Abstract
Background
Radiotracer imaging of the presynaptic nigrostriatal dopaminergic system is used to assess disease progression in Parkinson's disease (PD) and may provide a useful adjunct to clinical assessment during therapeutic trials of potential neuroprotective agents. Several clinical trials comparing dopamine agonists to L-DOPA or early vs. late L-DOPA have revealed differences between clinical assessment and imaging of the presynaptic dopaminergic system, hence questioning the comparability of these measures as neuroprotection outcome variables. Thus, results of these studies may have been affected by factors other than the primary biological process investigated.
Methodology/Principal Findings
We tested the possibility that L-DOPA might interfere with DAT binding. Post-mortem DAT binding was conducted in normal and MPTP-treated macaque monkeys that were administered L-DOPA, acutely or chronically. In parallel, DAT SPECT was conducted in MPTP-treated animals that were administered chronic L-DOPA. [99mTc]TRODAT-1 SPECT binding was similarly reduced in all MPTP monkeys regardless of L-DOPA treatment. L-DOPA had no significant effect on post-mortem DAT binding either in saline or in MPTP-lesioned animals.
Conclusions/Significance
These data indicate that L-DOPA does not induce modifications of DAT expression detectable by SPECT of by DAT binding autoradiography, suggesting that differences between clinical assessment and radiotracer imaging in clinical trials may not be specifically related to L-DOPA treatment.
Introduction
Radiotracer imaging of the presynaptic nigrostriatal dopaminergic system is a useful tool to assess the rate of disease progression in Parkinson's disease (PD). In longitudinal studies, F-DOPA PET and dopamine transporter (DAT) SPECT show a 4% to 13% yearly reduction in baseline putamen uptake compared with 0 to 2.5% in healthy controls [1], [2], [3], [4], [5], [6]. Such techniques may therefore provide a useful adjunct to clinical assessment in monitoring disease progression in therapeutic trials of potential disease modifying therapies.
However, three clinical trials comparing dopamine agonists to L-DOPA (CALM-PD and REAL-PET) or early vs. late L-DOPA (ELLDOPA) have revealed discrepancies between clinical assessment and radiotracer-based imaging of the dopaminergic system as assessed by β-CIT or F-DOPA PET [7], [8], [9], thus questioning the adequacy of these measures as outcome variables. Such a mismatch not only limits the interpretation of these studies but also hinders future development of disease modifying therapies since biomarkers and biological endpoints are mandatory to demonstrate a putative neuroprotective effect of a drug. The results of these clinical trials and the divergence between clinical and imaging endpoints have been the subject of an intense debate [10], [11], [12], [13], [14]. The reasons for such differences currently remain unknown and several hypotheses have been proposed, including L-DOPA-induced modification of DAT expression, interaction with radiotracers or accelerated loss of nigrostriatal terminals.
Since the results of these imaging studies may have been affected by factors other than the primary biological process under study, we tested the hypothesis that chronic L-DOPA treatment might interfere with DAT expression or with the imaging procedure. To determine whether L-DOPA may interfere with radioligand binding, we performed in vivo DAT SPECT in MPTP-treated macaque monkeys that were chronically treated with L-DOPA. We also assessed a potential effect of L-DOPA on DAT expression using post-mortem DAT binding in a cohort of normal and MPTP-treated macaque monkeys that were both administered L-DOPA, acutely or chronically.
Materials and Methods
Animals
Experiments were conducted on fifty-three female rhesus monkeys (Macaca mulatta, Xierxin, Beijing, PR of China; mean age = 5±1 years; mean weight = 5.3±0.8 kg). Animals were housed in individual primate cages under controlled conditions of humidity (50±5%), temperature (24±1°C) and light (12 h light/12 h dark cycles, time lights on 8:00 am), food and water were available ad libitum and animal care was supervised daily by veterinarians skilled in the healthcare and maintenance of non-human primates. Experiments were carried out in accordance with European Communities Council Directive of 24 November 1986 (86/609/EEC) for care of laboratory animals in an AAALAC-accredited facility following acceptance of study design by the Institute of Lab Animal Science IACUC (Chinese Academy of Medical Sciences, Beijing, China). Experiments followed previously published procedures [15], [16], [17], [18]. Even though animals were housed individually, the disposition of cages allowed each animal to have visual contacts and interact with monkeys housed in the adjacent cages. Stimulations for play are provided including rubber toys and mirrors that the monkeys use to view themselves and to get a greater look around the room. A radio is played daily from 8.00am–10.00am and 3.00pm–5.00pm to provide stimulation.
