Foxo3a in mice revealed a requirement for FOXO3A in HSCs, but not in myeloid progenitors. Foxo3a−/− HSCs were defective for colony formation in vitro and for repopulating capacity in vivo, had increased ROS levels and had reduced expression of SOD2 and catalase.
- 51. Tothova Z, et al. FoxOs are critical mediators of hematological resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. Increased ROS in Foxo-deleted HSCs, but not in myeloid progenitors, led the authors to show that INK4A induction, defective HSC repopulation and cell cycle control could be rescued by treatment of mice with NAC.
- 52. Marinkovic D, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J. Clin. Invest. 2007;117:2133–2144. doi: 10.1172/JCI31807. This work shows that loss of FOXO3A sensitizes mice to haemolytic anaemia induced by phenylhydrazine treatment. Loss of FOXO3A causes red blood cells to accumulate increased levels of ROS.
- 53. Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. Loss of ATM in the haematopoietic system results in reduced bone marrow cellularity, loss of stem cell self-renewal, increased INK4A expression and increased ROS.
- 54. Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic cells. Nature Med. 2006;12:446–451. doi: 10.1038/nm1388. Atm−/− HSCs showed increased p38 activity and inhibition of p38 with NAC, or with chemical inhibitors of p38 prevented induction of INK4A and restored HSC self-renewal.
- 55.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1–MAP kinase pathway in stress signaling. Biochim. Biophys. Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Ohtani N, et al. Opposing effects of Ets and Id proteins on p16INK4A expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 58.Tsai WB, Chung YL, Takahashi Y, Xu Z, Hu MCT. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nature Cell Biol. 2008;10:460–467. doi: 10.1038/ncb1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 61.Polager S, Kalma Y, Berkovich E, Ginsberg D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene. 2002;21:437–446. doi: 10.1038/sj.onc.1205102. [DOI] [PubMed] [Google Scholar]
- 62.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication and G2/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoskins EE, et al. Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene. 2008 Apr 28; doi: 10.1038/onc.2008.121. (doi: 10.1038/onc.2008.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Andrea AD, Grompe M. The Fanconi Anemia/BRCA pathway. Nature Rev. Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 65.O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nature Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 66.Venkitaraman AR. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nature Rev. Cancer. 2004;4:266–276. doi: 10.1038/nrc1321. [DOI] [PubMed] [Google Scholar]
- 67.Resnick IB, et al. Nijmegen breakage syndrome: clinical characteristics and mutation analysis in eight unrelated Russian families. J. Pediatr. 2002;140:355–361. doi: 10.1067/mpd.2002.122724. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi T, Morimoto K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage in lymphoblasts from Fanconi’s anemia patients due to possible catalase deficiency. Carcinogenesis. 1993;14:1115–1120. doi: 10.1093/carcin/14.6.1115. [DOI] [PubMed] [Google Scholar]
- 69.Clarke AA, Philpott NJ, Gordon-Smith EC, Rutherford TR. The sensitivity of Fanconi anaemia group C cells to apoptosis induced by mitomycin C is due to oxygen radical generation, not DNA cross-linking. Br. J. Haematol. 1997;96:240–247. doi: 10.1046/j.1365-2141.1997.d01-2023.x. [DOI] [PubMed] [Google Scholar]
- 70.Park SJ, et al. Oxidative stress/damage induces multimerization and interaction of Fanconi Anemia proteins. J. Biol. Chem. 2004;279:30053–30059. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- 71.Schindler D, Hoehn H. Fanconi anemia mutation causes cellular susceptibility to ambient oxygen. Am. J. Hum. Genet. 1988;43:429–435. [PMC free article] [PubMed] [Google Scholar]
- 72.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003;31:446–454. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 73.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 74.Tsantes AE, Bonovas S, Travlou A, Sitaras N. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid. Redox Signal. 2006;8:1205–1216. doi: 10.1089/ars.2006.8.1205. [DOI] [PubMed] [Google Scholar]
- 75.Neumann CA, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour supression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 76.Lee TH, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 77.Chan JY, Kwong M, Lo M, Emerson R, Kuypers FA. Reduced oxidative stress response in red blood cells from p45NFE2-deficient mice. Blood. 2001;97:2151–2158. doi: 10.1182/blood.v97.7.2151. [DOI] [PubMed] [Google Scholar]
- 78.Lan Q, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothman N, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C→T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57:2839–2842. [PubMed] [Google Scholar]
- 80.Hattangadi SM, Lodish HF. Regulation of erythrocyte lifespan: do reactive oxygen species set the clock? J. Clin. Invest. 2007;117:2075–2077. doi: 10.1172/JCI32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark AJ, Doyle KM, Humbert PO. Cell intrinsic functions of pRb in erythropoiesis. Blood. 2004;104:1324–1326. doi: 10.1182/blood-2004-02-0618. [DOI] [PubMed] [Google Scholar]
- 82.Kinross KM, Clark AJ, Iazzolino RM, Humbert PO. E2f4 regulates fetal erythropoiesis through the promotion of cellular proliferation. Blood. 2006;108:886–895. doi: 10.1182/blood-2005-09-008656. [DOI] [PubMed] [Google Scholar]
- 83.Dirlam A, Spike BT, Macleod KF. De-regulated E2f-2 activity underlies cell cycle and maturation defects in Rb null erythroblasts. Mol. Cell. Biol. 2007;27:8713–8728. doi: 10.1128/MCB.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cam H, et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. This work identifies overlapping sets of target genes regulated by E2F4 and NRF1.
