Abstract
Certain geographically distinct areas of the world have very high rates of esophageal cancer (EC). Previous studies have identified western Kenya as a high risk area for EC with an unusual percentage of cases in subjects 30 years of age or younger. To better understand EC in these young patients, we abstracted available data on all 109 young patients diagnosed with EC at Tenwek Hospital, Bomet District, Kenya from January 1996 through June 2009, including age at diagnosis, sex, ethnicity, tumor histology, residence location, and medical interventions. We also attempted to contact all patients or a family member and obtained information on ethnicity, tobacco and alcohol use, family history of cancer, and survival. Sixty (55%) representatives of the 109 young patients were successfully interviewed. The median survival time of these 60 patients was 6.4 months, the most common tumor histology was esophageal squamous cell carcinoma (ESCC) (98%), the M:F ratio was 1.4∶1, and only a few subjects used tobacco (15%) or alcohol (15%). Seventy-nine percent reported a family history of cancer and 43% reported having a family history of EC. In summary, this case series describes the largest number of young EC patients reported to date, and it highlights the uniqueness of the EC experience in western Kenya.
Introduction
Worldwide, esophageal cancer (EC) ranks eighth in cancer incidence and sixth in cancer mortality [1]. There are two primary cell types of EC, esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC); together these two types account for >95% of all cases of EC. In recent years, EAC rates have increased in most Western industrialized countries, and it has become the predominant form of EC in these populations; however, in other areas of the world, ESCC still predominates. About 80% of ECs occur in developing countries, and in these countries, nearly all of these cancers are ESCC [1].
The incidence of EC varies widely, and certain areas such as northern China [1], northeastern Iran [2], and South Africa [3] have very high rates of this disease, with age-standardized incidence rates from 50 to over 100 cases per 100,000 population per year. In contrast, most Western countries have much lower incidence rates of EC, from 4 to 10 cases per 100,000 population per year [4], [5].
Western Kenya also appears to have high rates of esophageal cancer. It has proven difficult to establish reliable cancer or death registries in this area, but case series reports from Tenwek Hospital, a tertiary care center in southwestern Rift Valley Province, and Moi Teaching and Referral Hospital, a tertiary care center in northern Rift Valley Province, show that EC is the most common cancer [6], [7], [8].
In both low- and high-incidence areas, EC is rare in individuals younger than age 30. In the US, the mean age of EC patients at diagnosis is 68 [4], and it rarely presents ≤30 years of age. EC cases in those ≤30 years of age in northern China, northeastern Iran, and the SEER registries in the US account for 0.7%, 1%, and 05% of cases, respectively [9], [10], [4]). At Tenwek Hospital, however, 6.3% of all EC cases are ≤30 [7]. To better understand the unusually frequent occurrence of EC in young people in this area, we conducted a retrospective study of all of the young EC patients diagnosed at Tenwek Hospital between January 1996 and June 2009.
Methods
Subject Identification and Data Gathering
We examined all pathology reports, endoscopy records, and patient files from Tenwek Hospital from January 1996 through June 2009 to identify all patients with a histologic or endoscopic diagnosis of EC who were ≤30 years of age (considered “young EC patients”). During this 13.5 year period, 109 such young EC patients were identified. We reviewed the following from the records of these patients: age at diagnosis, sex, ethnicity (specifically, tribal background), tumor histology, last known residence, and treatments.
To supplement the chart review and better understand the clinical course of EC in young patients at Tenwek, we attempted to locate all patients, their living family members, or another proxy familiar with their medical history. We successfully located a respondent for 60 of the 109 patients. Respondents were interviewed in their homes by a trained interviewer, using a structured questionnaire to obtain information on demographic characteristics, lifestyle, family history, and survival.
This study was approved by the human subjects review committee of Tenwek Hospital, and analysis of anonymized data was exempted from review by the Office of Human Subjects Research at the US National Cancer Institute.
Statistical analysis
The residence location of each subject was determined using the global positioning system coordinates from the GEOnet Names Server (http://www.nga.mil) and was mapped using Epi Info version 3.4.3 (CDC) software. Kaplan-Meir curves and median survival times were estimated using SAS 9.1 (SAS Institute, Inc, Cary, NC). Follow-up time was calculated using date of initial diagnosis and date of death. Date of initial diagnosis was identified from medical records. Date of death was obtained from medical records or interview responses.