Experimental protocol
Animals were randomly assigned to a particular treatment group. Six animals were kept as untreated controls (control group), six monkeys received a single dose of 20 mg/kg L-DOPA p.o (control acute L-DOPA), six monkeys received 20 mg/kg twice daily for three months (control chronic L-Dopa). The remaining 23 animals were treated daily (9:00 am) with MPTP hydrochloride (0.2 mg/kg, i.v., Sigma, St Louis, MO) dissolved in saline according to a previously described protocol [19], [20]. Monkeys were injected in the femoral vein under gentle restraint. Following stabilization of the MPTP-induced syndrome, animals received either saline (MPTP), either a single dose of 20 mg/kg L-DOPA (MPTP acute L-DOPA) or twice daily for three months (MPTP chronic L-DOPA). TRODAT SPECT was performed in twelve additional monkeys: four untreated controls, four MPTP and four MPTP chronically treated with L-DOPA according to exact same methods and timeline. The experimental flowchart is described on Figure 1 . All animals were killed by sodium pentobarbital overdose (150 mg/kg, i.v.) 1 hr after the last dose of vehicle or L-DOPA, and the brains were removed quickly after death. Each brain was bisected along the midline and the two hemispheres were immediately frozen by immersion in isopentane (−45°C) and then stored at −80°C. Tissue was sectioned coronaly at 20 µm in a cryostat at −17°C, thaw-mounted onto gelatine-coated slides, dried on a slide warmer and stored at −80°C.
Figure 1. Experimental flowchart illustrating study design, treatments and group assignments.
Behavioural assessment
Animal behaviour was assessed in their home cages. All observers were blinded with regard to the experimental protocol. During each session, two blinded examiners evaluated the level of motor performance of each animal, by coaxing them to perform various tasks by offering appetizing fruits. Animals received supplemental feeding from day 7 onwards to maintain their body weight as constant as possible. The degree of parkinsonism was assessed daily (9 a.m.) for 30 min using a validated parkinsonian macaque clinical scale [15], [20], [21] which rates the following symptoms of parkinsonian disability: tremor, variations in the general level of activity, body posture (flexion of spine), vocalization, freezing and frequency of arm movements (reaching for food for each upper limb) and rigidity (for each upper limb). The minimal disability score was 0 and the maximum score was 25 [21]. The severity of dyskinesia was rated using the Dyskinesia Disability Scale [22], [23], [24]: 0, dyskinesia absent; 1, mild, fleeting, and rare dyskinetic postures and movements; 2, moderate, more prominent abnormal movements, but not interfering significantly with normal behaviour; 3, marked, frequent and, at times, continuous dyskinesia intruding on the normal repertoire of activity; or, 4, severe, virtually continuous dyskinetic activity, disabling to the animal and replacing normal behaviour. Daily behavioural assessments were performed before and after L-Dopa administration. Median rating scores and SEM were calculated daily for each group.
[99mTc]TRODAT-1 SPECT
Anaesthesia was induced by ketamine (i.m., 15 mg/kg, Imalgene, Centravet, France) in animals having received their last dose of vehicle or L-DOPA the day before scanning. A polycarbonated custom-made stereotaxic device (Blueprint by Primate Research Center, University of Washington, Seattle, USA) maintained the monkey's head in the SPECT camera to allow reproducible positioning of the animal. Reproducibility of the tomographic technique had been ensured by repeating the assessment twice in one normal and one fully parkinsonian animal and on the basis of our previous experience with another SPECT system [25]. Single-vial kit (National Laboratory of Nuclear Medicine, Suzhou, China) containing 100 µg of TRODAT-1, 20 µg of stannous chloride anhydrous, 10 mg of sodium gluco-heptonate was added with 3.7 GBq (0.5 to 1.0 ml) of [99mTc] pertechnetate. The reaction mixture was heated for 30 minutes at 100°C and then cooled to room temperature. The radiochemical purity was kept at >90% as evaluated by thin layer chromatography. To diminish non-specific binding of pertechnetate to the choroid plexus (i.e. to increase the signal to noise ratio as per manufacturer recommendation) 10 mg/kg of KClO4 was administered by bolus injection 30 min before 10 mCi [99mTc]-TRODAT-1 was injected in the femoral vein. SPECT was performed 3 hours after the injection of radiopharmaceuticals. Data were acquired at 20 s per view, using 64 views and a 128×128 matrix, and were reconstructed using Butterworth filtered back projection with a slice thickness of 3.9 mm. All images were obtained on the same double-head camera (e.camduet Signature Series multipurpose gamma camera Siemens, USA). SPECT was performed with low-energy-high-resolution parallel hole Fan-beam collimators at 3.2 enlargement (collimators specially designed for brain scanning). Parameters were as follows: energy window: 140 keV, matrix: 128_128, 6°/projection, 40 s per projection and 180 projection angles per detector over 360°. After back-projection, a Butterworth low-pass filter with an order of 8.0 and a cut-off of 0.2 was applied. Attenuation correction was accomplished using Chang's first order correction method.