- 85.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 86. Tracy K, et al. BNIP3 is a RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell. Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. The authors identify BNIP3 as a direct transcriptional target of RB-E2f and show a functional interaction with HIF at the BNIP3 promoter. Loss of RB increases BNIP3 expression and induces cell death associated with defective autophagy.
- 87.Bindra RS, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2006;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 88.Schlisio S, Halperin T, Vidal M, Nevins JR. Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. EMBO J. 2002;21:5775–5786. doi: 10.1093/emboj/cdf577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giangrande PH, Hallstrom TC, Tunyaplin C, Calame K, Nevins JR. Identification of E-box factor TFE3 as a functional partner for the E2F3 transcription factor. Mol. Cell Biol. 2003;23:3707–3720. doi: 10.1128/MCB.23.11.3707-3720.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giangrande PH, Zhu W, Rempel RE, Laasko N, Nevins JR. Combinatorial gene control involving E2F and E box family members. EMBO J. 2004;23:1336–1347. doi: 10.1038/sj.emboj.7600134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sankaran VG, Orkin SH, Walkley CR. Rb instrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 2008;22:463–475. doi: 10.1101/gad.1627208. The authors show that Rb1-null erythroblasts have uncoupled mitochondrial mass regulation from other aspects of terminal maturation, such as cell cycle exit and expression of key maturation markers.
- 92.Schweers RL, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl Acad. Sci. USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandoval H, et al. Essential role for Nix in autophagic maturation of red cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang C, et al. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc. Natl Acad. Sci. USA. 2006;103:11567–11572. doi: 10.1073/pnas.0603363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang H, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of c-Myc activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. The authors present data proposing that one method of coping with hypoxia is to reduce mitochondrial mass by inhibiting mitochondrial biogenesis.
- 96. Zhang H, et al. Mitochondrial autophagy is a HIF-1 dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. The authors present data suggesting that BNIP3 promotes mitophagy by titrating the inhibitory activity of BCL2 away from the crucial autophagy regulator beclin 1.