Literature review
The literature was abstracted using the MEDLINE and PubMed databases (National Library of Medicine), initially using keywords: “esophageal cancer young” and/or “esophageal carcinoma young” with limits of: Humans, Case Reports, Core clinical journals, Cancer, MEDLINE, PubMed Central, All Infant: birth-23 months, All Child: 0–18 years, All Adult: 19+ years, Newborn: birth-1 month, Infant: 1–23 months, Preschool Child: 2–5 years, Child: 6–12 years, Adolescent: 13–18 years. Other keywords included combinations with: childhood cancer of the esophagus, young squamous cell carcinoma, young adenocarinoma, barrett's esophagus, and adolescence. Reference lists of all selected references were used as a secondary source. The search yielded 37 useful articles with 145 reports of esophageal malignancies in patients ≤30 years of age.
Results
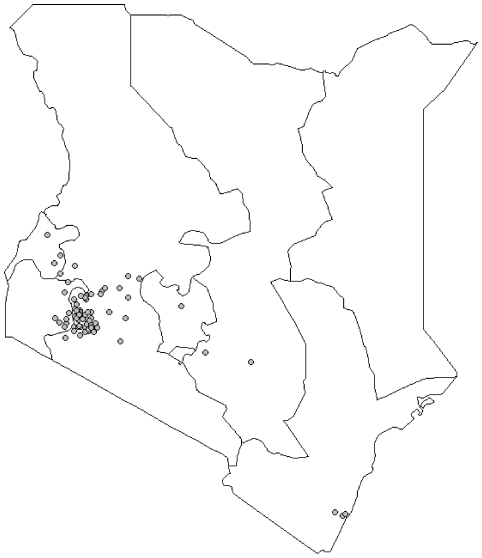
Between 1996 and 2009, 109 patients 30 years of age or younger were diagnosed with EC at Tenwek Hospital, with the youngest subject 14 years of age. This included 65 males and 44 females, a M:F ratio of 1.5∶1 ( Table 1 ). Eighty-seven (95%) of the 92 cases with known histology were ESCCs. Eighty percent of the young patients were of the Kalenjin ethnic group. Figure 1 is a map showing the residence locations of all of the 109 patients.
Table 1. Distributions of 109 esophageal cancer patients ≤30 years of age seen at Tenwek Hospital from January 1996 through June 2009.
| N | 109 |
| Age, years, mean (SD) | 25 (4) |
| Sex, M:F | 1.5∶1 |
| Male, N (%) | 65 (60) |
| Female, N (%) | 44 (40) |
| Histology | |
| Known, N (%) | 92 (84) |
| ESCC, N (%) | 87 (95) |
| EAC, N (%) | 5 (5) |
| Unknown, N (%) | 17 (16) |
| Ethnic group | |
| Kalenjin, N (%) | 87 (80) |
| Non-Kalenjin, N (%) | 22 (20) |
Figure 1. Home villages of young esophageal cancer patients.
Locations of the home villages of the 109 esophageal cancer patients ≤30 years of age seen at Tenwek Hospital (star) from January 1996 through June 2009.
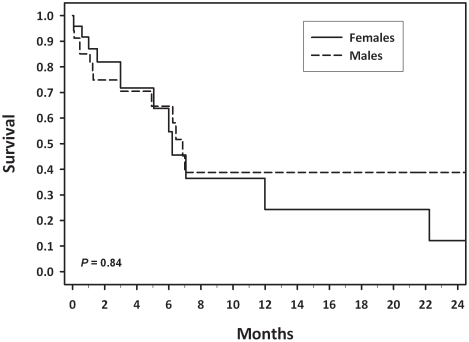
We successfully collected follow-up information on 60 (55%) of the 109 young patients. In the subgroup with follow-up information ( Table 2 ), the M:F ratio was 1.4∶1, 98% were pathology-confirmed ESCC cases, and 95% were part of the Kalenjin ethnic group, which is composed of seven related tribes living in southwestern Kenya. Thirty-five (58%) of the 60 patients elected to forgo palliative therapy ( Table 2 ). Twenty-one subjects (35%) received palliative interventions. Only four cases (7%) were candidates for esophagectomy, and one survived more than 5 years. Most patients had short survival times, and there was no significant difference by sex (P = 0.84), with median survival times of 6.9 months in males and 6.2 months in females ( Figure 2 ).