The values are the mean of one acquisition in each individual. In normal animals, standardized oval regions of interest (ROI) for right and left striata (ST) and for the cerebellum (CB) have been determined on transverse slice according to a median sagittal line and a perpendicular line passing through the temporal lobes as previously described [26]. These geometrical ROIs had a volume of 0.3 ml for striatum according to macaque brain anatomical data [27]. In MPTP-treated animals we used the same size of ROIs without considering potential striatal atrophy consecutive to MPTP intoxication. The striatal specific uptake of [99mTc]-TRODAT-1 was calculated (Mean ± S.E.M.) according to the ratio of (ST-CB)/CB.
DAT binding in the striatum
DAT binding using [125I]-(E)-N-(3-iodoprop-2-enyl)-2β-carboxymethyl-3β-(4′-methylphenyl)-nortropane (PE2I; Chelatec, France) was measured on fresh-frozen cryostat-cut 20 µm thick slide-mounted coronal sections as previously described [15], [20]. Sections incubated in the presence of 100 µM cocaine (Sigma) were used to define nonspecific binding [15], [20]. After substraction of background for non-specific staining, densitometric analysis of autoradiographs was performed using an image analysis system (Densirag V2.0, Explora Nova, La Rochelle, France). Two rostrocaudal levels were analyzed in accordance with the functional organization of the striatum: a rostral (associative) level including the caudate, putamen and nucleus accumbens (anterior commissure +2 mm); and a caudal (motoric) level including the body of the caudate, the putamen and both parts of the globus pallidus (i.e. pars externalis and pars internalis) (anterior commissure −4 mm). Where appropriate, both caudate and putamen were divided into dorsolateral, dorsomedial, ventrolateral and ventromedial quadrants for analysis. Two sections per animal and per striatal level were analysed by an examiner blind with regard to the experimental condition. Optical densities (mean ± SEM) were averaged for each region in each animal.
Statistical analysis
SPECT data were analyzed using one-way ANOVA followed by Bonferroni post-hoc test. DAT binding data were analysed using two-way ANOVA followed by post-hoc t-tests corrected for multiple comparisons by the method of Bonferroni [28]. Comparison of DAT binding intensity among striatal subregions in MPTP-treated monkeys was performed using one-way ANOVA followed by Bonferroni post-hoc test. These analyses were completed using STATA program (Intercooled Stata 6.0, Stata Corporation, College Station, TX). A probability level of 5% (p<0.05) was considered significant.
Results
Evolution of symptoms and behavioural assessment
MPTP-treated animals displayed the classic progression of symptoms previously shown with this intoxication regimen [17], [20]. Monkeys became increasingly bradykinetic, adopted a stooped posture, with increased rigidity of the limbs and decreased vocalization. Movement accuracy progressively deteriorated and there were occasional episodes of freezing as well as postural tremor. Stable parkinsonism was obtained after 19±0.5 MPTP injections (parkinsonian score = 9.3±0.34). All MPTP groups displayed a similar parkinsonian score (MPTP: 8.5±0.5, MPTP acute L-Dopa: 9.5±0.5, MPTP chronic L-Dopa (dyskinetic): 9.33±0.4, MPTP chronic L-Dopa (non dyskinetic): 9.83±0.87). Parkinonian scores of all MPTP groups were significantly different from controls (F6,35 = 88.12, p<0.0001) and identical between them. Animals who developed L-Dopa induced dyskinesia following chronic L-Dopa treatment had a dyskinetic score of 2.33±0.21.
In vivo DAT imaging
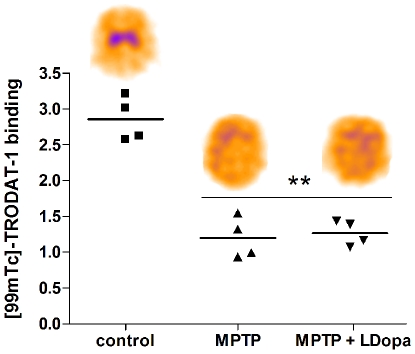
In vivo [99mTc]-TRODAT-1 binding using SPECT was measured in in control, MPTP and MPTP chronically treated with L-DOPA ( Fig. 2 ). ANOVA analysis demonstrated a significant effect of MPTP (F(2,9) = 50.93, p<0.0001). Post-hoc analysis further revealed that scans performed in MPTP and MPTP with chronic L-DOPA treatment showed a significant decrease in [99mTc]-TRODAT-1 binding compared with control animals ( Fig. 2 ). There was no difference between MPTP and MPTP + chronic L-DOPA conditions.
Figure 2. In vivo assessment of DAT density using [99mTc]TRODAT-1 SPECT in control, MPTP and MPTP-monkeys treated chronically with L-Dopa.
** indicates significant difference compared with control group (p<0.001 using Bonferonni test following one-way ANOVA). Representative SPECT images are shown for each group.
