Abstract
Adiponectin is an adipocyte-secreted protein involved in a variety of metabolic processes, including glucose regulation and fatty acid catabolism. We conducted a genome-wide association study to investigate the genetic loci associated with plasma adiponectin in 1776 unrelated Filipino women from the Cebu Longitudinal Health and Nutrition Survey (CLHNS). Our strongest signal for adiponectin mapped to the gene CDH13 (rs3865188, P ≤ 7.2 × 10−16), which encodes a receptor for high-molecular-weight forms of adiponectin. Strong association was also detected near the ADIPOQ gene (rs864265, P = 3.8 × 10−9) and at a novel signal 100 kb upstream near KNG1 (rs11924390, P = 7.6 × 10−7). All three signals were also observed in 1774 young adult CLHNS offspring and in combined analysis including all 3550 mothers and offspring samples (all P ≤ 1.6 × 10−9). An uncommon haplotype of rs11924390 and rs864265 (haplotype frequency = 0.050) was strongly associated with lower adiponectin compared with the most common C–G haplotype in both CLHNS mothers (P = 1.8 × 10−25) and offspring (P = 8.7 × 10−32). Comprehensive imputation of 2653 SNPs in a 2 Mb region using as reference combined CHB, JPT and CEU haplotypes from the 1000 Genomes Project revealed no variants that perfectly tagged this haplotype. Our findings provide the first genome-wide significant evidence of association with plasma adiponectin at the CDH13 locus and identify a novel uncommon KNG1–ADIPOQ haplotype strongly associated with adiponectin levels in Filipinos.
INTRODUCTION
Adiponectin, an adipokine secreted by adipocytes, is believed to play important roles in various metabolic processes, including glucose regulation and fatty acid catabolism (1). Plasma adiponectin level is negatively correlated with body mass index (BMI), glucose, insulin and triglyceride levels and is positively associated with high-density lipoprotein cholesterol (HDL-C) concentration and insulin-stimulated glucose disposal (1). Animal studies have reported that administration of adiponectin attenuated insulin resistance and improved endothelial dysfunction (2,3). The protective effect of adiponectin is further supported by epidemiological studies in which individuals with obesity, type 2 diabetes and coronary artery disease had decreased circulating adiponectin concentrations (4,5). Therefore, hypoadiponectinemia has been suggested as an independent risk factor for metabolic syndrome and may lead to obesity, type 2 diabetes and atherosclerosis (6,7).
Circulating adiponectin level is under substantial genetic influence. An estimated 30–70% of the variability in plasma adiponectin is explained by genetic variation (8–10). Genome-wide linkage scans for adiponectin level produced linkage signals, but replications were inconsistent across different ethnic populations (8–10). Encoding the adiponectin protein, the gene ADIPOQ has been widely investigated for variants associated with circulating adiponectin. Several SNPs including rs17300539 (−11391G>A), rs2241766 (+45T>G) and rs1501299 (+276G>T) have been reproducibly reported to be associated with circulating adiponectin (11–16). Two reports suggested the presence of distinct association signals for adiponectin levels represented by 5′-promoter SNP rs17300539 and either rs6773957 (17) or rs182052 (18), and a gene-wide tagSNP investigation revealed that two haplotype blocks mapping to the ADIPOQ promoter and the relevant exons showed association with adiponectin (19). Only limited evidence has been provided for the functional relevance of these SNPs (20,21), and the common genetic variants identified thus far explain only a fraction of the estimated heritability for adiponectin (22).
Genome-wide association studies (GWAS) provide a more complete characterization of common genetic determinants across the genome. The first GWAS for adiponectin, conducted in a population of northern and western European origin, confirmed the strong association with variants in ADIPOQ and reported initial evidence of association with CDH13 (rs7195409, P = 2.0 × 10−5), although the latter finding did not reach a level of genome-wide significance (23). A recent meta-analysis of three GWAS revealed a new locus, ARL15 (rs4311394, P = 2.9 × 10−8), and provided further evidence that variants at ARL15 may be associated with risk of type 2 diabetes in European populations (24). The evidence for loci associated with circulating adiponectin remains unclear in populations of non-European ancestry. Therefore, the primary goal of this study was to perform a GWAS to investigate SNP associations with plasma adiponectin level in 1776 Filipino mothers from the Cebu Longitudinal Health and Nutrition Survey (CLHNS). We also genotyped selected SNPs and tested their association with adiponectin concentration in 1774 young adult offspring of the CLHNS mothers to test evidence in both sexes and in a younger Asian population.
RESULTS
GWAS results in CLHNS mothers
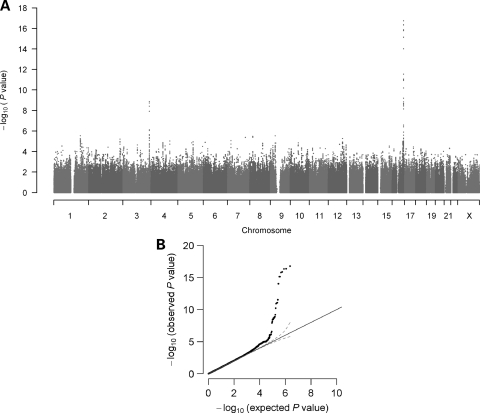
A GWAS for plasma adiponectin in 1776 CLHNS women (Table 1) showed 38 SNPs associated at P < 10−6 that clustered in three chromosome regions. The observed genomic control inflation factor (λGC) was 1.03, suggesting no substantial population stratification among the CLHNS mothers sample (Fig. 1). The strongest signal mapped to CDH13 locus on chromosome 16 (rs3865188, P = 7.2 × 10−16) and two signals were located on chromosome 3, near ADIPOQ (rs864265, P = 3.8 × 10−9) and ∼100 kb upstream of ADIPOQ near KNG1 (rs11924390, P = 7.6 × 10−7). Conditioning on rs3865188, the remaining 22 nearby CDH13 SNPs showed no evidence for a secondary signal (P > 0.13, Supplementary Material, Fig. S1). Conditional analysis for SNPs within the KNG1–ADIPOQ gene region on chromosome 3 showed that the two nearby loci were not completely independent (Supplementary Material, Fig. S2). In models accounting for the ADIPOQ variant rs864265, the strength of association slightly increased for the KNG1 SNP rs11924390 (conditioned P = 8.7 × 10−8), but was greatly attenuated for other ADIPOQ variants (P > 0.13). Similarly, when the KNG1 variant rs11924390 was included in the statistical model, the strength of association for the ADIPOQ SNP rs864265 became more significant (conditioned P = 4.4 × 10−10), whereas the association strength with other KNG1 SNPs was attenuated (P > 0.53).
Table 1.