Table 2. Distributions of 60 esophageal cancer patients ≤30 years of age seen at Tenwek Hospital from January 1996 through June 2009 who had follow-up information.
| N | 60 |
| Age, years, mean (SD) | 25 (4) |
| Sex, M:F | 1.4∶1 |
| Male, N (%) | 35 (58) |
| Female, N (%) | 25 (42) |
| Histology | |
| ESCC, N (%) | 59 (98) |
| EAC, N (%) | 1 (2) |
| Ethnic group | |
| Kalenjin, N (%) | 57 (95) |
| Non-Kalenjin, N (%) | 3 (5) |
| Interventions | |
| Stent, N (%) | 14 (23) |
| Esophagectomy, N (%) | 4 (7) |
| Other, N (%) | 7 (12) |
| None, N (%) | 35 (58) |
| Survival | |
| Range, days | 2 – 2920 |
| Median, months | 6.4 |
| Alive at end of follow-up (%) | 3 (5) |
| Survival unknown, N (%) | 8 (13) |
Figure 2. Survival with esophageal cancer in young patients by sex.
Survival by sex of the 60 esophageal cancer patients ≤30 years of age seen at Tenwek Hospital from January 1996 through June 2009 who had follow-up information.
We examined several known risk factors for EC in the 60 followed patients ( Table 3 ). None of the followed female patients (n = 25) smoked tobacco, and only one had ever consumed alcoholic beverages. Among the male patients (n = 35), 9 (26%) smoked tobacco and 8 (23%) drank alcoholic beverages. A family history of cancer was present in 45 (79%) of the 57 subjects in which such a history was known, and there was a family history of EC in 21 (43%) of the 49 subjects with such data, including 5 (10%) with multiple EC cases in their families.
Table 3. Distributions of risk factors overall and by sex for esophageal cancer among patients ≤30 years of age seen at Tenwek Hospital from January 1996 through June 2009 who had follow-up information.
| Total | Male | Female | |
| N | 60 | 35 | 25 |
| Tobacco smoking | |||
| Yes, N (%) | 9 (15) | 9 (26) | 0 (0) |
| No, N (%) | 51 (85) | 26 (74) | 25 (100) |
| Alcoholic beverage drinking | |||
| Yes, N (%) | 9 (15) | 8 (23) | 1 (4) |
| No, N (%) | 51 (85) | 27 (77) | 24 (96) |
| Family history of cancer | |||
| Known, N (%) | 57 (95) | 32 (91) | 25 (100) |
| Yes, N (%) | 45 (79) | 23 (72) | 22 (88) |
| No, N (%) | 12 (21) | 9 (28) | 3 (12) |
| In first degree relative, N (%) | 25 (44) | 10 (31) | 15 (60) |
| Multiple CA family hx, N (%) | 16 (28) | 6 (19) | 10 (40) |
| Unknown, N (%) | 3 (5) | 3 (9) | 0 (0) |
| Family history of esophageal cancer | |||
| Known, N (%) | 49 (82) | 28 (80) | 21 (84) |
| Yes, N (%) | 21 (43) | 9 (32) | 12 (57) |
| No, N (%) | 28 (57) | 19 (68) | 9 (43) |
| Multiple EC family hx, N (%) | 5 (10) | 2 (7) | 3 (14) |
| Unknown, N (%) | 11 (18) | 7 (20) | 4 (16) |
A search of the literature found 37 articles describing 145 cases of EC patients 30 years of age or younger. 102 (70%) of the cases were in case series in which the exact ages were not indicated, and for 104 cases (72%), the tumor histology was not given ( Table 4 ). Of the 43 cases with reported ages, the median age was 17 years and the age range was from 8 years to 30 years. The M:F ratio in the 145 reported cases was 1.8∶1. Of the 41 cases with reported histology, 17 (41%) were ESCC, 16 (39%) were EAC, and 8 (20%) reported as EC not otherwise specified (NOS) ( Table 5 ).