Ex vivo DAT binding autoradiography
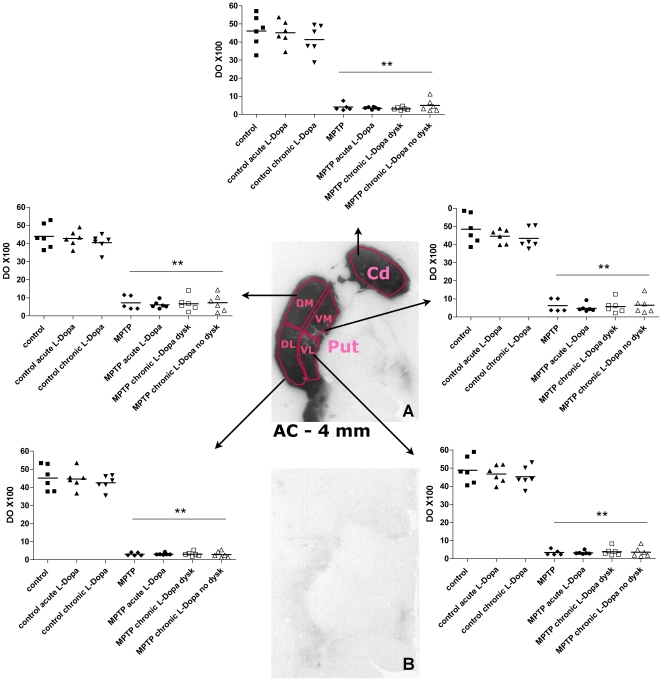
DAT binding autoradiography using [125I]-PE2I was performed at two rostrocaudal levels of the striatum. At the rostral (associative) level, there was a significant effect of MPTP in all striatal subregions investigated both in the caudate nucleus (dorsomedial: F1,36 = 1344.12, p<0.0001, dorsolateral: F1,36 = 1063.6, p<0.0001, ventromedial: F1,36 = 788.01, p<0.0001, ventrolateral: F1,36 = 740.23, p<0.0001) and in the putamen (dorsomedial: F1,36 = 916.92, p<0.0001, dorsolateral: F1,36 = 1146.09, p<0.0001, ventromedial: F1,36 = 335.47, p<0.0001, ventrolateral: F1,36 = 466.53, p<0.0001). (Fig. S1). At the caudal (motoric) level, DAT binding was also drastically reduced in all MPTP groups both in the caudate (F1,34 = 461.23, p<0.0001) and in the putamen (dorsomedial: F1,34 = 542.34, p<0.0001, dorsolateral: F1,34 = 985.22, p<0.0001, ventromedial: F1,34 = 576.71, p<0.0001, ventrolateral: F1,34 = 886.54, p<0.0001) ( Fig. 3 ). In MPTP-treated monkeys, DAT binding was found to be differentially affected among striatal subregions. At the rostral level, there was a significant regional effect of MPTP both in the caudate (F1,19 = 8.214, p<0.01) and in the putamen (F1,19 = 49.80, p<0.0001, Fig. S1). Post hoc analysis revealed a greater loss of DAT binding in the dorsolateral and dorsomedial caudate compared with the ventrolateral subregion (p<0.05). In the putamen, the loss of DAT binding was more pronounced in the dorsolateral and dorsomedial subregions compared with the ventromedial (p<0.001) and ventrolateral subregions (p<0.001). Such differential vulnerability was also found in the putamen at the caudal level (F1,19 = 8.601, p<0.01, Fig.3 ). Post-hoc analysis revealed a greater effect of MPTP in the dorsolateral compared with dorsomedial (p<0.05) and ventromedial subregions (p<0.05). However, there was no significant effect of acute or chronic L-Dopa treatment and no significant interaction between MPTP and L-DOPA on DAT binding in any experimental group and striatal subregions.
Figure 3. DAT binding autoradiography in the posterior striatum (AC −4 mm).
A. Representative DAT binding autoradiogram from a control animal illustrating the position of striatal subregions. B. Representative autoradiogram from a MPTP-treated monkey. Cd: caudate nucleus, Put: putamen DL: dorsolateral, DM: dorsomedial, VL: ventrolateral, VM: ventromedial. ** indicates significant difference compared with corresponding control group (p<0.001 using Bonferonni test following two-way ANOVA).
Discussion
The unexpected divergence between clinical and imaging endpoints reported in several clinical trials in PD is not only puzzling but also an obstacle for the development of future therapeutic trials. Even though the validity of DAT imaging as a surrogate marker for clinical trial has been legitimately questioned [29], the reproduction of this mismatch in three independent investigations and using different markers (F-DOPA and β-CIT) suggests that factors other than the imaging technique may have influenced the outcomes. Among such possibilities, pharmacological interactions between radiotracers and L-DOPA and/or L-DOPA-induced reduction of DAT expression have been suggested [10], [30]. In addition, compensatory mechanisms involving changes in binding sites or enzyme activities may also be involved [31]. Indeed, partial lesions of the nigrostriatal projection are associated with a reduction of the density of high affinity DAT binding sites together with a large increase of the density of low affinity binding sites and a further reduction of their affinity [32]. These data suggest that the mechanisms underlying altered DAT function may have an impact when using DAT radioligands.