General characteristics of CLHNS mothers and young adult offspring
| Mothers | Independent offspring | All offspring | |
|---|---|---|---|
| N | 1776 | 336 | 1774 |
| Female (%) | 100 | 44.4 | 47.3 |
| Adiponectin (µg/ml) | 2.48 (1.90, 3.32) | 2.37 (1.88, 3.12) | 2.47 (1.94, 3.10) |
| Age in 2005 (years) | 48.4 ± 6.1 | 21.5 ± 0.3 | 21.5 ± 0.3 |
| Household income in 2005 (pesos/week) | 396.3 (243.7, 623.0) | 326.7 (196.1, 563.8) | 358.1 (213.6, 585.2) |
| Household assets in 2005 (0 to 11) | 5.2 ± 2.0 | 5.1 ± 2.1 | 5.2 ± 2.0 |
| Number of previous pregnancies | 6.5 ± 3.0 | – | – |
| Post-menopausal (%) | 38.2 | – | – |
| Waist circumference (cm) | 81.1 ± 10.9 | 71.2 ± 8.3 | 70.3 ± 7.8 |
| BMI (kg/m2) | 24.1 (21.4, 27.0) | 20.5 (18.8, 22.7) | 20.2 (18.7, 22.2) |
Data are mean ± SD, median (25th percentile, 75th percentile) or %. Independent offspring (n = 336) are a subgroup of individuals within the 1774 offspring sample whose mother was not included in the 1776 CLHNS mothers cohort for GWA analysis.
Figure 1.
Genome-wide association with plasma adiponectin in CLHNS mothers cohort. (A) Manhattan plot, (B) quantile–quantile plot of observed and expected P-values for association with the adiponectin level. The observed genomic control inflation factor (λGC) was 1.03.
We next tested whether these associations with adiponectin were affected by adjustment for waist circumference. The association became stronger for CDH13 (P = 4.1 × 10−21) and ADIPOQ (P = 9.2 × 10−11), but did not change substantially for KNG1 (P = 2.4 × 10−6). These findings may indicate that adjusting for waist circumference removes an independent source of variation in adiponectin levels.
Follow-up genotyping results in CLHNS mothers and offspring samples
To confirm the evidence of the adiponectin association based on imputed SNPs, we directly genotyped CDH13 rs3865188 and KNG1 rs11924390 in 1776 CLHNS mothers. Compared with the original association based on imputed SNPs, the evidence remained comparably significant when using experimentally determined genotypes (rs3865188 P = 3.8 × 10−16; rs11924390 P = 2.2 × 10−6, Table 2); high quality imputed genotypes were analyzed for rs864265 (MACH r2 > 0.99, see also Materials and Methods).
Table 2.
Strongest loci associated with adiponectin in CLHNS mother and young adult offspring cohorts
| SNP | Gene | Allele 1 | Allele 2 | Mothers (n = 1776) |
Independent offspring (n = 336) |
All offspring (n = 1774) |
Combined (n = 3550) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | β (SE) | P-value | β (SE) | P-value | β (SE) | P-value | β (SE) | P-value | ||||
| rs3865188 | CDH13 | T | A | T: 0.467 | −0.111 (0.014) | 3.8E−16 | −0.131 (0.028) | 3.7E-06 | −0.106 (0.012) | 2.3E−18 | −0.109 (0.009) | 4.1E−30 |
| rs864265 | ADIPOQ | G | T | T: 0.124 | 0.123 (0.021) | 3.8E−09 | 0.144 (0.041) | 0.0005 | 0.141 (0.018) | 1.3E−14 | 0.130 (0.014) | 1.4E−19 |
| rs11924390 | KNG1 | C | T | T: 0.499 | −0.067 (0.014) | 2.2E−06 | −0.086 (0.031) | 0.0059 | −0.046 (0.012) | 2.2E−04 | −0.059 (0.010) | 1.6E−09 |
Directly genotyped SNPs were used in analysis except rs864265, which was imputed in CLHNS mothers (MACH r2> 0.99). CLHNS mothers analysis was adjusted for age, age2, household assets, natural log-transformed household income and menopausal status; CLHNS offspring analyses were adjusted for sex, household assets and natural log-transformed household income. The mixed model analysis of combined samples was adjusted for age, age2, sex, household assets, natural log-transformed household income and generation (mothers/offspring). MAF, minor allele frequency; β, change in natural log-transformed adiponectin (µg/ml) with each additional copy of Allele 1. All SNPs are aligned to the HapMap forward DNA strand.
We also genotyped the three index SNPs in the CLHNS young adult offspring (Table 1). We first examined the association in a subgroup of the offspring sample consisting of 336 individuals whose mothers were not present in CLHNS mothers GWAS sample. Significant association (Bonferroni corrected P < 0.017, 0.05/3 tests) was observed for rs3865188 (P = 3.7 × 10−6), rs864265 (P = 5.1 × 10−4) and rs11924390 (P = 0.0059) (Table 2). When all 1774 offspring were included in the analysis, we observed significant evidence of association for rs3865188 (P = 2.3 × 10−18), rs864265 (P = 1.3 × 10−14) and rs11924390 (P = 2.2 × 10−4) (Table 2). When the mother and offspring samples were combined using a linear mixed effect model that accounted for their relationships, the associations reached genome-wide significance (rs3865188, P = 4.1 × 10−30; rs864265, P = 1.4 × 10−19 and rs11924390, P = 1.6 × 10−9, Table 2).
Two-SNP haplotype effect of rs11924390 (KNG1) and rs864265 (ADIPOQ) on plasma adiponectin
Given the close proximity of the KNG1 and ADIPOQ loci, we performed haplotype analyses to investigate the association between adiponectin and the specific two-SNP haplotypes consisting of the lead SNPs at KNG1 (rs11924390) and ADIPOQ (rs864265) in both the CLHNS mothers and the offspring. The effect on adiponectin of each additional copy of the specific haplotype compared with the homozygote reference haplotype was assessed. The uncommon C–T haplotype with a frequency of 0.050 was significantly associated with a decreased level of adiponectin compared with the most common C–G haplotype in CLHNS mothers (P = 1.8 × 10−25, Table 3). Similar findings of haplotype association were observed in the 336 independent offspring (P = 9.5 × 10−12) and in the entire set of 1774 offspring (P = 8.7 × 10−32, Table 3). Further analyses that computed score statistics provided significant evidence for an overall association between haplotypes and adiponectin level (global P = 1.1 × 10−27, 5.0 × 10−4 and 5.9 × 10−35 in CLHNS mothers, independent offspring subgroup and all offspring, respectively). In both mothers and offspring, the uncommon C–T haplotype showed the strongest evidence of association, suggesting that this haplotype is largely responsible for the observed associations between adiponectin and the individual SNPs. Comprehensive imputation of 2653 SNPs in a 2 Mb region using as reference combined CHB, JPT and CEU haplotypes from the 1000 Genomes Project revealed no variants that perfectly tagged this haplotype (Supplementary Material, Fig. S3).