Table 4. Published papers presenting information on esophageal cancer in young patients.
| Histology | ||||||||
| Date | Reference | No. Cases | Mean Age | No. Males | No. ESCC | No. EAC | No. EC NOS | Location |
| 1925 | Jackson [24] | 2 | 23 | unknown | USA | |||
| 1929 | Kaufuman [25] | 1 | 21 | 0 | 0 | 0 | 1 | Germany |
| 1955 | Saettler [26] | 1 | 24 | 0 | 0 | 0 | 1 | Germany |
| 1961 | Hahlbrock [27] | 1 | 13 | 1 | 0 | 0 | 1 | Germany |
| 1963 | Birzel [28] | 1 | 12 | 1 | 0 | 0 | 1 | Germany |
| 1967 | Sanowaski [29] | 1 | 24 | 1 | 1 | 0 | 0 | USA |
| 1967 | Wright [30] | 2 | 21 | 2 | 1 | 0 | 1 | England |
| 1968 | Kinnman [31] | 1 | 15 | 1 | 1 | 0 | 0 | Korea |
| 1968 | Paymaster * [23] | 86 | 25 | 58 | unknown | India | ||
| 1971 | Das * [32] | 11 | ≤30 | 3 | unknown | India | ||
| 1976 | Oberiter [33] | 1 | 12 | 0 | 0 | 0 | 1 | Croatia |
| 1977 | Morota [34] | 1 | 18 | 1 | 0 | 0 | 1 | Japan |
| 1977 | Poleynard [35] | 1 | 25 | 1 | 0 | 1 | 0 | USA |
| 1979 | Tata [36] | 1 | 17 | 0 | 0 | 0 | 1 | India |
| 1979 | Singh [37] | 1 | 14 | 1 | 1 | 0 | 0 | India |
| 1980 | Soni [38] | 1 | 8 | 0 | 1 | 0 | 0 | India |
| 1983 | Elliott [39] | 1 | 14 | 1 | 0 | 1 | 0 | England |
| 1984 | Hilou [40] | 1 | 15 | 1 | 0 | 1 | 0 | England |
| 1986 | Bright [41] | 1 | 20 | 1 | 0 | 1 | 0 | Australia |
| 1988 | Khastgir [42] | 1 | 18 | 0 | 1 | 0 | 0 | India |
| 1988 | Dewar [43] | 1 | 20 | 0 | 1 | 0 | 0 | Australia |
| 1989 | Adzick [44] | 1 | 20 | 0 | 0 | 1 | 0 | USA |
| 1989 | Shahi [45] | 1 | 14 | 1 | 1 | 0 | 0 | India |
| 1989 | Hoeffel [46] | 2 | 13 | 2 | 0 | 2 | 0 | France |
| 1992 | Kumar [47] | 7 | 17 | 5 | 4 | 3 | 0 | India |
| 1993 | Hassall [48] | 1 | 17 | 1 | 0 | 1 | 0 | Canada |
| 1993 | Aryya [49] | 1 | 10 | 1 | 1 | 0 | 0 | India |
| 1997 | Gangopadhyay [50] | 1 | 8 | 1 | 0 | 1 | 0 | India |
| 1998 | Schettini [51] | 1 | 11 | 0 | 1 | 0 | 0 | Brazil |
| 1999 | Karwasra [52] | 1 | 17 | 1 | 1 | 0 | 0 | India |
| 2001 | Singh [53] | 1 | 18 | 1 | 1 | 0 | 0 | India |
| 2001 | Zotter [54] | 1 | 16 | 1 | 0 | 1 | 0 | Austria |
| 2003 | Al-Hilli * [55] | 5 | 25 | 3 | unknown | Bahrain | ||
| 2005 | Pultrum [56] | 1 | 22 | 0 | 0 | 1 | 0 | Netherlands |
| 2005 | Tampi [57] | 1 | 15 | 1 | 1 | 0 | 0 | India |
| 2007 | Moreels [58] | 1 | 28 | 1 | 0 | 1 | 0 | Netherlands |
| 2007 | Shinohara [59] | 1 | 27 | 0 | 0 | 1 | 0 | USA |
*These references did not give exact ages, so the center of the range is given.