In the present study, we sought to determine whether acute or chronic L-DOPA treatment at therapeutically relevant doses may interfere with the radiotracer or induce modifications of DAT expression that could be detected either by in vivo by SPECT or ex vivo using DAT binding autoradiography.
[99mTc]-TRODAT-1 SPECT performed 24 h after the last L-DOPA administration was significantly reduced following MPTP and L-DOPA treatment did not induce an additional reduction of TRODAT-1 binding, suggesting that L-DOPA does not interfere with TRODAT-1 binding to the DAT. As we and others have previously shown, DAT reduction estimated in vivo by SPECT is lower compared with ex vivo quantification of dopaminergic cell bodies or terminals [33], [34]. Even though a previous study in rats showed a significant decrease of TRODAT SPECT binding in rats receiving 125 or 150 mg/kg L-DOPA [35], our SPECT results using therapeutically relevant doses are in agreement with previous work showing that [123I]beta-CIT or [123I]FP-CIT binding is not affected by L-DOPA treatment in PD patients [36], [37]. Therefore, factors specifically related to L-DOPA treatment such as dose, duration of treatment and wash-out period do not appear to have a strong influence on DAT imaging. However, in some of the clinical trials the greater reduction of DAT binding in patients receiving L-Dopa was observed compared with patients receiving various regimens of dopamine agonists (ropinirole or pramipexole) [7], [9]. Therefore, a specific effect of dopamine agonists on DAT binding may also be involved and cannot be inferred from the present study.
Indeed, DAT availability can be pharmacologically regulated since several drugs including antidepressants, opioïds and CNS stimulants may influence DAT imaging (for review, see Booij and Kemp, 2008). Genetic regulation is also involved as polymorphisms in the 3′ untranslated region of the DAT gene are associated with higher striatal DAT availability [38], [39]. DAT SPECT can also be influenced by a number of other factors including smoking [40], body mass index [41], or anxiety and depression [42].
In order to investigate DAT density with a higher sensitivity, we performed ex-vivo DAT binding autoradiography at two rostrocaudal levels of the caudate and putamen using [125I]-PE2I. While DAT binding was dramatically reduced in all MPTP groups, there was no significant difference between saline and L-DOPA-treated MPTP animals (acute or chronic) in any of the striatal levels and subregions investigated. However, out of 13 striatal subregions investigated, there was a small non-significant decrease in MPTP monkeys treated with L-DOPA in 4 putaminal subregions at the rostral, i.e. associative, level (Fig S1). This trend was not found in the rostral associative caudate nor in the caudal motoric caudate or putamen. While these observations are consistent with a previous study showing a 5% decrease in size of the DAT terminal tree in normal rats treated chronically with L-DOPA [43], acute or chronic treatment with L-DOPA did not influence DAT binding in normal animals in our study. In addition, the occurrence of L-DOPA-induced dyskinesia was not associated with differences in DAT binding in agreement with previous results [44]. Previous studies investigating the effects of dopamine agonists or L-DOPA on DAT binding in normal or dopamine-depleted animals have shown contrasting results [45]. Interestingly, all ex-vivo binding studies showing a significant effect of L-DOPA on dopamine uptake sites in the striatum were performed with [3H]-mazindol [46], [47], [48]. Conversely, [3H]-GBR-12935, [3H]-WIN-35,428, [3H]-cocaine, [123I]-β-CIT or [125I]-PE2I did not disclose any effect of L-DOPA on DAT binding [49], [50], [51], [52]. One noticeable difference between these DAT ligands is the very high affinity of mazindol for the norepinephrine transporter (Ki = 3 nM) [53]. Conversely, PE2I has a very low affinity for the norepinephrine transporter (>1000 nM) [54], [55]. In addition, the drugs used to define non-specific binding and duration of film exposure may have played a role in the apparent variable effects of L-DOPA on DAT density when measured ex vivo.