Table 3.
Adiponectin association with haplotypes consisting of SNPs rs11924390 (KNG1) and rs864265 (ADIPOQ)
| rs11924390 | rs864265 | Estimated haplotype frequency | β | SE | P-value |
|---|---|---|---|---|---|
| CLHNS mothers (n = 1776) | |||||
| C | G | 0.450 | Reference | ||
| T | G | 0.426 | 0.021 | 0.015 | 0.16 |
| T | T | 0.074 | 0.064 | 0.028 | 0.020 |
| C | T | 0.050 | −0.385 | 0.036 | 1.8E−25 |
| Independent CLHNS offspring (n = 336) | |||||
| C | G | 0.442 | Reference | ||
| T | G | 0.405 | 0.027 | 0.030 | 0.38 |
| T | T | 0.096 | 0.098 | 0.052 | 0.063 |
| C | T | 0.056 | −0.504 | 0.071 | 9.5E−12 |
| All CLHNS offspring (n = 1774) | |||||
| C | G | 0.444 | Reference | ||
| T | G | 0.427 | 0.004 | 0.013 | 0.78 |
| T | T | 0.076 | 0.027 | 0.025 | 0.27 |
| C | T | 0.052 | −0.386 | 0.032 | 8.7E−32 |
The ‘haplo.glm' function implemented in the ‘haplo.stats' R package was used to calculate the coefficient β and P-value for each haplotype compared with the reference haplotype, which was set as the most common haplotype C–G. The same covariates used for genotype analysis were applied in haplotype analysis. Using the ‘haplo.score' function implemented in the ‘haplo.stats' R package, the global P-values for association between haplotypes and adiponectin level were 1.1 × 10−27, 5.0 × 10−4 and 5.9 × 10−35 in CLHNS mothers, independent offspring subgroup and all offspring sample, respectively.
Adiponectin association with previously reported SNPs
We next examined whether associations in the CLHNS mother sample replicated evidence for loci previously reported in either candidate gene studies or GWAS (Table 4). Among ADIPOQ variants identified by a candidate gene approach, the widely reported promoter SNP rs17300539 (−11391 G>A) with potential biological function (16) was monomorphic in the CLHNS mothers. Four other candidate gene variants rs266729 (−11377 C>G), rs2241766 (+45 T>G), rs1501299 (+276 G>T) and rs1063537 (11,13,16) were marginally associated with adiponectin (P = 0.018, 0.0077, 0.059 and 0.017, respectively). The GWAS SNP rs3774261 (23) displayed significant association in the same direction in CLHNS (P = 7.0 × 10−5). Although rs3774261 was in weak LD with the ADIPOQ index SNP rs864265 (r2 = 0.02 based on HapMap CHB and JPT combined sample), reciprocal conditional analyses suggested that adiponectin associations with these variants are not independent (P for rs3774261 conditional on rs864265 = 0.066, Table 4; P for rs864265 conditional on rs3774261 = 2.4 × 10−6). We did not replicate associations with other GWAS SNPs (23,24) in ARL15 (rs4311394, P = 0.53) and LYZL1 (rs1774950, P = 0.73). Notably, the most strongly associated CDH13 SNP reported previously (rs7195409) (23) was not significantly associated with adiponectin in the CLHNS mothers (P = 0.16). The strongest CDH13 SNP in the CLHNS mothers (rs3865188, P= 3.8 × 10−16) exhibited only limited LD with rs7195409 (r2 = 0 and D′ = 0.06 in HapMap CHB and JPT combined samples, Table 4).
Table 4.
Evidence of association in CLHNS mothers of SNPs previously reported to be associated with adiponectin
| SNP (alias) | Closest gene | Chr | Position | Allele 1 > 2 | MAF | Imputation qualitya | Baseline model |
bLD r2 | Conditioned Pc | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P-value | ||||||||||
| rs16861194 (−11426 A>G) | ADIPOQ | 3 | 188,042,119 | A>G | G: 0.18 | 0.95 | −0.021 (0.019) | 0.26 | 0.013 | 0.95 | (21) |
| rs17300539 (−11391 G>A) | ADIPOQ | 3 | 188,042,154 | G>A | A: 0.00 | – | – | – | ND | ND | (16) |
| rs266729 (−11377 C>G) | ADIPOQ | 3 | 188,042,168 | G>C | G: 0.27 | 0.96 | −0.038 (0.016) | 0.018 | 0.029 | 1.7E−04 | (16) |
| rs182052 | ADIPOQ | 3 | 188,043,476 | A>G | A: 0.45 | 1.00 | −0.017 (0.014) | 0.22 | 0.057 | 9.1E−04 | (19) |
| rs17366568 | ADIPOQ | 3 | 188,053,147 | G>A | A: 0.03 | 0.98 | 0.034 (0.043) | 0.44 | 0.001 | 0.54 | (29) |
| rs2241766 (+45 T>G) | ADIPOQ | 3 | 188,053,594 | G>T | G: 0.26 | 0.79 | 0.047 (0.017) | 0.0077 | ND | 0.10 | (13) |
| rs1501299 (+276 G>T) | ADIPOQ | 3 | 188,053,825 | G>T | T:0.39 | 0.90 | −0.028 (0.015) | 0.059 | 0.000 | 0.75 | (13) |
| rs3774261 | ADIPOQ | 3 | 188,054,253 | G>A | G:0.37 | 1.00 | −0.056 (0.014) | 7.0E−05 | 0.019 | 0.066 | (23) |
| rs6773957 | ADIPOQ | 3 | 188,056,407 | G>A | G:0.37 | 1.00 | −0.056 (0.014) | 7.0E−05 | 0.019 | 0.066 | (23) |
| rs1063537 | ADIPOQ | 3 | 188,056,769 | C>T | T:0.22 | 0.96 | −0.041 (0.017) | 0.017 | 0.020 | 0.12 | (11) |
| rs4311394 | ARL15 | 5 | 53,336,419 | A>G | G:0.44 | 0.99 | 0.009 (0.014) | 0.53 | ND | ND | (24) |
| rs1774950 | LYZL1 | 10 | 82,085,093 | T>C | T:0.44 | 0.99 | 0.005 (0.014) | 0.73 | ND | ND | (23) |
| rs7195409 | CDH13 | 16 | 82,085,093 | A>G | G:0.10 | 0.99 | 0.032 (0.023) | 0.16 | 0 | 0.10 | (23) |
Analyses were adjusted for age, age2, household assets, natural log-transformed household income and menopausal status (n = 1776). β (SE) indicates the effect size and standard error of each additional copy of Allele 1 on natural log-transformed adiponectin level. The SNP rs17300539 (−11391G>A) was monomorphic in direct TaqMan genotyping.
aMACH r2 was applied to indicate the imputation quality of the imputed SNPs.
bLinkage disequilibrium (LD) measure r2 with the index SNP of each locus (ADIPOQ: rs864265 and CDH13: rs3865188), based on the HapMap CHB + JPT combined sample.
cP-values for SNP association when conditional analysis was performed using the index SNP of each locus; ND, no data. Position for all SNPs is relative to NCBI Build 36 assembly.