Table 5. Summary of age, sex and histologic data from published reports of esophageal cancer in young persons, overall and separately in developing and developed countries.
| Total | Developing countries | Developed countries | |
| Cases, N (%) | 145 | 122 (84) | 23 (16) |
| Sex | |||
| Male, N (%) | 92 (64) | 77 (63) | 15 (71) |
| Female, N (%) | 51 (46) | 45 (37) | 6 (29) |
| M:F | 1.8∶1 | 1.7∶1 | 2.5∶1 |
| Histology | |||
| Known, N (%) | 41 (28) | 20 (16) | 21 (91) |
| ESCC cases, N (%) | 17 (41) | 14 (70) | 3 (14) |
| ACA cases, N (%) | 16 (39) | 4 (20) | 12 (57) |
| EC NOS, N (%) | 8 (20) | 2 (10) | 6 (29) |
| Unknown, N (%) | 104 (72) | 102 (84) | 2 (9) |
One hundred twenty-two (84%) of the young EC cases reported in the literature lived in developing countries, including 114 cases (79%) in India alone. The M:F ratio was 1.7∶1 in developing countries and 2.5∶1 in developed countries. Of the cases with a specified histological cell type, ESCC predominated in the developing countries (14/18, 78%), whereas EAC predominated in developed countries (12/15, 80%) ( Table 5 ).
Discussion
Western Kenya has been identified as an area with a common occurrence of ESCC. Of patients that are diagnosed at Tenwek Hospital, about 6% are ≤30 years of age [7]. This high percentage has not been reported anywhere else in the world. From January 1996 – June 2009, 109 such young EC cases were identified in this case series from Tenwek Hospital.
Among the total 109 cases and the 60 cases with follow-up information, the M:F ratio was close to 1.5∶1. This is similar to the gender distribution of cases found in all EC patients seen at Tenwek (1.6∶1) [7] and in other high-risk populations in developing areas, such as Linxian, China [11] and Golestan Province in northwestern Iran, [12]. This M:F ratio is much lower than those found in industrialized countries [13]. By far the most common histologically confirmed tumor type among the young EC patients seen at Tenwek was ESCC (95%), which is also the most common tumor type in adult patients at Tenwek [7] and in other known high risk areas [14]. This large percentage of ESCC may even be an underestimate, because some of the small number of EAC cases identified at Tenwek Hospital may have originated in the gastric cardia.
Two primary risk factors for esophageal cancer in Western populations are smoking tobacco and drinking alcoholic beverages in excess [15]. We found that tobacco and alcohol consumption were reported by only a minority of young EC cases at Tenwek, which supports the argument that although these exposures are associated with EC in developed countries, they do not seem to be major etiologic factors in this area. This finding is similar to other developing, high-risk ESCC areas in China [16] and Iran [17]. Notably, almost 80% of patients in this case series had a family history of cancer, including a 43% with a specific family history of EC, which is a higher percentage than in cases from a high risk area in China [16] but lower than in cases from a high-risk area in Iran [18]. The contribution of other risk factors will require formal etiologic studies, but may include consumption of very hot tea [19], limited diet [20], exposure to polycyclic aromatic hydrocarbons [21], or genetics.
It is also important to note that most young patients in this series were of Kalenjin ethnicity, although the meaning of this is difficult to assess in a case-series. A similar proportion of Kalenjins has been reported among EC patients from the traditional catchment area around Tenwek Hospital [7]. The high proportion of cases with a family history of EC and the apparent restriction to a specific ethnic background both suggest that genetic factors could be important in the etiology of EC in this area, but these observations could also reflect shared environmental risk factors such as socioeconomic status, diet, use of similar traditional medicines [22] or foods, or communicable diseases.
Of the followed patients, survival was poor, with a median of 6.4 months, which is shorter than the still poor survival of 9.2 months seen for all EC cases in the United States [13]. In all populations, the majority of EC cases are diagnosed at an advanced stage, and it appears that this is especially true among young patients at Tenwek. Local knowledge of the high case fatality rate may further discourage cases from coming to the hospital until the cancer is very advanced.
Our literature review shows that little is known about EC in young people in any population. We found several case series of young patients from India, but only limited reports from other countries. Taken together, these reports suggest that the occurrence of EC in patients ≤30 years of age is rare throughout the world; even in the large case series presented by Paymaster et al [23] young EC patients comprised only around 1% of their cases. These literature reports also suggest that the demographic and tumor characteristics of EC in young patients are similar to those of EC in older patients from the same populations: the M:F ratio in the reported young patients was close to one (1.8∶1) in cases from developing countries and was greater (2.5∶1) in cases from developed countries, and the proportion of ESCC tumors was high (14/18, 78%) in cases from developing countries and it was low (3/15, 20%) in cases from developed countries.
