Abstract
The centromere is perhaps the most iconic feature on a eukaryotic chromosome. An amateur enthusiast equipped with a light microscope can easily identify the center of each metacentric chromosome, marking the spot responsible for accurate genome segregation. This review will highlight findings which provide novel insights into how centromeres are assembled and disassembled, the role centromeric proteins play in repair, epigenetic features uniquely found at the centromere, and the three dimensional organization of centromeres caught in the act of mitosis. These advances have unveiled a veritable wonderland of non-canonical features that drive centromere function.
Introduction
In eukaryotes, centromeres are organized into three discrete strata. The inner most unit is the centromeric chromatin, which serves as the platform to recruit inner and outer kinetochore proteins, which in turn facilitate binding to spindle microtubules at mitosis [1]. The centromeric chromatin is composed of AT-rich centromeric DNA repeats, packaged by specialized nucleosomes containing a centromere-specific histone H3 variant (CENH3). CENH3 homologs include CENP-A in humans, Cse4/Cnp1 in yeast, CENH3 in Arabidopsis, Cid in flies, and HCP-3 in worms [2] (Table 1). CENH3 depletion results in mitotic arrest, and overexpressed CENH3s can seed centromeres at ectopic locations [3–5]. Thus, it is thought that key inner kinetochore proteins recognize some features of centromeric chromatin. However, CENH3s are only ~50% conserved [6], and CENH3 chromatin domains vary widely, ranging from a single centromeric nucleosome per chromosome in budding yeast, to large arrays of CENH3/H2A nucleosomes alternating with arrays of H3/H2A.Z nucleosomes in metazoan chromosomes.
Table 1. Organization of Centromere Domains.
Point centromeres (budding yeast), Regional centromeres (fission yeast, insects, mammals, plants) and Holocentric centromeres (nematodes) have conserved organization. Homologs are indicated in italics. Ambiguity regarding function is denoted (?).
| Organism | Centromeric DNA (>80% AT) |
Centromere -specific histone H3 |
Assembly/ Chaperone Complex |
CENH3 Nucleosome Components |
Inner Kinetochore Proteins |
Outer Kinetochore Proteins |
Chromatin Remodelers |
|---|---|---|---|---|---|---|---|
|
Saccharomyces cerevisiae |
120bp unique Centromere- Determining Element |
CSE4 | Mis16 (RbAp48) Mis18 Scm3 |
CSE4+H4 + Scm3; CSE4+H4 + H2AB |
Mif2p (CENP-C) Ndc10/CBF3 CBF-1 |
KMN complex* Ctf19/COMA complex |
Swi/Snf2 (RSC) GCN5p |
|
Schizosaccharomyces pombe |
1.5kb unique Central Core domain |
CNP-1 | Mis16 Mis18 Sim3 Scm3 |
CNP-1+H4 + Scm3; CNP-1+H4+H2AB |
CNP-3 (CENP-C) |
KMN complex | Swi/Snf2 |
|
Drosophila melanogaster |
5bp repeats | CID | RbAp48 | CID+H4+H2AB | CENP-C Cal-1 (?) |
KMN complex | unreported |
|
Homo sapiens Note exceptions i) Neocentromeres: no specific sequences ii) Mouse: 120bp minor B repeats |
171bp α-satellite repeats |
CENP-A | RbAp48 HJURP NPM |
CENP-A+H4 +H2AB |
CENP-C CENP-B CENP-N CENP-H |
KMN complex CENP-E INCENP CENP-K CENP-M CENP-O CENP-T CENP-U CENP-V CENP-W |
RSF FACT CHD1 Swi/Snf2B |
| Arabidopsis thaliana | 180bp repeats | CENH3 | unreported | unreported | CENP-C | KMN complex | unreported |
|
Caenorhabditis elegans |
Holocentric: coats entire chromosome |
HCP-3 | KLN-2 (Mis18BP) |
unreported | HCP-4 (CENP-C) |
KMN complex | unreported |
Despite the diversity noted above, centromere organization across most species is similar (Table 1), and centromere ultra-structure probably derives from a common ancestral identity. Swap experiments support the view that the leitmotif lies within the CENH3 nucleosome itself. When introduced into fly cells, yeast Cse4, human CENP-A, and worm HCP-3 localize to Drosophila centromeres [7]; Cse4 can localize to and functionally complement human CENP-A depletion [8]; residues of CENH3 swapped into H3 allow the chimera to localize to centromeres [9–10] and, avian CENP-A can preferentially associate with mouse 120bp centromeric repeats [11]. Consequently, studies dissecting CENH3 assembly, localization, and structure are of considerable interest to achieve a unitary model for centromere function [12–13].