Association of CDH13, ADIPOQ and KNG1 variants with other metabolic-related traits
Consistent with findings in previous studies (25,26), plasma adiponectin was highly correlated with other metabolic-related traits including BMI, waist circumference, lipid profiles, insulin and homeostasis model assessment (HOMA) index in both the mothers and offspring (Supplementary Material, Table S1). We next assessed the strongest adiponectin SNPs at CDH13, ADIPOQ and KNG1 for their associations with these related quantitative traits (Table 5). In the CLHNS mothers, nominal associations were detected for CDH13 SNP rs3865188 with waist circumference (P = 0.042) and for ADIPOQ SNP rs864265 with triglycerides (P = 0.047). In the offspring, the G allele of ADIPOQ SNP rs864265 that was associated with higher adiponectin level displayed nominally significant association with lower BMI (P = 0.0086), lower waist circumference (P = 0.0066), lower insulin level (P = 0.0011), decreased HOMA-IR (P = 0.0027) and decreased HOMA-β (P = 6.3 × 10−4). The association between rs864265 and HOMA-β remained significant after Bonferroni correction for multiple testing (P < 0.0008, 0.05/60 tests). After including adiponectin level as a covariate in the model, the P-values for the associations with insulin, HOMA-IR and HOMA-β remained nominally significant at 0.014, 0.026 and 0.0072, respectively, indicating that the genetic effects of rs864265 on these traits may be partially independent of adiponectin level.
Table 5.
Association of adiponectin-associated SNPs with other metabolic traits
| SNP | Log_BMI | Waist circumference | Log_TG | Log_HDL-C | Log_LDL-C | Log_glucose | Log_insulin | Log_HOMA-IR | Log_HOMA-β | |
|---|---|---|---|---|---|---|---|---|---|---|
| CLHNS mothers (n = 1776) | ||||||||||
| rs3865188 CDH13 | β (SE) | −0.007 (0.006) | −0.72 (0.35) | −0.009 (0.016) | −0.000 (0.009) | 0.004 (0.010) | −0.005 (0.007) | −0.022 (0.021) | −0.027 (0.024) | −0.008 (0.021) |
| P-value | 0.25 | 0.042 | 0.58 | 0.97 | 0.66 | 0.49 | 0.29 | 0.25 | 0.72 | |
| rs864265 ADIPOQ | β (SE) | 0.004 (0.009) | 0.30 (0.54) | 0.049 (0.025) | 0.002 (0.013) | 0.016 (0.015) | 0.005 (0.011) | 0.041 (0.031) | 0.046 (0.036) | 0.026 (0.032) |
| P-value | 0.64 | 0.58 | 0.047 | 0.89 | 0.26 | 0.68 | 0.19 | 0.20 | 0.42 | |
| rs11924390 KNG1 | β (SE) | 0.006 (0.006) | 0.30 (0.36) | 0.005 (0.017) | 0.000 (0.009) | 0.011 (0.010) | −0.004 (0.007) | 0.032 (0.021) | 0.028 (0.024) | 0.040 (0.022) |
| P-value | 0.34 | 0.41 | 0.77 | 0.99 | 0.25 | 0.56 | 0.13 | 0.25 | 0.063 | |
| CLHNS offspring (n = 1774) | ||||||||||
| rs3865188 CDH13 | β (SE) | −0.001 (0.005) | 0.08 (0.26) | −0.017 (0.017) | −0.007 (0.008) | −0.000 (0.010) | −0.005 (0.003) | −0.021 (0.019) | −0.026 (0.020) | −0.008 (0.019) |
| P-value | 0.77 | 0.75 | 0.32 | 0.38 | 0.99 | 0.091 | 0.27 | 0.18 | 0.67 | |
| rs864265 ADIPOQ | β (SE) | −0.019 (0.007) | −1.07 (0.39) | −0.008 (0.025) | 0.021 (0.013) | −0.007 (0.015) | 0.002 (0.005) | −0.091 (0.028) | −0.089 (0.030) | −0.097 (0.028) |
| P-value | 0.0086 | 0.0066 | 0.76 | 0.091 | 0.63 | 0.61 | 0.0011 | 0.0027 | 6.3E−04 | |
| rs11924390 KNG1 | β (SE) | −0.003 (0.005) | −0.06 (0.26) | −0.009 (0.017) | 0.000 (0.008) | 0.002 (0.010) | 0.003 (0.003) | 0.007 (0.019) | 0.010 (0.020) | −0.004 (0.019) |
| P-value | 0.51 | 0.82 | 0.58 | 0.99 | 0.88 | 0.34 | 0.73 | 0.63 | 0.85 | |
CLHNS mothers analysis was adjusted for age, age2, household assets, natural log-transformed household income and menopausal status; CLHNS offspring analysis was adjusted for sex, household assets and natural log-transformed household income. The reference alleles are the same as those indicated in Table 2.
DISCUSSION
The present study provides strong evidence of association with adiponectin at CDH13, ADIPOQ and KNG1 that together explain approximately 7.5 and 8.9% of the variability in natural log-transformed adiponectin levels in 1776 Filipino mothers and 1774 offspring, respectively. These data provide the first genome-wide significant association at CDH13 and compelling evidence for a novel uncommon haplotype near ADIPOQ. Only nominally significant associations were detected between ADIPOQ SNP rs864265 and multiple metabolic-related traits in the CLHNS offspring, and the effects of the associations with insulin, HOMA-IR and HOMA-β were partially independent of adiponectin level.