In summary, this case series describes the largest number of young EC patients reported to date, and it highlights the uniqueness of the EC experience in western Kenya. The causes of the overall high incidence and the particularly high incidence in young people remain unknown and will require detailed epidemiologic studies of the local population.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by intramural funds from the Division of Cancer Epidemiology and Genetics of the National Cancer Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Ca-A Cancer Journal for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Kamangar F, Nasrollahzadeh D, Møller H, Boffetta P, et al. Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran - A review. European Journal of Cancer. 2009;45:3156–3165. doi: 10.1016/j.ejca.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Sumeruk R, Segal I, Te Winkel W, Van Der Merwe CF. Oesophageal cancer in three regions of South Africa. South African Medical Journal. 1992;81:91–93. [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969-2006), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. Available: http://www.seer.cancer.gov/popdata. Accessed 2009 February.
- 5.Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, et al. Trends in oesophageal cancer incidence and mortality in Europe. International Journal of Cancer. 2008;122:1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 6.White RE, Abnet CC, Mungatana CK, Dawsey SM. Oesophageal cancer: A common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462–463. doi: 10.1016/S0140-6736(02)09639-3. [DOI] [PubMed] [Google Scholar]
- 7.Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in western Kenya. Dis Esophagus. 2010;23:128–135. doi: 10.1111/j.1442-2050.2009.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of western Kenya. African Health Sciences. 2005;5:157–163. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Chen SH, Li YM. Epidemiological investigation of esophageal carcinoma. World Journal of Gastroenterology. 2004;10:1834–1835. doi: 10.3748/wjg.v10.i12.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semnani SH, Besharat S, Abdolahi N, Kalavi KH, Fazeli SA, et al. Esophageal cancer in northeastern Iran [1]. Indian Journal of Gastroenterology. 2005;24:224. [PubMed] [Google Scholar]
- 11.Lu JB, Yang WX, Liu JM. Trends in morbidity and mortality for oesophageal cancer in Linxian county, 1959-1983. International Journal of Cancer. 1985;36:643–645. doi: 10.1002/ijc.2910360603. [DOI] [PubMed] [Google Scholar]
- 12.Mahboubi E, Kmet J, Cook PJ. Oesophageal cancer studies in the Caspian Littoral of Iran: The Caspian Cancer Registry. British Journal of Cancer. 1973;28:197–214. doi: 10.1038/bjc.1973.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ries LAG YJ, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. In: National Cancer Institute SP, NIH Pub. No 07-6215, editor. Bethesda, MD, 2007.
- 14.Blot W, McLaughlin J, Fraumeni J. Esophageal cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 15.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57, vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian General Population Trial cohort in China. International Journal of Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 17.Nasrollahzadeh D, Kamangar F, Aghcheli K, Sotoudeh M, Islami F, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. British Journal of Cancer. 2008;98:1857–1863. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Sun P, et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. International Journal of Cancer. 2006;119:1047–1051. doi: 10.1002/ijc.21906. [DOI] [PubMed] [Google Scholar]
- 19.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental Causes of Esophageal Cancer. Gastroenterology Clinics of North America. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abedi-Ardekani B, Kamangar F, Hewitt SM, Hainaut P, Sotoudeh M, et al. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut. 2010;59:1178–1183. doi: 10.1136/gut.2010.210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sewram V, Shephard GS, Van Der Merwe L, Jacobs TV. Mycotoxin contamination of dietary and medicinal wild plants in the Eastern Cape Province of South Africa. Journal of Agricultural and Food Chemistry. 2006;54:5688–5693. doi: 10.1021/jf060483b. [DOI] [PubMed] [Google Scholar]