Several important differences between clinical trials and experimental studies should be emphasized: the degree of dopaminergic lesion in MPTP-Treated monkeys in this study is greater than that of PD patients enrolled in clinical trials. It is thus possible that the extent of the lesion in our study may have prevented to observe an effect that may exist in patients. Indeed, adaptative mechanisms including modified DAT binding properties have been shown following partial nigrostriatal lesions [32]. Experimental studies in primates include limited number of animals even though the present study is unprecedented in that respect. They thus have a limited power to detect subtle differences that can be observed in clinical studies involving large cohorts of patients (i.e. >40–60 patients). A power analysis (power 80%, alpha = 0.05) reveals that at the caudal motoric level of the striatum, between 44 and 145 animals would have been needed to detect a difference between MPTP and MPTP-L-DOPA animals in the caudate nucleus (i.e. the less motor involved part of the caudal striatum). However, in the four putaminal quadrants, (i.e. in the motor part of the striatum), between 340 and 6600 animals would have been needed to obtain a significant difference.
Nevertheless, our results suggest that regulation of DAT expression is not influenced by an acute or chronic treatment with L-DOPA both in normal and MPTP-lesioned monkeys at therapeutically relevant doses. Even though the 3-month L-DOPA treatment performed in the present study is shorter than the duration of treatment in the human disease, it is sufficient for the occurrence of plastic changes, transcriptional and post-transcriptional adaptations as shown with LID and by far longer than the vast majority of previous preclinical experiments. One should however acknowledge that the MPTP monkey model of PD, although it recapitulates many features of the human disease [56], is not the disease. The present results suggest that L-DOPA itself is unlikely to be solely responsible for the decreased DAT binding observed in PD patients in clinical trials. Further studies should investigate the involvement of other factors, one being the specific regulation of DAT expression in diseased neurons and/or terminals.
Supporting Information
DAT binding autoradiography in the rostral striatum (AC+2 mm). A. Representative DAT binding autoradiogram from a control animal illustrating the position of striatal subregions. B. Representative autoradiogram from a MPTP-treated monkey. Cd: caudate nucleus, Put: putamen DL: dorsolateral, DM: dorsomedial, VL: ventrolateral, VM: ventromedial. ** indicates significant difference compared with corresponding control group (p<0.001 using Bonferonni test following two-way ANOVA).
(3.52 MB TIF)
Footnotes
Competing Interests: MH and PR are employees of Motac Neuroscience Ltd. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by Agence Nationale de la Recherche grants (ANR-07-JCJC-0090; ANR-08-MNP-018; ANR-07-MNP-Trafinlid) and the National High Technology Research and Development Program of China, No. 2006AA02A116. Funding for the research was provided by Motac Neuroscience Ltd to MH and PR who are employees of Motac Neuroscience. The funders had a role in study design, data collection and analysis.
References
- 1.Marek K, Innis R, van Dyck C, Fussell B, Early M, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology. 2001;57:2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- 2.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64:314–319. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurmi E, Ruottinen HM, Kaasinen V, Bergman J, Haaparanta M, et al. Progression in Parkinson's disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol. 2000;47:804–808. [PubMed] [Google Scholar]
- 4.Winogrodzka A, Bergmans P, Booij J, van Royen EA, Janssen AG, et al. [123I]FP-CIT SPECT is a useful method to monitor the rate of dopaminergic degeneration in early-stage Parkinson's disease. J Neural Transm. 2001;108:1011–1019. doi: 10.1007/s007020170019. [DOI] [PubMed] [Google Scholar]
- 5.Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, et al. Progression of dopaminergic degeneration in Parkinson's disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord. 2002;17:45–53. doi: 10.1002/mds.1265. [DOI] [PubMed] [Google Scholar]
- 6.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–1661. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 9.Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 10.Ahlskog JE. Slowing Parkinson's disease progression: recent dopamine agonist trials. Neurology. 2003;60:381–389. doi: 10.1212/01.wnl.0000044047.58984.2f. [DOI] [PubMed] [Google Scholar]