Our strongest main effect signal mapped to the gene CDH13 on chromosome 16 and explained 3.7% of the variability in the mothers and 4.3% in the offspring. The CDH13 gene encodes the protein T-cadherin, which is a receptor for hexameric and high-molecular-weight forms of adiponectin (27). T-cadherin is highly expressed in endothelial and smooth muscle cells, where it interacts with adiponectin and may protect vascular endothelial cells from apoptosis (28). The association of adiponectin with a SNP near CDH13 was recently reported in a GWAS consisting of 2280 northern and western Europeans (rs7195409, P = 1.99 × 10−5) (23), but we did not strongly support association with that SNP in the CLHNS mothers (P = 0.16). Nonetheless, our data provided solid evidence that the CDH13 locus is associated within adiponectin levels. We observed a stronger and separate signal for adiponectin in the CDH13 locus at rs3865188 (P = 6.7 × 10−16), which resides in the gene's promoter region, about 887 kb upstream of the reported variant rs7195409. Very weak linkage disequilibrium exists between rs7195409 and rs3865188 (r2 = 0, D′ = 0.12 in CEU and r2 = 0, D′ = 0.06 in CHB and JPT combined samples), and conditional analyses further supported their independence (P for rs3865188 conditional on rs7195409 = 5.0 × 10−16; P for rs7195409 conditional on rs3865188 = 0.11). In addition, for the CDH13 variant rs11646213, which was previously reported in a study of 4659 European individuals (P = 0.10), we observed stronger association in the 1776 CLHNS mothers (P = 1.27 × 10−6) (29). Together, these results suggest that a difference in genetic architecture between Europeans and Asians within the CDH13 locus may explain our lack of replication of the previous SNP as well as our strong association with other SNPs near the same gene.
The CLHNS samples also showed strong evidence for an association between adiponectin and rs11924390 near the gene KNG1, which has not been reported previously. KNG1 encodes two different proteins of high-molecular-weight kininogen (HMWK) and low-molecular-weight kininogen via alternative splicing. HMWK is essential for blood coagulation and bradykinin release. A common variant (rs710446) was recently reported to associate with activated partial thromboplastin time (aPTT), which is related to the risk of thrombosis and coagulation disorders (30). The SNP rs710446 is located approximately 27 kb away from rs11924390 and we found no association of this SNP with adiponectin in the CLHNS mothers (P = 0.45). The two SNPs are in weak pair-wise linkage disequilibrium (r2 = 0.07, D' = 0.59 based on HapMap CHB and JPT combined samples), suggesting that the aPTT signal in KNG1 is distinct from the adiponectin signal we detected.
The gene region of ADIPOQ had strong prior evidence of association with adiponectin level as a candidate gene and was validated by recent GWA studies (11,16–18,20,21,23,29). The Framingham Offspring Study, which systematically examined the associations with 22 SNPs that captured all common variation at r2 > 0.8 across ADIPOQ and its flanking regions, observed that adiponectin level was significantly associated with SNPs in two different regulatory regions, the 5′-promoter (rs17300539) and the 3′-untranslated regions (rs6773957) (17). A recent study dissecting the genetic architecture of plasma adiponectin suggested that two ADIPOQ variants (rs17300539 and rs182052) exhibited independent association (18), and a recent GWAS of 4659 European individuals suggested association with at least nine SNP groups near ADIPOQ (29). In the CLHNS samples, conditional analyses suggest that common SNPs in or near the ADIPOQ gene (chr3:188 032–188 070 kb, spanning 11 kb up- and downstream of exons) likely represent a single signal for plasma adiponectin (Supplementary Material, Fig. S2), although none of the SNPs imputed with high quality perfectly tags the strongly associated rs11924390–rs864265 haplotype.
Great efforts have been made by previous studies to pinpoint the functional variant(s) that influence circulating adiponectin levels. Two ADIPOQ promoter SNPs, rs17300539 (−11391G>A) and rs266729 (−11377C>G), have been investigated frequently in functional experiments (9,11,12,31) due to their strong reproducible correlations with circulating adiponectin and to the potential role of the promoter region in regulating gene transcriptional activity. In vitro data revealed a functional effect of rs17300539 on adiponectin, suggesting that the A allele was likely to increase ADIPOQ promoter activity and enhance transcription (31). A subsequent study demonstrated that SNPs rs17300539, rs266729 and a third ADIPOQ promoter variant rs16861194 (−11426A>G) modulate gene transcription by altering the DNA binding activity in mouse 3T3-L1 adipocytes; the rs16861194 G allele substantially reduced basal promoter and DNA binding activity, although the exact binding factors remained largely unclear (21). Bioinformatic prediction suggests that SNP rs266729 (−11377C>G) alters the sequence of one of four transcriptional stimulatory protein (SP1) binding sites in the ADIPOQ promoter region (32). Our results, however, did not support the importance of these promoter SNPs in CLHNS mothers who reside in Metro Cebu, Philippines. SNP rs17300539 is monomorphic in mothers, while rs266729 showed only modest association with plasma adiponectin (P = 0.018), and no evidence for association was found for rs16861194 (P = 0.26). While rs266729 shows similar minor allele frequencies between Europeans and Asians (CEU: G 0.30 and CLHNS: G 0.27), frequencies differ for rs17300539 (CEU: A 0.08 and CLHNS: A 0.00) and rs16861194 (CEU: G 0.07 and CLHNS: G 0.18), suggesting different genetic architecture in the ADIPOQ promoter region between populations.
Given the differences in local genetic structure, the possible functional relevance of other nearby genetic variants should be considered. The SNP rs864265 (chr3: 188 036 986), located 5 kb upstream of the widely discussed ADIPOQ promoter region and 16 kb upstream of the translation start site, showed the strongest association with plasma adiponectin in the CLHNS sample (P = 3.8 × 10−9). Perhaps more relevant, our findings of association with the uncommon C–T haplotype of the rs11924390 and rs864265 SNPs suggest that a functional variant might be tagged by the uncommon haplotype. Compared with the previously reported haplotypes that cover the ADIPOQ gene (19), this novel haplotype spans the KNG1–ADIPOQ locus and suggests a more extensive gene region where the potential causal SNP(s) may reside. We investigated additional variation within 2 Mb (187–189 Mb) flanking KNG1 and ADIPOQ by testing the association of SNPs imputed based on 1000 Genomes Project Pilot data, but no additional SNPs showed stronger association than SNPs imputed based on HapMap (Supplementary Material, Fig. S3). The failure to identify a stronger signal suggests either that the 1000 Genomes Project Pilot did not include the single functional variant, that such a variant was poorly imputed and thus its association with adiponectin was inaccurate or that more than one functional variant exists. Non-synonymous amino acid substitutions may contribute to the variation of circulating adiponectin by affecting the spatial organization of the protein and interfering with post-transcriptional modification, multimerization or interaction of the protein with its receptors (16). Variants including G84R, G90S, H111Y and R112C have been reported to be associated with adiponectin level and suggested to be functionally relevant (16,33,34). However, these variants were rare in initial studies (MAF<0.02) and are not included as HapMap or 1000 Genomes Project Pilot imputed SNPs. Thus, their association with adiponectin level in the CLHNS sample remains unknown.