Assembly of CENH3 nucleosomes
Chaperones guiding CENH3 assembly to centromeres have been pursued for over a decade. The prevalent model posits that specific complexes target CENH3 precisely to the centromere [14], while others have argued that CENH3 can occupy gaps in chromatin [15]. The earliest experimental indication of how CENH3 assembles into chromatin comes from genetic dissections in yeast. Deletions of two proteins, Mis16 and Mis18, results in loss of centromeric features and mis-segregation [16]. Mis18 occupies centromeric locations ahead of newly incorporated Cse4 [16], and recruits the fungal protein Scm3, which associates with Sim3 to facilitate Cse4 incorporation [17]. Synthetic hyper-acetylation rescues Mis18 deletion [18], suggesting that Mis18 may drive acetylation and mobilization of H3 nucleosomes, providing gaps for CENH3 assembly. In worms, a Mis18 binding protein, KLN-2, escorts HCP-3 to chromatin, indicating a conserved role for Mis18 in holocentric centromeres [19]. Similarly, in fly cells, a Mis16 homolog, RbAp48 co-purifies with the pre-nucleosomal Cid/H4 complex. RbAp48 is found in all H3 assembly complexes, and in vitro, chaperones Cid/H2A/H2B/H4 into chromatin [20]. Thus, its identification as a Cid chaperone supports non-specific CENH3 assembly.
A prediction from the gap-filling model is that excess CENH3 should enrich in regions of high nucleosome turnover. Results from a seminal study in budding yeast suggest this may be the case. Chromatin immunoprecipitation followed by deep sequencing (ChIPSeq) has revealed that in addition to 16 centromeric locations, Cse4 occupies 132 promoters of highly expressed genes [21]. How do kinetochore proteins distinguish between Cse4 at centromeric versus ectopic locations? In yeast, the key yeast inner kinetochore complex, CBF3, binds directly to centromeric DNA, and is thus anchored to a single location [22]. Lack of kinetochore specificity in metazoans may explain why these organisms restrict CENH3 expression to late G2, and incorporation to M/early G1 [23–24], where M-phase specific chromosome compaction serves as a natural barrier to CENH3 mis-incorporation. However, G2/M timing of CENH3 expression/incorporation leads to dilution of CENH3 during DNA replication. Equal inheritance of centromere location would then require old CENH3 to be divided equally between daughter chromatids (Figure 1). Another problem is that during early M phase, unincorporated CENH3 could diffuse away. How is this prevented? Using a genome-wide RNAi screen in fly cells, researchers determined that Cal-1 co-localizes with CENH3 at interphase and mitosis [25]. When Cal-1 is depleted, new CENH3 is unable to deposit. Conversely, Cal-1 binding is disrupted if CENH3 or CENP-C is depleted, suggesting that Cal-1 itself requires a pre-existing platform of centromeric chromatin. Cal-1 may serve as a mitosis-specific bridge between centromeric chromatin and soluble CENH3/H4/RbAp48 complexes, pointing to an attractive convergence of gap-filling and targeting mechanisms.
Figure 1.