- 97.Tracy K, Macleod KF. Regulation of mitochondrial integrity, autophagy and cell survival by BNIP3. Autophagy. 2007;3:616–619. doi: 10.4161/auto.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamacher-Brady A, et al. Response to myocardial ischemia/reperfusion injury involves BNip3 and autophagy. Cell Death Diff. 2006;13:1–12. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 99.Mammucari C, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27:4860–4864. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- 101.Altiok S, Xu M, Spiegelman BM. PPARγ induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fajas L, et al. E2Fs regulate adipocyte differentiation. Dev. Cell. 2002;3:39–49. doi: 10.1016/s1534-5807(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 103.Scime A, et al. Rb and p107 regulate pre-adipocyte differentiation into white versus brown fat through repression of PGC-1a. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Esposito F, Russo L, Russo T, Cimino F. Retinoblastoma protein dephosphorylation is an early event of cellular response to pro-oxidant conditions. FEBS Lett. 2000;470:211–215. doi: 10.1016/s0014-5793(00)01318-1. [DOI] [PubMed] [Google Scholar]
- 105.Magenta AFP, Romani S, Di Stefano V, Capogrossi MC, Martelli F. PP2A-PR70 interacts with pRb and mediates its de-phosphorylation. Mol. Cell. Biol. 2008;28:873–882. doi: 10.1128/MCB.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamauchi A, Bloom ET. Control of cell cycle progression in human natural killer cells through redox regulation of expression and phosphorylation of retinoblastoma gene product protein. Blood. 1997;89:4092–4099. [PubMed] [Google Scholar]
- 107.Guardavaccaro D, Pagano M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell. 2006;22:1–4. doi: 10.1016/j.molcel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylatiton-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ji P, et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell. 2004;16:47–58. doi: 10.1016/j.molcel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 110.Binne UK, et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nature Cell Biol. 2006;9:225–232. doi: 10.1038/ncb1532. [DOI] [PubMed] [Google Scholar]
- 111.Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature. 2005;432:640–645. doi: 10.1038/nature03173. [DOI] [PubMed] [Google Scholar]
- 112.Chan HM, Krstic-Demonacos M, Smith L, Demonacos C, La Thangue N. Acetylation control of the retinoblastoma tumour suppressor protein. Nature Cell Biol. 2001;3:667–674. doi: 10.1038/35083062. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen DX, Baglia LA, Huang SM, Baker CM, McCance DJ. Acetylation regulates the differentiation-specific functions of the retinoblastoma protein. EMBO J. 2004;23:1609–1618. doi: 10.1038/sj.emboj.7600176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong S, Weber S. Deacteylation of the retinoblastoma tumour suppressor protein by SIRT1. Biochem. J. 2007;407:451–460. doi: 10.1042/BJ20070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo J, et al. Negative control of p53 by Sir2a promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 116.Vaziri H, et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 117.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1a and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 118.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1a. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Fulco M, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 120.Puri PL, et al. Class 1 histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell. 2001;8:885–897. doi: 10.1016/s1097-2765(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 121.Di Carlo A, et al. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 2004;279:16332–16338. doi: 10.1074/jbc.M313931200. [DOI] [PubMed] [Google Scholar]
- 122. Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol. Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. CITED2 is an inhibitor of p300–CBP and feeds back to inhibit p300-dependent transactivation by HIF. Induction of CITED2 by hypoxia and by FOXO3A is HIF-dependent. Knockdown of FOXO3 sensitized tumour cells to hypoxia-induced cell death.
- 123.Parmar K, Mauch P, Vergillo JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gustaffson MV, et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev. Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 126.Lee AC, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 127.Di Mocco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 128.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 129.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 130. Dolado I, et al. p38α MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. Upregulation of glutathione S-transferase m2 (Gstm2) in HRAS-transformed cells is p38α-dependent. Overexpression of Gstm2 limited p38α activation and desensitized cells to ROS-induced apoptosis.
- 131. Gauthier ML, et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12:479–491. doi: 10.1016/j.ccr.2007.10.017. Shows that overexpression of COX2 induced INK4A expression and growth arrest. This arrest is RB-dependent and, conversely, COX2 expression is induced by inactivation of RB.