- 23.Paymaster JC, Sanghvi LD, Gangadharan P. Cancer in the gastrointestinal tract in western India. Epidemiologic study. Cancer. 1968;21:279–288. doi: 10.1002/1097-0142(196802)21:2<279::aid-cncr2820210218>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Jackson C. Carcinoma and Sarcoma of the Esophagus: A Plea for Early Diagnosis. The American Journal of the Medical Sciences. 1925;169:625–648. [Google Scholar]
- 25.Kaufmann E. Philadelphia: P. Blakiston's sons & co; 1929. Pathology for students and practitioners; authorized translation of the Lehrbuch der pathologischen anatomie, by Dr. Edward Kaufmann ... translated by Stanley P. Reimann ... Stanley P. Reimann MD, translator.2452 [Google Scholar]
- 26.Sattler A. Esophagus carcinoma in young adults. Dtsch Gesundheitsw. 1955;10:529–530. [PubMed] [Google Scholar]
- 27.Hahlbrock KH. Unusual esophageal carcinoma in a 12-3/4-year-old boy. HNO. 1961;9:110–112. [PubMed] [Google Scholar]
- 28.Birzle H. Esophageal carcinoma in a 12-year-old. Fortschr Geb Rontgenstr Nuklearmed. 1963;98:495–496. [PubMed] [Google Scholar]
- 29.Sanowski RA, DiBianco J. Carcinoma of the esophagus in a young adult. Gastrointestinal Endoscopy. 1967;13:21–23. [PubMed] [Google Scholar]
- 30.Wright JT, Richardson PC. Squamous carcinoma of the thoracic oesophagus in malabsorption syndrome. British medical journal. 1967;1:540–542. doi: 10.1136/bmj.1.5539.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinnman J, Shin HI, Wetteland P. Carcinoma of the oesophagus after lye corrosion. Report of a case in a 15-year-old korean male. Acta Chirurgica Scandinavica. 1968;134:489–493. [PubMed] [Google Scholar]
- 32.Das MM, Ray SC, Venkatesan SR, Ray M, Chatterjee BP. Carcinoma oesophagus. (A review of 326 cases). Journal of the Indian Medical Association. 1971;57:44–52. [PubMed] [Google Scholar]
- 33.Oberiter V, Fabecic Sabadi V, Bajakic D. Esophageal carcinoma in a 12 year old girl (Serbocroatian). Lijecnicki Vjesnik. 1976;98:422–424. [PubMed] [Google Scholar]
- 34.Morota N. Carcinoma of the esophagus associated with lung abscess and pyothorax seen in an 18 year-old boy. Journal of the Japanese Association for Thoracic Surgery. 1977;25:791–797. [PubMed] [Google Scholar]
- 35.Poleynard GD, Marty AT, Birnbaum WB. Adenocarcinoma in the columnar lined (Barrett) esophagus: case report and review of the literature. Archives of Surgery. 1977;112:997–1000. doi: 10.1001/archsurg.1977.01370080095016. [DOI] [PubMed] [Google Scholar]
- 36.Tata HR, Fletcher AG. Carcinoma oesophagus. Indian Journal of Surgery. 1979;41:121–130. [Google Scholar]
- 37.Singh H, Suri RK, Gujral JS. Carcinoma oesophagus in childhood. (A case report and review of the literature). Indian Journal of Surgery. 1979;41:118–119. [Google Scholar]
- 38.Soni NK, Chatterji P. Carcinoma of the esophagus in an eight year old child. Journal of Laryngology and Otology. 1980;94:327–329. doi: 10.1017/s002221510008885x. [DOI] [PubMed] [Google Scholar]
- 39.Elliott MJ, Ashcroft T. Primary adenocarcinoma of the gastro-oesophageal junction in childhood. A case report. Scandinavian Journal of Thoracic and Cardiovascular Surgery. 1983;17:65–66. doi: 10.3109/14017438309102382. [DOI] [PubMed] [Google Scholar]
- 40.Hilou RA, Atkins J, Matthews HR. Oesophageal adenocarcinoma in a boy of fifteen years. Journal of Laryngology and Otology. 1984;98:643–646. doi: 10.1017/s002221510014722x. [DOI] [PubMed] [Google Scholar]
- 41.Bright N, Marshall RD, Wallis P. Barrett's oesophagus and adenocarcinoma - A case report. Australian and New Zealand Journal of Surgery. 1986;56:661–664. doi: 10.1111/j.1445-2197.1986.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 42.Khastgir T, Kar P, Kulpati DD. Carcinoma oesophagus in a young girl masquerading as anorexia nervosa. The Journal of the Association of Physicians of India. 1988;36:679. [PubMed] [Google Scholar]