- 11.Marek K, Jennings D, Seibyl J. Do dopamine agonists or levodopa modify Parkinson's disease progression? Eur J Neurol. 2002;9(Suppl 3):15–22. doi: 10.1046/j.1468-1331.9.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery EB., Jr Agonists versus levodopa in PD: the thrilla of whitha. Neurology. 2003;61:1462; author reply 1462–1463. doi: 10.1212/wnl.61.10.1462. [DOI] [PubMed] [Google Scholar]
- 13.Morrish PK. Real-PET and CONSORT. Ann Neurol. 2003;54:692; author reply 692–693. doi: 10.1002/ana.10774. [DOI] [PubMed] [Google Scholar]
- 14.Morrish PK. REAL and CALM: what have we learned? Mov Disord. 2003;18:839–840. doi: 10.1002/mds.10448. [DOI] [PubMed] [Google Scholar]
- 15.Bezard E, Ferry S, Mach U, Stark H, Leriche L, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9:762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, et al. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2:28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezard E, Gerlach I, Moratalla R, Gross CE, Jork R. 5-HT1A receptor agonist-mediated protection from MPTP toxicity in mouse and macaque models of Parkinson's disease. Neurobiol Dis. 2006;23:77–86. doi: 10.1016/j.nbd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson's disease. J Neurosci. 2007;27:14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezard E, Imbert C, Deloire X, Bioulac B, Gross CE. A chronic MPTP model reproducing the slow evolution of Parkinson's disease: evolution of motor symptoms in the monkey. Brain Res. 1997;766:107–112. doi: 10.1016/s0006-8993(97)00531-3. [DOI] [PubMed] [Google Scholar]
- 20.Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE. Comparison of eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the Macaque monkey. J Neurosci Methods. 2000;96:71–76. doi: 10.1016/s0165-0270(99)00184-3. [DOI] [PubMed] [Google Scholar]
- 22.Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD. Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine-treated common marmoset (Callithrix Jacchus). Mov Disord. 1995;10:731–740. doi: 10.1002/mds.870100606. [DOI] [PubMed] [Google Scholar]
- 23.Brotchie JM, Fox SH. Quantitative assessment of dyskinesias in subhuman primates. Mov Disord. 1999;14(Suppl 1):40–47. [PubMed] [Google Scholar]
- 24.Hill MP, Ravenscroft P, Bezard E, Crossman AR, Brotchie JM, et al. Levetiracetam potentiates the antidyskinetic action of amantadine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson's disease. J Pharmacol Exp Ther. 2004;310:386–394. doi: 10.1124/jpet.104.066191. [DOI] [PubMed] [Google Scholar]
- 25.Prunier C, Bezard E, Montharu J, Mantzarides M, Besnard JC, et al. Presymptomatic diagnosis of experimental Parkinsonism with 123I-PE2I SPECT. Neuroimage. 2003;19:810–816. doi: 10.1016/s1053-8119(03)00163-0. [DOI] [PubMed] [Google Scholar]
- 26.Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, et al. Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson's disease. Mol Neurobiol. 2003;28:209–218. doi: 10.1385/MN:28:3:209. [DOI] [PubMed] [Google Scholar]
- 27.Francois C, Yelnik J, Percheron G. A stereotaxic atlas of the basal ganglia in macaques. Brain Res Bull. 1996;41:151–158. doi: 10.1016/0361-9230(96)00161-x. [DOI] [PubMed] [Google Scholar]
- 28.Miller RG. New York: Springer; 1981. Simultaneous statistical inference. [Google Scholar]
- 29.Morrish PK. How valid is dopamine transporter imaging as a surrogate marker in research trials in Parkinson's disease? Mov Disord. 2003;18(Suppl 7):S63–70. doi: 10.1002/mds.10581. [DOI] [PubMed] [Google Scholar]
- 30.Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–215. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis. 1997;4:247–253. doi: 10.1006/nbdi.1997.0157. [DOI] [PubMed] [Google Scholar]
- 32.Stanic D, Parish CL, Zhu WM, Krstew EV, Lawrence AJ, et al. Changes in function and ultrastructure of striatal dopaminergic terminals that regenerate following partial lesions of the SNpc. J Neurochem. 2003;86:329–343. doi: 10.1046/j.1471-4159.2003.01843.x. [DOI] [PubMed] [Google Scholar]
- 33.Scheller D, Chan P, Li Q, Wu T, Zhang R, et al. Rotigotine treatment partially protects from MPTP toxicity in a progressive macaque model of Parkinson's disease. Exp Neurol. 2007;203:415–422. doi: 10.1016/j.expneurol.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Scherfler C, Donnemiller E, Schocke M, Dierkes K, Decristoforo C, et al. Evaluation of striatal dopamine transporter function in rats by in vivo beta-[123I]CIT pinhole SPECT. Neuroimage. 2002;17:128–141. doi: 10.1006/nimg.2002.1158. [DOI] [PubMed] [Google Scholar]
- 35.Dresel SH, Kung MP, Plossl K, Meegalla SK, Kung HF. Pharmacological effects of dopaminergic drugs on in vivo binding of [99mTc]TRODAT-1 to the central dopamine transporters in rats. Eur J Nucl Med. 1998;25:31–39. doi: 10.1007/s002590050191. [DOI] [PubMed] [Google Scholar]