A model for how HJURP/RbAp48-mediated CENH3 assembly is regulated by the cell cycle. Majority of CENH3 nucleosomes are in the tetrameric configuration during G2/M. At M phase, newly expressed CenH3 is weakly associated with centromeric chromatin. At early G1, HJURP/RbAp48 drive CENH3 incorporation into centromeres, and there is a greater propensity to equilibrate towards the octameric form. During replication, CENH3 nucleosomes split into the stable tetrameric configuration allowing equal segregation. Color Legend: magenta/dark blue: CenH3; orange/light blue: H2A; tan/terra cotta: H2B; grey/green: H4; red: DNA; green propeller: RbAp48.
An exception to the mechanisms of CENH3 deposition discussed above comes from plants, which deposit CENH3 during G2 [26], leaving open the potential for mis-incorporation within euchromatin. Therefore, it is likely that plants have evolved additional epigenetic mechanisms to restrict CENH3 assembly [27]. As discussed later, one such trick might be to couple DNA methylation to inner kinetochore assembly.
A key tenet of the targeted assembly model requires a specific complex to deliver CenH3 to centromeres exclusively. Three recent studies have identified a CENP-A-specific assembly chaperone that satisfies the first requirement [28–30]. CENP-A pre-assembly complexes were found to contain one copy of histones H4 and CENP-A (endogenous and ectopic CENP-A do not mix in this complex), Holliday Junction Recognition Protein (HJURP), Nucleophosmin (NPM), and the histone chaperone RbAp48. One of the groups also identified H2a and H2b within CENP-A- and H3- assembly complexes [30], leading to the tantalizing possibility that histones deposit as half-nucleosomal intermediates.
HJURP satisfies the requirement for specificity because GST-fusion assays show that it can specifically discriminate loop 1/helix 2 regions of CENP-A, previously identified as the CATD region, necessary for CENP-A localization to centromeres. Reciprocal experiments show that ectopically expressed HJURP associates with endogenous CENP-A and H4 from human cells. HJURP co-localizes with GFP-CENP-A when the latter is incorporated into the centromere. Concurrently, HJURP depletion results in loss of GFP-CENP-A from the centromere [28]. These studies support the view that HJURP is indeed a CENP-A-specific chaperone.
A notable finding is that 80 amino acids within HJURP’s N-terminus are necessary for its association with CENP-A [30]. This is an exciting discovery because HJURP and the fungal protein Scm3 contain evolutionarily conserved TYTL motifs spanning this region [31]. Scm3 is required for Cse4 assembly and retention on yeast centromeric DNA [17, 32–34], and in the absence of deep sequence homology, Cse4 can functionally rescue CENP-A depletion in human cells [8]. Furthermore, HJURP is present in all mammals and Scm3 is present in all fungi, implying similar CENH3 assembly pathways exist in evolutionary distant groups.
Paradoxically, HJURP also localizes to Holliday junctions at breakpoints across the human genome [35], which contradicts the second requirement for targeted assembly: exclusive location. Indeed, GFP-CENP-A localizes to sequence-specific double strand breaks (DSB), presumably through its association with HJURP [36]. HJURP guiding CENP-A to both centromeric and ectopic locations presents an obvious conundrum. How are metazoan centromere domains distinguished from ectopic CENH3 nucleosomes? A speculative hypothesis is that there are two modes of CENH3 assembly, resulting in two types of CENH3 nucleosomes. One CENH3 form predominates at centromeres, reinforced by key kinetochore partners, while the other is an outcome of promiscuous assembly in euchromatin. The latter mode would have self-imposed constraints, such as blocking domains recognized by kinetochore proteins.