In conclusion, this study expands our understanding of genetic association with adiponectin in a population of mostly non-European ancestry and underscores a difference in the genetic architecture of plasma adiponectin levels between Europeans and Asians. Our findings provide compelling evidence of a strong association for plasma adiponectin with the CDH13 locus. We also observed a haplotype association with plasma adiponectin of an uncommon C–T haplotype consisting of two variants at the KNG1–ADIPOQ locus, which may motivate further studies to investigate potential functional variants and to elucidate the underlying biological mechanisms.
MATERIALS AND METHODS
The original study population, study design and recruitment protocols of the CLHNS have been described in detail previously (35). The CLHNS is a community-based birth cohort study which originally enrolled 3327 pregnant women in 1983–1984, and the women and their offspring have since been followed. In the 2005 survey, the available study samples consisted of 1895 Filipino mothers and 1779 offspring. Among the mothers, we excluded 81 estimated first-degree relatives, 14 participants without genotyping data and 24 individuals with missing adiponectin outcome or covariates, and among the offspring we excluded one of each twin pair (n = 5). The final sample set included 1776 CLHNS women and 1774 offspring, of which 336 independent offspring did not have a mother in the CLHNS mothers GWA cohort and 1438 are members of offspring–mother pairs.
GWA SNP genotyping, quality control and genotype imputation based on HapMap CEU + CHB + JPT using MACH have also been previously described (36). Briefly, SNP genotyping was performed using the Affymetrix Genomewide Human SNP Array 5.0, according to the manufacturer's standard protocol. Genotyping calling was performed using Birdseed (Version 2). Genotyping was conducted on 1895 unique CLHNS mothers samples, 40 CLHNS duplicates and 5 HapMap CEPH trios. Fourteen CLHNS samples were excluded due to genotyping failure (n = 10) or a <97% call rate (n = 4). The final sample call rate was 99.6% in the remaining 1881 CLHNS mother samples. In the marker quality control for the initial 424 670 genotyping SNPs, those with poor mapping, call rate <90% and/or deviation from Hardy–Weinberg equilibrium (P < 10−6) were removed from the follow-up imputation (n = 13 287). An additional 3277 SNPs were discarded due to three or more discrepancies between genotypes in 40 duplicate pairs, Mendelian inheritance errors in five CEPH trios and/or three or more genotype discrepancies with HapMap. We applied a hidden Markov model algorithm implemented in MACH software version 1.0 (37) to impute genotypes in CLHNS mothers samples for 352 264 directly genotyped SNPs that were polymorphic in both the 60 CEU founders and the 89 combined CHB + JPT HapMap samples. After exclusion of SNPs with poor imputation quality (MACH r2 < 0.3) or with low minor allele frequency (MAF ≤ 0.01), a total of 2 073 674 HapMap imputed SNPs were tested for association with plasma adiponectin level in 1776 CLHNS mothers. Additional imputation within 2 Mb gene region (187–189 Mb) of KNG1–ADIPOQ locus was performed based on haplotypes created from the 1000 Genomes Project pilot release (August 2009) of CEU+CHB+JPT samples. Regional plots were created using LocusZoom (38).
We constructed principal components (PCs) using the software EIGENSOFT to capture population substructure among CLHNS subjects (39). We assessed the association between each of the first 10 PCs and the adiponectin level to identify any potential ancestry explanatory PC; none were significantly associated and thus no PCs were included as covariates in the linear regression models for GWAS (All P > 0.06, Supplementary Material, Table S2). The genomic control value (λGC) was calculated to evaluate the type I error inflation due to population stratification.
Plasma samples were analyzed for adiponectin with a commercially available enzyme-linked immunosorbent assay (R&D Systems #DY1065). All samples were assayed in duplicate, and control samples were included with each assay to monitor between-assay variation. The percent coefficient of variation (SD/mean) for low, middle and high controls was 9.5, 9.6 and 7.8, respectively. Informed consent was obtained from all CLHNS participants, and the study protocol was approved by the University of North Carolina Institutional Review Board for the Protection of Human Subjects.
Follow-up genotyping was performed for four SNPs (rs3865188, rs11924390, rs864265 and rs17300539). The first three SNPs were genotyped to validate their significant associations with adiponectin observed in the mothers and also to test for supporting evidence in the offspring. The fourth SNP (rs17300539) was genotyped because it was a reproducibly reported associated SNP for adiponectin, but imputed genotypes were unavailable in the CLHNS mothers. Three SNPs (rs17300539, rs3865188 and rs11924390) were genotyped using TaqMan allelic discrimination (Applied Biosystems, Foster City, CA, USA) in both the CLHNS mothers and offspring. The genotyping success rate was >97%, and no discrepancies among 80 duplicate pairs were observed. The ADIPOQ SNP rs864265 was successfully genotyped in all 1774 CLHNS offspring using the Cardio-MetaboChip (Illumina, San Diego, CA, USA). Direct genotype data for rs864265 from the Cardio-MetaboChip were also available for 85 (4.8%) mothers. Due to both the high imputation quality of rs864265 (r2 > 0.99) and the high allele concordance rate (>99.4%) between the Cardio-MetaboChip genotypes and the posterior expected genotypes based on HapMap imputed genotypes in the 85 mothers, rs864265 was not directly genotyped in the remaining 1691 mothers. The imputed genotypes were then used in statistical analyses.
Adiponectin level was natural log-transformed (log-adiponectin) to satisfy the model assumption of normally distributed residuals. GWA analyses were performed in Array Studio software version 3.6 (Omicsoft Corporation, Research Triangle Park, NC, USA). A multiple linear regression model assuming an additive effect and adjusting for age, age2, household assets, natural log-transformed household income and menopausal status during the 2005 survey was applied to test for the association between each SNP and log-adiponectin in CLHNS mothers (Supplementary Material, Table S2). A one degree-of-freedom likelihood ratio test was used to examine the statistical significance.
In follow-up analyses, multiple linear regression models were performed to test the association of three genotyped SNPs (rs3865188, rs864265 and rs11924390) with log-adiponectin in both the mother and offspring samples. In the offspring, the analyses were adjusted for sex, household assets and natural log-transformed household income. Age, age2 and menopausal status that were used as covariates for the analysis of CLHNS mothers were not included in offspring analyses because the ages of all offspring were within 2 years of each other (mean ± SD = 21.5 ± 0.3, Table 1) and none had reached menopause. In light of the relatedness between mother–child pairs, follow-up analysis in offspring was first conducted in a subgroup sample consisting of 336 CLHNS offspring whose mothers were not included in the CLHNS mother GWA samples. Partial correlations were determined for each SNP to estimate the proportion of variation in log-adiponectin explained by these genetic loci. To assess the association on the combined sample of mothers and offspring, a general linear mixed model was used to account for the correlation of adiponectin levels between mother–child pairs due to shared genetic and environmental exposures.