- 43.Dewar JM, Courtney JT, Byrne MJ, Joske RA. Esophageal cancer in a young woman after treatment for osteosarcoma. Medical and Pediatric Oncology. 1988;16:287–289. doi: 10.1002/mpo.2950160414. [DOI] [PubMed] [Google Scholar]
- 44.Adzick NS, Fisher JH, Winter HS, Sandler RH, Hendren WH. Esophageal adenocarcinoma 20 years after esophageal atresia repair. Journal of Pediatric Surgery. 1989;24:741–744. doi: 10.1016/s0022-3468(89)80528-7. [DOI] [PubMed] [Google Scholar]
- 45.Shahi UP, Sudarsan, Dattagupta S, Singhal S, Kumar L, et al. Carcinoma oesophagus in a 14 year old child: report of a case and review of literature. Tropical gastroenterology: official journal of the Digestive Diseases Foundation. 1989;10:225–228. [PubMed] [Google Scholar]
- 46.Hoeffel JC, Nihoul-Fekete C, Schmitt M. Esophageal adenocarcinoma after gastroesophageal reflux in children. Journal of Pediatrics. 1989;115:259–261. doi: 10.1016/s0022-3476(89)80076-9. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Shukla NK, Mishra MC, Shahi UP, Kapur BML. Primary oesophageal carcinoma in teens. Journal of Surgical Oncology. 1992;50:254–257. doi: 10.1002/jso.2930500412. [DOI] [PubMed] [Google Scholar]
- 48.Hassall E, Dimmick JE, Magee JF. Adenocarcinoma in childhood Barrett's esophagus: Case documentation and the need for surveillance in children. American Journal of Gastroenterology. 1993;88:282–288. [PubMed] [Google Scholar]
- 49.Aryya NC, Lahiri TK, Gangopadhyay AN, Asthana AK. Carcinoma of the esophagus in childhood. Pediatric Surgery International. 1993;8:251–252. [Google Scholar]
- 50.Gangopadhyay AN, Mohanty PK, Chooramani Gopal S, Gupta DK, Sahi UP, et al. Adenocarcinoma of the esophagus in an 8-year-old boy [1]. Journal of Pediatric Surgery. 1997;32:1259–1260. doi: 10.1016/s0022-3468(97)90698-9. [DOI] [PubMed] [Google Scholar]
- 51.Schettini ST, Ganc A, Saba L. Esophageal carcinoma secondary to a chemical injury in a child. Pediatric Surgery International. 1998;13:519–520. doi: 10.1007/s003830050388. [DOI] [PubMed] [Google Scholar]
- 52.Karwasra RK, Yadav V, Bansal AR. Esophageal carcinoma in a 17-year-old man. Am J Gastroenterol. 1999;94:1122–1123. doi: 10.1111/j.1572-0241.1999.01122.x. [DOI] [PubMed] [Google Scholar]
- 53.Singh S, Lal P, Sikora SS, Datta NR. Squamous cell carcinoma arising from a congenital duplication cyst of the esophagus in a young adult. Diseases of the Esophagus. 2001;14:258–261. doi: 10.1046/j.1442-2050.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 54.Zotter H, Schwinger W, Kerbl R, Urban C, Smolle Juettner FM, et al. Management of a 16-year-old boy with adenocarcinoma at the esophageal gastric junction. Med Pediatr Oncol. 2001;37:557. doi: 10.1002/mpo.1255. [DOI] [PubMed] [Google Scholar]
- 55.Al-Hilli F, Malik AK. Oesophageal cancer in Bahrain. East Mediterr Health J. 2003;9:372–376. [PubMed] [Google Scholar]
- 56.Pultrum BB, Bijleveld CM, De Langen ZJ, Plukker JTM. Development of an adenocarcinoma of the esophagus 22 years after primary repair of a congenital atresia. Journal of Pediatric Surgery. 2005;40 doi: 10.1016/j.jpedsurg.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 57.Tampi C, Pai S, Doctor VM, Plumber S, Jagannath P. HPV-associated carcinoma of esophagus in the young: A case report and review of literature. International Journal of Gastrointestinal Cancer. 2005;35:135–142. doi: 10.1385/IJGC:35:2:135. [DOI] [PubMed] [Google Scholar]
- 58.Moreels TG, van Vliet EPM, Tilanus HW, Tran TCK, Kuipers EJ, et al. Down syndrome and esophageal cancer. Diseases of the Esophagus. 2007;20:183–186. doi: 10.1111/j.1442-2050.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 59.Shinohara ET, Swisher-McClure S, Husson M, Sun W, Metz JM. Esophageal cancer in a young woman with bulimia nervosa: A case report. Journal of Medical Case Reports. 2007;1 doi: 10.1186/1752-1947-1-160. [DOI] [PMC free article] [PubMed] [Google Scholar]