Bi-stability in CENH3 nucleosome structure
Evidence suggests that the CENH3 nucleosome can exist in stable non-canonical forms (Figure 2). In S. cereviseae, the non-histone protein Scm3 associates with the Cse4/H4 nucleosome via Cse4’s CATD region [37], and in vitro, Scm3 substitutes one copy of H2A/H2B in the Cse4 octamer, resulting in a hexameric nucleosome [33]. However, Cse4/H4 also co-purifies with H2A/H2B from CEN DNA [38], thus it is unclear if Scm3/Cse4 is mutually exclusive with H2A/H2B in vivo. In S. pombe, Cnp1 co-purifies with reduced amounts of H2A, H2B, and its association with Scm3 appears cell-cycle dependent [17, 32], and with the Swi/Snf2h chromatin remodeling complex [39]. In contrast to in vitro, where fly Cid easily salt-assembles into cross-linkable octamers, native Cid nucleosomes in Drosophila cells co-IP with equal amounts of H2A, H2B and H4, cross-link as tetramers, and have half the height of canonical octamers [40]. In-solution recognition imaging by atomic force microscopy pinpoints a single N-terminal epitope per native Cid nucleosome [41], supporting the concept of “hemisome” tetramers [42].
Figure 2.
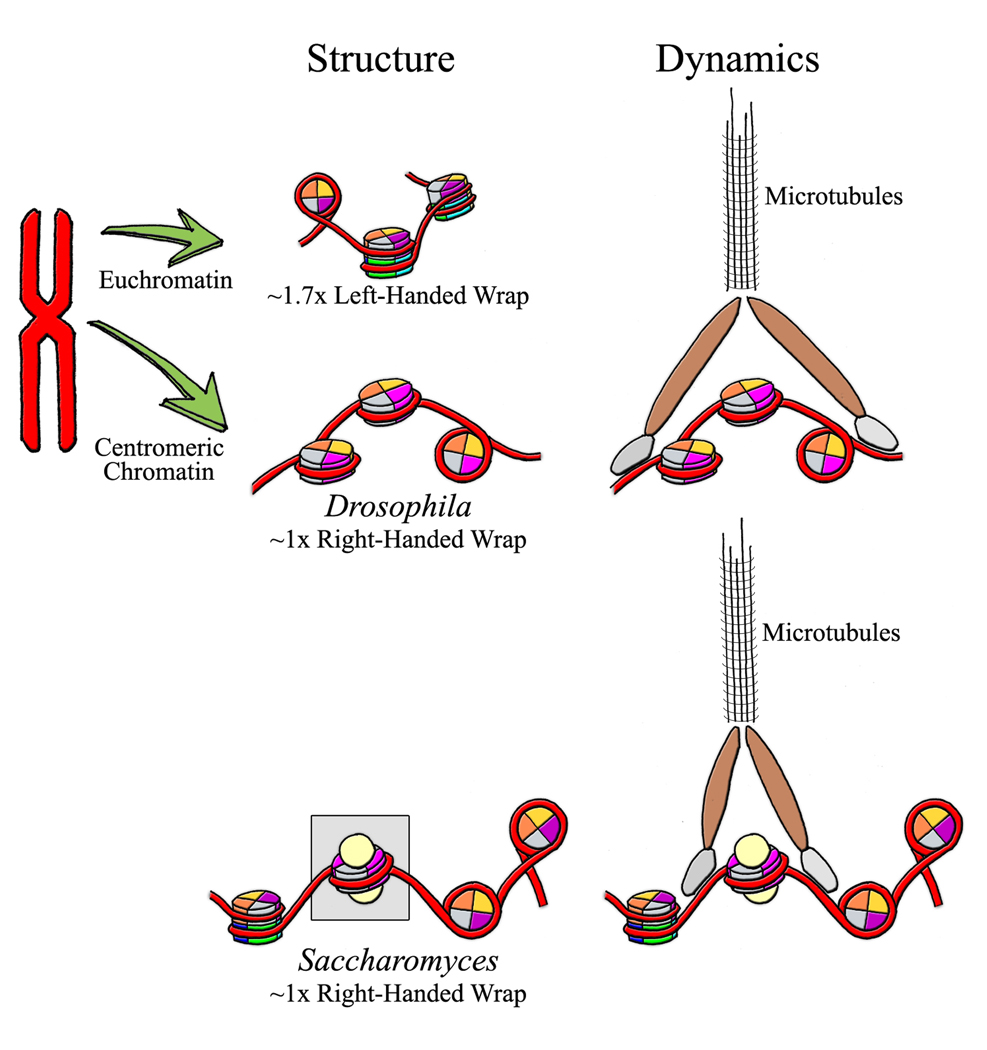
CENH3 structure intersects with dynamics at mitosis. Euchromatic CENH3 is depicted with DNA that wraps octameric nucleosomes ~1.7 times in a left-handed orientation. In Drosophila centromeric chromatin, tetramers of CENH3, H2A, H2B, and H4 wrap DNA in one right-handed turn. Saccharomyces spp. ‘point’ centromeres (boxed in grey) are depicted tetramers consisting of two each of CENH3 and H4 histones, wrapping DNA in one right-handed turn, and capped at either end with the nonhistone protein Scm3 (beige spheres). During mitosis, microtubules attach to centromeric chromatin via interactions with Ndc80 (brown), Cbf3, and Ndc10 (grey) in Saccharomyces spp. Similar dynamics are expected in other organisms. Color Legend: magenta/dark blue: CenH3; orange/light blue: H2A; yellow: H2B; grey/green: H4; red: DNA. Structures were modeled in the program CN3D using the published crystallographic coordinates of the canonical nucleosome.
