Abstract
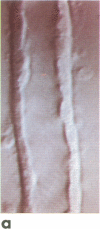
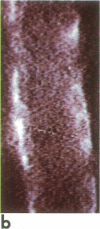
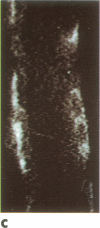
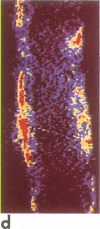
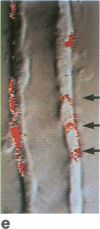
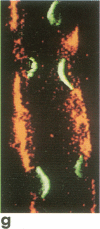
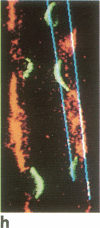
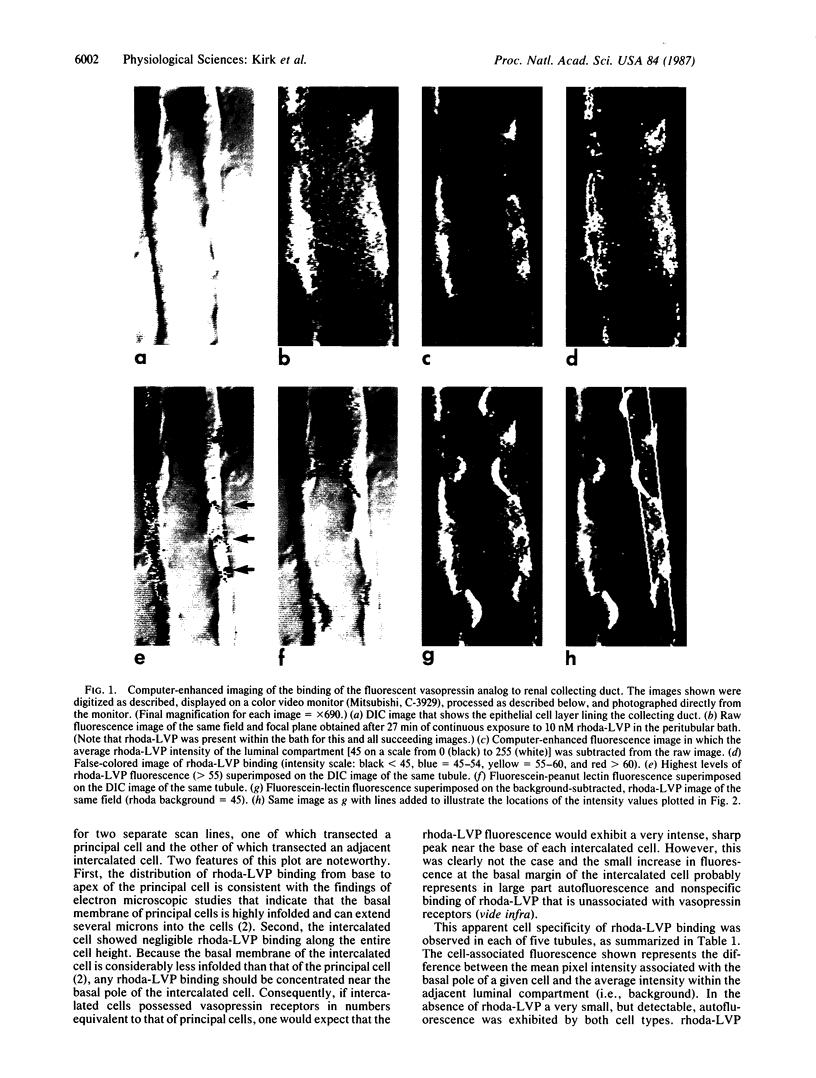
A noninvasive microscopic method was used to assess the cell specificity of vasopressin binding within the heterogeneous collecting duct. The binding of a fluorescent vasopressin analog (1-desamino-8-rhodamine-L-lysine vasopressin) to cells of the microperfused rabbit cortical collecting tubule was visualized and quantitated with image-intensified video microscopy and digital image processing. Binding to the basolateral membranes of a subpopulation of cells could be detected within 1-2 min of addition of the fluorescent analog (10 nM) to the peritubular bath. Binding could be prevented or reversed by the addition of a 10-fold excess of the native hormone, which indicates that the fluorescent analog binds specifically to vasopressin receptors. The time course of binding paralleled and slightly preceded hyperpolarization of the lumen-negative transepithelial voltage, an electrical response that is also elicited by the native hormone. Double-label experiments in which the intercalated cell population was stained with fluorescein-labeled peanut lectin revealed that binding of the vasopressin analog was localized to the remaining cell type, the principal cell. Our results support the following conclusions. First, the principal cell constitutes the primary target cell for vasopressin in the rabbit cortical collecting tubule, although the intercalated cell may possess a limited number of receptors at a density below the detection limit of this optical approach. Second, computer-enhanced video microscopy is a powerful, noninvasive method for assessing the kinetics and spatial pattern of hormone binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Buku A., Schwartz I. L., Gazis D., Ma C. L., Eggena P. Synthesis and biological activities of a fluorescent photoaffinity analog of vasopressin. Endocrinology. 1985 Jul;117(1):196–200. doi: 10.1210/endo-117-1-196. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Bulger R. E. Renal carbonic anhydrase. Am J Physiol. 1982 Oct;243(4):F311–F324. doi: 10.1152/ajprenal.1982.243.4.F311. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Grantham J. J., Moses H. L., Burg M. B., Orloff J. Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol. 1968 Feb;36(2):355–367. doi: 10.1083/jcb.36.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Kriz W. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol. 1979;56:1–123. doi: 10.1007/978-3-642-67147-0. [DOI] [PubMed] [Google Scholar]
- Kashgarian M., Biemesderfer D., Caplan M., Forbush B., 3rd Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int. 1985 Dec;28(6):899–913. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., DiBona D. R., Schafer J. A. Morphologic response of the rabbit cortical collecting tubule to peritubular hypotonicity: quantitative examination with differential interference contrast microscopy. J Membr Biol. 1984;79(1):53–64. doi: 10.1007/BF01868526. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Schafer J. A., DiBona D. R. Quantitative analysis of the structural events associated with antidiuretic hormone-induced volume reabsorption in the rabbit cortical collecting tubule. J Membr Biol. 1984;79(1):65–74. doi: 10.1007/BF01868527. [DOI] [PubMed] [Google Scholar]
- LeHir M., Kaissling B., Koeppen B. M., Wade J. B. Binding of peanut lectin to specific epithelial cell types in kidney. Am J Physiol. 1982 Jan;242(1):C117–C120. doi: 10.1152/ajpcell.1982.242.1.C117. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Hayhurst R. A. Functional differentiation of cell types of cortical collecting duct. Am J Physiol. 1985 Mar;248(3 Pt 2):F449–F453. doi: 10.1152/ajprenal.1985.248.3.F449. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Barasch J., Al-Awqati Q. Plasticity of functional epithelial polarity. 1985 Nov 28-Dec 4Nature. 318(6044):368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Evan A. P., Welling D. J. Shape of cells and extracellular channels in rabbit cortical collecting ducts. Kidney Int. 1981 Aug;20(2):211–222. doi: 10.1038/ki.1981.123. [DOI] [PubMed] [Google Scholar]