Partial correlation analyses were used to evaluate the relationship between adiponectin level and other metabolic-related traits including BMI, waist circumference, triglycerides, HDL-C, low-density lipoprotein cholesterol (LDL-C), fasting glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-β). HOMA-IR was calculated as fasting glucose (mmol/l) × fasting insulin (µU/ml)/22.5 while HOMA-β was computed as 20 × fasting insulin (µU/ml)/[fasting glucose (mmol/l) − 3.5]. All traits except for waist circumference were natural log-transformed before analyses. Multiple linear regression models, accounting for the same covariates as for the log-adiponectin analyses, were performed to test for genotype effects on the metabolic-related traits. All analyses for follow-up association with log-adiponectin and other metabolic-related traits were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA).
Haplotype analyses were performed using the ‘haplo.stat' R package. Haplotypes and haplotype frequencies were estimated using the R function ‘haplo.em'. The association between haplotypes and adiponectin was assessed using the R function ‘haplo.glm'. An additive model was assumed, in which the regression coefficient β represents the expected change in natural log-transformed adiponectin level with each additional copy of the specific haplotype compared with the reference haplotype. The most common C–G haplotype of KNG1 (rs11924390)–ADIPOQ (rs864265) was set as the reference haplotype. The R function ‘haplo.score' was used to compute the global score statistics to test the overall association between haplotypes and adiponectin. The same covariates used for genotype analysis were also applied in the haplotype analysis models.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by National Institutes of Health grants (DK078150, TW05596, HL085144), pilot funds (RR20649, ES10126, DK56350) and a training grant (T32 GM007092). K.L.M. is a Pew Scholar in the Biological Sciences.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Office of Population Studies Foundation research and data collection teams and the study participants who generously provided their time for this study.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Diez J.J., Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. doi:10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. doi:10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 3.Ouedraogo R., Gong Y., Berzins B., Wu X., Mahadev K., Hough K., Chan L., Goldstein B.J., Scalia R. Adiponectin deficiency increases leukocyte–endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. doi:10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotta K., Funahashi T., Arita Y., Takahashi M., Matsuda M., Okamoto Y., Iwahashi H., Kuriyama H., Ouchi N., Maeda K., et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R.E., Tataranni P.A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. doi:10.1210/jc.86.5.1930. [DOI] [PubMed] [Google Scholar]
- 6.Gable D.R., Hurel S.J., Humphries S.E. Adiponectin and its gene variants as risk factors for insulin resistance, the metabolic syndrome and cardiovascular disease. Atherosclerosis. 2006;188:231–244. doi: 10.1016/j.atherosclerosis.2006.02.010. doi:10.1016/j.atherosclerosis.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Renaldi O., Pramono B., Sinorita H., Purnomo L.B., Asdie R.H., Asdie A.H. Hypoadiponectinemia: a risk factor for metabolic syndrome. Acta Med. Indones. 2009;41:20–24. [PubMed] [Google Scholar]
- 8.Comuzzie A.G., Funahashi T., Sonnenberg G., Martin L.J., Jacob H.J., Black A.E., Maas D., Takahashi M., Kihara S., Tanaka S., et al. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2001;86:4321–4325. doi: 10.1210/jcem.86.9.7878. doi:10.1210/jc.86.9.4321. [DOI] [PubMed] [Google Scholar]
- 9.Chuang L.M., Chiu Y.F., Sheu W.H., Hung Y.J., Ho L.T., Grove J., Rodriguez B., Quertermous T., Chen Y.D., Hsiung C.A., et al. Biethnic comparisons of autosomal genomic scan for loci linked to plasma adiponectin in populations of Chinese and Japanese origin. J. Clin. Endocrinol. Metab. 2004;89:5772–5778. doi: 10.1210/jc.2004-0640. doi:10.1210/jc.2004-0640. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay R.S., Funahashi T., Krakoff J., Matsuzawa Y., Tanaka S., Kobes S., Bennett P.H., Tataranni P.A., Knowler W.C., Hanson R.L. Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes. 2003;52:2419–2425. doi: 10.2337/diabetes.52.9.2419. doi:10.2337/diabetes.52.9.2419. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakou T., Collins L.J., Spencer-Jones N.J., Malcolm C., Wang X., Snieder H., Swaminathan R., Burling K.A., Hart D.J., Spector T.D., et al. Adiponectin gene ADIPOQ SNP associations with serum adiponectin in two female populations and effects of SNPs on promoter activity. J. Hum. Genet. 2008;53:718–727. doi: 10.1007/s10038-008-0303-1. doi:10.1007/s10038-008-0303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasseur F., Helbecque N., Lobbens S., Vasseur-Delannoy V., Dina C., Clement K., Boutin P., Kadowaki T., Scherer P.E., Froguel P. Hypoadiponectinaemia and high risk of type 2 diabetes are associated with adiponectin-encoding (ACDC) gene promoter variants in morbid obesity: evidence for a role of ACDC in diabesity. Diabetologia. 2005;48:892–899. doi: 10.1007/s00125-005-1729-z. doi:10.1007/s00125-005-1729-z. [DOI] [PubMed] [Google Scholar]
- 13.Hara K., Boutin P., Mori Y., Tobe K., Dina C., Yasuda K., Yamauchi T., Otabe S., Okada T., Eto K., et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–540. doi: 10.2337/diabetes.51.2.536. doi:10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 14.Mousavinasab F., Tahtinen T., Jokelainen J., Koskela P., Vanhala M., Oikarinen J., Keinanen-Kiukaanniemi S., Laakso M. Common polymorphisms (single-nucleotide polymorphisms SNP + 45 and SNP + 276) of the adiponectin gene regulate serum adiponectin concentrations and blood pressure in young Finnish men. Mol. Genet. Metab. 2006;87:147–151. doi: 10.1016/j.ymgme.2005.08.010. doi:10.1016/j.ymgme.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Gu H.F. Biomarkers of adiponectin: plasma protein variation and genomic DNA polymorphisms. Biomark. Insights. 2009;4:123–133. doi: 10.4137/bmi.s3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasseur F., Helbecque N., Dina C., Lobbens S., Delannoy V., Gaget S., Boutin P., Vaxillaire M., Lepretre F., Dupont S., et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum. Mol. Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. doi:10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 17.Hivert M.F., Manning A.K., McAteer J.B., Florez J.C., Dupuis J., Fox C.S., O'Donnell C.J., Cupples L.A., Meigs J.B. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57:3353–3359. doi: 10.2337/db08-0700. doi:10.2337/db08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henneman P., Aulchenko Y.S., Frants R.R., Zorkoltseva I.V., Zillikens M.C., Frolich M., Oostra B.A., van Dijk K.W., van Duijn C.M. The genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome related traits. Diabetes Care. 2010;33:908–913. doi: 10.2337/dc09-1385. doi:10.2337/dc09-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heid I.M., Wagner S.A., Gohlke H., Iglseder B., Mueller J.C., Cip P., Ladurner G., Reiter R., Stadlmayr A., Mackevics V., et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55:375–384. doi: 10.2337/diabetes.55.02.06.db05-0747. doi:10.2337/diabetes.55.02.06.db05-0747. [DOI] [PubMed] [Google Scholar]