Arguing against non-canonical models, a recent study reports that while Scm3 deletions are lethal as previously documented [33, 37], over-expressed Cse4 rescues Scm3 deletions, and, Cse4 co-IPs with H2A, H2B, and H4, leading the authors to conclude that Cse4 makes conventional nucleosomes [34]. However, it should be noted that rescued yeast cells have mitotic defects, suggesting Scm3-depleted Cse4 nucleosomes are compromised in function. The study also maps Cse4 lethal mutations in vivo, most of which impact Cse4:DNA and Cse4:H4 interactions, and concurrently, fail to assemble in vitro as well. One critical mutation targets His207, required for CENH3:CENH3 dimer interface in octamers [43]. Concurrently, this mutant yields <10% octamers in vitro. In sharp disagreement, the same His207 mutant co-IPs efficiently with another tagged Cse4 molecule from whole cell extracts. Further, a mutation in Leu220, which does not affect reconstitution efficiency in vitro, inhibits co-IPs in vivo. One possibility is that co-IPs reflect non-nucleosomal Cse4 interactions. Alternatively, salt-mediated in vitro assembly may not adequately reflect CENH3 behavior at centromeres. Indeed, a recent study has reported that saltmediated Cse4 octamers cannot occupy centromere-like DNA, whereas Cse4 assembled by Scm3 localizes to centromere-like DNA exclusively [44].
What is the biological significance of structural duality in Cse4 nucleosomes? Genome-wide Cse4 occupancy data reveal that Cse4 is present at centromeric DNA and at euchromatic locations [21]. Thus, an exciting possibility is that a distinctive CENH3 structural form specifically marks centric loci. Evidence in support of this idea comes from analyzing CENH3 nucleosomal topology in vitro and in vivo. Using RbAp48-mediated assembly, fly CENH3/H2A/H2B/H4 wrap 120bp of DNA, forming stable nucleosomes with a normal twist [20]. However, the topological state of DNA in these CENH3 nucleosomes is unique- instead of 2 left handed turns, they wrap DNA in a single right-handed super-helix [45]. The relevance of in vitro topological non-conformity was tested directly in yeast, using mini-plasmids with 0, 1, or 2 copies of the yeast 120bp centromeric DNA known to recruit Cse4. Remarkably, echoing an earlier finding [46], Cse4 nucleosomes on these circles wrap DNA in the right-handed topological state in vivo. Importantly, the topological changes are quantized: for every 0, 1, or 2 Cse4 nucleosomes added to the circle, precisely 0, 1, or 2 linking number changes in topology occur. Genetic deletions rule out mitotic torque and other kinetochore proteins involvement in the altered topology. Because right-handed wrapping is structurally incompatible with canonical octamers, previously described CENH3 tetramers provide a sound structural basis for the topology [47] (Figure 2).