- 36.Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, et al. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]beta-CIT. Mov Disord. 1999;14:436–442. doi: 10.1002/1531-8257(199905)14:3<436::aid-mds1008>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Schillaci O, Pierantozzi M, Filippi L, Manni C, Brusa L, et al. The effect of levodopa therapy on dopamine transporter SPECT imaging with(123)I-FP-CIT in patients with Parkinson's disease. Eur J Nucl Med Mol Imaging. 2005;32:1452–1456. doi: 10.1007/s00259-005-1922-9. [DOI] [PubMed] [Google Scholar]
- 38.van de Giessen EM, de Win MM, Tanck MW, van den Brink W, Baas F, et al. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- 39.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- 40.Newberg A, Lerman C, Wintering N, Ploessl K, Mozley PD. Dopamine transporter binding in smokers and nonsmokers. Clin Nucl Med. 2007;32:452–455. doi: 10.1097/01.rlu.0000262980.98342.dd. [DOI] [PubMed] [Google Scholar]
- 41.Chen PS, Yang YK, Yeh TL, Lee IH, Yao WJ, et al. Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers–a SPECT study. Neuroimage. 2008;40:275–279. doi: 10.1016/j.neuroimage.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, et al. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson's disease. J Nucl Med. 2005;46:227–232. [PubMed] [Google Scholar]
- 43.Parish CL, Stanic D, Drago J, Borrelli E, Finkelstein DI, et al. Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur J Neurosci. 2002;16:787–794. doi: 10.1046/j.1460-9568.2002.02132.x. [DOI] [PubMed] [Google Scholar]
- 44.Guigoni C, Dovero S, Aubert I, Li Q, Bioulac BH, et al. Levodopa-induced dyskinesia in MPTP-treated macaques is not dependent on the extent and pattern of nigrostrial lesioning. Eur J Neurosci. 2005;22:283–287. doi: 10.1111/j.1460-9568.2005.04196.x. [DOI] [PubMed] [Google Scholar]
- 45.Winogrodzka A, Booij J, Wolters E. Disease-related and drug-induced changes in dopamine transporter expression might undermine the reliability of imaging studies of disease progression in Parkinson's disease. Parkinsonism Relat Disord. 2005;11:475–484. doi: 10.1016/j.parkreldis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Gnanalingham KK, Robertson RG. The effects of chronic continuous versus intermittent levodopa treatments on striatal and extrastriatal D1 and D2 dopamine receptors and dopamine uptake sites in the 6-hydroxydopamine lesioned rat–an autoradiographic study. Brain Res. 1994;640:185–194. doi: 10.1016/0006-8993(94)91872-4. [DOI] [PubMed] [Google Scholar]
- 47.Ikawa K, Watanabe A, Kaneno S, Toru M. Modulation of [3H]mazindol binding sites in rat striatum by dopaminergic agents. Eur J Pharmacol. 1993;250:261–266. doi: 10.1016/0014-2999(93)90390-4. [DOI] [PubMed] [Google Scholar]
- 48.Rioux L, Frohna PA, Joyce JN, Schneider JS. The effects of chronic levodopa treatment on pre- and postsynaptic markers of dopaminergic function in striatum of parkinsonian monkeys. Mov Disord. 1997;12:148–158. doi: 10.1002/mds.870120204. [DOI] [PubMed] [Google Scholar]
- 49.Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol. 1996;298:27–30. doi: 10.1016/0014-2999(95)00770-9. [DOI] [PubMed] [Google Scholar]
- 50.Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, et al. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993;13:295–309. doi: 10.1002/syn.890130402. [DOI] [PubMed] [Google Scholar]
- 51.Moody CA, Granneman JG, Bannon MJ. Dopamine transporter binding in rat striatum and nucleus accumbens is unaltered following chronic changes in dopamine levels. Neurosci Lett. 1996;217:55–57. doi: 10.1016/0304-3940(96)13048-2. [DOI] [PubMed] [Google Scholar]
- 52.Thibaut F, Bonnet JJ, Vaugeois JM, Costentin J. Pharmacological modifications of dopamine transmission do not influence the striatal in vivo binding of [3H]mazindol or [3H]cocaine in mice. Neurosci Lett. 1996;205:145–148. doi: 10.1016/0304-3940(96)12399-5. [DOI] [PubMed] [Google Scholar]
- 53.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 54.Emond P, Garreau L, Chalon S, Boazi M, Caillet M, et al. Synthesis and ligand binding of nortropane derivatives: N-substituted 2beta-carbomethoxy-3beta-(4′-iodophenyl)nortropane and N-(3-iodoprop-(2E)-enyl)-2beta-carbomethoxy-3beta-(3′,4′-disubstituted phenyl)nortropane. New high-affinity and selective compounds for the dopamine transporter. J Med Chem. 1997;40:1366–1372. doi: 10.1021/jm960795d. [DOI] [PubMed] [Google Scholar]
- 55.Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, et al. Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage. 1999;9:108–116. doi: 10.1006/nimg.1998.0366. [DOI] [PubMed] [Google Scholar]
- 56.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DAT binding autoradiography in the rostral striatum (AC+2 mm). A. Representative DAT binding autoradiogram from a control animal illustrating the position of striatal subregions. B. Representative autoradiogram from a MPTP-treated monkey. Cd: caudate nucleus, Put: putamen DL: dorsolateral, DM: dorsomedial, VL: ventrolateral, VM: ventromedial. ** indicates significant difference compared with corresponding control group (p<0.001 using Bonferonni test following two-way ANOVA).
(3.52 MB TIF)