- 20.Morandi A., Maffeis C., Lobbens S., Bouatia-Naji N., Heude B., Pinelli L., Meyre D., Froguel P. Early detrimental metabolic outcomes of rs17300539-A allele of ADIPOQ gene despite higher adiponectinemia. Obesity (Silver Spring) 2009;18:1469–1473. doi: 10.1038/oby.2009.403. doi:10.1038/oby.2009.403. [DOI] [PubMed] [Google Scholar]
- 21.Laumen H., Saningong A.D., Heid I.M., Hess J., Herder C., Claussnitzer M., Baumert J., Lamina C., Rathmann W., Sedlmeier E.M., et al. Functional characterization of promoter variants of the adiponectin gene complemented by epidemiological data. Diabetes. 2009;58:984–991. doi: 10.2337/db07-1646. doi:10.2337/db07-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore J.H., Williams S.M. Epistasis and its implications for personal genetics. Am. J. Hum. Genet. 2009;85:309–320. doi: 10.1016/j.ajhg.2009.08.006. doi:10.1016/j.ajhg.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling H., Waterworth D.M., Stirnadel H.A., Pollin T.I., Barter P.J., Kesaniemi Y.A., Mahley R.W., McPherson R., Waeber G., Bersot T.P., et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–744. doi: 10.1038/oby.2008.625. doi:10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards J.B., Waterworth D., O'Rahilly S., Hivert M.F., Loos R.J., Perry J.R., Tanaka T., Timpson N.J., Semple R.K., Soranzo N., et al. A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. doi:10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto Y., Hirose H., Saito I., Tomita M., Taniyama M., Matsubara K., Okazaki Y., Ishii T., Nishikai K., Saruta T. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin. Sci. (Lond.) 2002;103:137–142. doi: 10.1042/cs1030137. doi:10.1042/CS20010336. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Li H., Franco O.H., Yu Z., Liu Y., Lin X. Adiponectin and metabolic syndrome in middle-aged and elderly Chinese. Obesity (Silver Spring) 2008;16:172–178. doi: 10.1038/oby.2007.42. doi:10.1038/oby.2007.42. [DOI] [PubMed] [Google Scholar]
- 27.Hug C., Wang J., Ahmad N.S., Bogan J.S., Tsao T.S., Lodish H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl Acad. Sci. USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. doi:10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi T., Adachi Y., Ohtsuki Y., Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med. Mol. Morphol. 2007;40:115–120. doi: 10.1007/s00795-007-0364-9. doi:10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- 29.Heid I.M., Henneman P., Hicks A., Coassin S., Winkler T., Aulchenko Y.S., Fuchsberger C., Song K., Hivert M.F., Waterworth D.M., et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis. 2010;208:412–420. doi: 10.1016/j.atherosclerosis.2009.11.035. doi:10.1016/j.atherosclerosis.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houlihan L.M., Davies G., Tenesa A., Harris S.E., Luciano M., Gow A.J., McGhee K.A., Liewald D.C., Porteous D.J., Starr J.M., et al. Common variants of large effect in F12, KNG1, and HRG are associated with activated partial thromboplastin time. Am. J. Hum. Genet. 2010;86:626–631. doi: 10.1016/j.ajhg.2010.02.016. doi:10.1016/j.ajhg.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouatia-Naji N., Meyre D., Lobbens S., Seron K., Fumeron F., Balkau B., Heude B., Jouret B., Scherer P.E., Dina C., et al. ACDC/adiponectin polymorphisms are associated with severe childhood and adult obesity. Diabetes. 2006;55:545–550. doi: 10.2337/diabetes.55.02.06.db05-0971. doi:10.2337/diabetes.55.02.06.db05-0971. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D., Ma J., Brismar K., Efendic S., Gu H.F. A single nucleotide polymorphism alters the sequence of SP1 binding site in the adiponectin promoter region and is associated with diabetic nephropathy among type 1 diabetic patients in the Genetics of Kidneys in Diabetes Study. J. Diabetes Complicat. 2009;23:265–272. doi: 10.1016/j.jdiacomp.2008.05.004. doi:10.1016/j.jdiacomp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi M., Arita Y., Yamagata K., Matsukawa Y., Okutomi K., Horie M., Shimomura I., Hotta K., Kuriyama H., Kihara S., et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int. J. Obes. Relat. Metab. Disord. 2000;24:861–868. doi: 10.1038/sj.ijo.0801244. doi:10.1038/sj.ijo.0801244. [DOI] [PubMed] [Google Scholar]
- 34.Bowden D.W., An S.S., Palmer N.D., Brown W.M., Norris J.M., Haffner S.M., Hawkins G.A., Guo X., Rotter J.I., Chen Y.D., et al. Molecular basis of a linkage peak: exome sequencing and family-based analysis identifies a rare genetic variant in the ADIPOQ gene in the IRAS Family Study. Hum. Mol. Genet. 2010 doi: 10.1093/hmg/ddq327. 2010 [Epub ahead of print 5 August] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adair L.S., Popkin B.M., Akin J.S., Guilkey D.K., Gultiano S., Borja J., Perez L., Kuzawa C.W., McDade T., Hindin M.J. Cohort profile: the Cebu Longitudinal Health and Nutrition Survey. Int. J. Epidemiol. 2010 doi: 10.1093/ije/dyq085. 2010 [Epub ahead of print 27 May] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange L.A., Croteau-Chonka D.C., Marvelle A.F., Qin L., Gaulton K.J., Kuzawa C.W., McDade T.W., Wang Y., Li Y., Levy S., et al. Genome-wide association study of homocysteine levels in Filipinos provides evidence for CPS1 in women and a stronger MTHFR effect in young adults. Hum. Mol. Genet. 2010;19:2050–2058. doi: 10.1093/hmg/ddq062. doi:10.1093/hmg/ddq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Willer C.J., Ding J., Scheet P., Abecasis G.R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 2010;32:1–19. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruim R.J., Welch R.P., Sanna S., Teslovich T.M., Chines P.S., Gliedt T.P., Boehnke M., Abecasis G.R., Willer C.J. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. doi:10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904–909. doi: 10.1038/ng1847. doi:10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.