- 132.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nature Rev. Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim H, et al. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J. Biol. Chem. 2005;280:21237–21245. doi: 10.1074/jbc.M413842200. [DOI] [PubMed] [Google Scholar]
- 134.Gauthier ML, et al. p38 regulates cyclooxygenase-2 in human mammary epithelial cells and is activated in pre-malignant tissue. Cancer Res. 2005;65:1792–1799. doi: 10.1158/0008-5472.CAN-04-3507. [DOI] [PubMed] [Google Scholar]
- 135.Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo A. Cyclo-oxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 136.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 137.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocynate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 139.Wikemheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell. Mol. Life Sci. 2006;63:767–780. doi: 10.1007/s00018-005-5487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bremner R, et al. Direct transcriptional repression by Rb and its reversal by specific cyclins. Mol. Cell. Biol. 1995;15:3256–3265. doi: 10.1128/mcb.15.6.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weintraub SJ, et al. Mechanisms of active transcriptional repression by Rb. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 142.Brehm A, Kouzarides T. Retinoblastoma protein meets chromatin. Trends Biochem. Sci. 1999;24:142–145. doi: 10.1016/s0968-0004(99)01368-7. [DOI] [PubMed] [Google Scholar]
- 143.Hernando E, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 144.Mittnacht S. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 145.Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- 146.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nature Rev. Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 147.Chau BN, Wang JYJ. Coordinated regulation of life and death by RB. Nature Rev. Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- 148.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at Complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 149.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumor suppression: the senesence connection. Nature Rev. Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 151.Wu L, et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- 152.Wenzel PL, et al. Rb is critical in a mammalian tissue stem cell population. Genes Dev. 2007;21:85–97. doi: 10.1101/gad.1485307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lipinski M, Macleod KF, Crowley D, Mullaney T, Jacks T. Cell-autonomous and non-cell autonomous developmental functions of the retinoblastoma tumor suppressor gene in vivo. EMBO J. 2001;20:3402–3413. doi: 10.1093/emboj/20.13.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spike BT, Macleod KF. Effects of hypoxia on heterotypic macrophage interactions. Cell Cycle. 2007;6:2620–2624. doi: 10.4161/cc.6.21.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spike BT, Dibling BC, Macleod KF. Hypoxic stress underlies defects in erythroblast island formation in the Rb null mouse. Blood. 2007;110:2173–2181. doi: 10.1182/blood-2007-01-069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iavarone A, et al. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature. 2004;432:1040–1045. doi: 10.1038/nature03068. [DOI] [PubMed] [Google Scholar]
- 157.Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 158.Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–3495. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- 159.Dickson C, et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995;90:43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- 160.Foster CS, et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871–5879. doi: 10.1038/sj.onc.1207800. [DOI] [PubMed] [Google Scholar]
- 161.Oeggerli M, et al. E2F3 is the main target gene of the 6p22 amplicon with high specificity for human bladder cancer. Oncogene. 2006;25:6538–6543. doi: 10.1038/sj.onc.1209946. [DOI] [PubMed] [Google Scholar]
- 162.Moroni MC, et al. Apaf-1 is a transcriptional target for E2F and p53. Nature Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 163.Nahle Z, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nature Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 164. Hallstrom T, Mori S, Nevins JR. An E2F-1 dependent gene expression program that determines the balance betweeen proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. Expression profiling was used to identify pro-apoptotic E2F1 target genes that are repressed by phosphoinositide 3-kinase and identified AMPKα2, CYO26B1, SOS2 and GRB7, amongst others.
- 165.Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F-1 mediates apoptosis. J. Biol. Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 166.Brugarolas J, Bronson RT, Jacks T. p21 is a critical Cdk2 regulator essential for proliferation control in Rb-deficient cells. J. Cell. Biol. 1998;141:503–514. doi: 10.1083/jcb.141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith KS, et al. Bmi-1 regulation of INK4A–ARF is a downstream requirement for transformation of hematopoietic progenitors by E2a–Pbx1. Mol. Cell. 2003;12:393–400. doi: 10.1016/s1097-2765(03)00277-6. [DOI] [PubMed] [Google Scholar]
- 168.Gilley J, Coffer PJ, Ham J. FoxO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell. Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kranc KR, et al. Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via INk4a/ARF. Mol. Cell. Biol. 2003;23:7658–7666. doi: 10.1128/MCB.23.21.7658-7666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hershko T, Korotayev K, Polager S, Ginsberg D. E2F-1 modulates p38 MAPK phosphorylation via transcriptional regulation of Ask1 and Wip1. J. Biol. Chem. 2006;281:31309–31316. doi: 10.1074/jbc.M601758200. [DOI] [PubMed] [Google Scholar]
- 171.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim. Biophys. Acta. 2007;1769:244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 172.Tanaka H, et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell. 2002;9:1017–1029. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- 173.Rius J, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen J, et al. Generation of normal lymphocyte populations of Rb-deficient ES cells: analysis of gene function by RAG-2-deficient blastocyst complementation. Curr. Biol. 1993;3:405–413. doi: 10.1016/0960-9822(93)90347-q. [DOI] [PubMed] [Google Scholar]
- 175.Lasorella A, Noseda M, Beyna M, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- 176.de Bruin A, et al. Rb function in extraembryonic tissue lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc. Natl Acad. Sci. USA. 2003;100:6546–6551. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ye M, et al. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multi-lineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]