Yeast and fly CENH3s are 70% dissimilar in sequence, and derive from organisms whose last common ancestor lies at the root of the eukaryotic tree. Common structural motifs within CENH3 nucleosomes, such as topology, probably orginate from a shared ancestry, and should persist in other species. Indirect evidence comes from studies in human cells, where CENP-A occupies 80% AT-rich satellites, and co-IPs with H2A, H2B and H4 [48]. A recent human study has found that two distinct populations of CENP-A nucleosomes exist: a salt-sensitive and mitosis-capable form during G2/M; and, a salt-resistant RSF-associated form that prevails during mid-G1 [49]. In Drosophila, the chromatin remodeler RSF substitutes the fly H2A variant into nucleosomes [50], which could strongly influence nucleosome stability [51]. Consequently, dependent on location, chromatin remodelers could drive CENH3 to pair with different H2A variants to modulate stability, and kinetochore binding.
Kinetochore protein recognition of CENH3 chromatin
An ongoing quest in centromere biology is to uncover how kinetochore proteins recognize centromeric regions. Most members of the centromere-associated network (CAN) proteins have been identified [52–54]. Key CAN members such as CENP-C have homologs across all species, and participate with CENH3 to create the distinctive centromeric domain (Table 1). Concurrently, CENP-C genetic deletions are lethal. CENP-C co-localizes with CENH3 in both metazoan centromeres [55], and at ectopic locations [3,56,57].
How does CENP-C recognize CENH3 chromatin? CENP-C has three domains of significant homology across eukaryotes. Of these, the Mif2/CENP-C domain binds human alpha satellite DNA directly, and intriguing evidence from bi-fluorescence complementation (biFC) experiments suggests that the same domain interacts with the C-terminus of CENP-A [58]. However, biochemical experiments show that CENP-A/CENP-C co-IPs yield smeary DNA ladders ranging from 100bp-1000bp, [59–60], not single nucleosomes. In extensively nucleasedigested chromatin, CENP-C instead co-purifies with H3, which may have been deposited at centromeres during replication because of CENH3 dilution [60]. A potential concern is that intrinsic instability in the CENP-A nucleosome [61] could be exacerbated during some chromatin preparation procedures [62], resulting in kinetochore protein re-localization to H3 chromatin.
To investigate the reversible nature of centromere establishment, researchers created an alpha-satellite based human artificial chromosome, whose centromere can be synthetically inactivated [56]. Surprisingly, they observed rapid loss of CENP-C, even when CENP-A-domains are intact. Centromere re-establishment was also examined in maize, wherein an inactive smaller second centromere was reactivated by excision [27]. This system reveals that CENP-C is weakly loaded during late anaphase at reactivated centromeres, in the absence of any known epigenetic mark. Thus, CENP-C recognizes multiple epigenetic features at centromeres. Indeed, the CENP-C domain which binds alpha satellite DNA also interacts with the DNA methyltransferase DNMT3b at metaphase [63], targeting de novo DNA methylation to flanking chromatin. Because CENH3 nucleosomes themselves are anti-correlated with DNA methylation in A. thaliana and Z. mays [64], DNA methylation likely targets pericentric heterochromatin. Concurrently, when CENP-C or DNMT3b are depleted, pericentric and centric transcription increases, even though CENP-A amounts are constant [65]. Concurrently, in fission yeast, CENP-C depletion results in loss of H2A.Z restriction, and increased transcription at centromeres, also without affecting Cnp-1 [66–67]. Studies in plants and fission yeast indicate that CENP-C is involved in chromosome orientation at mitosis and meiosis as well [68]. Thus, this fascinating protein plays myriad roles in regional centromeres.
Another key mammalian inner kinetochore protein, CENP-N, immuno-precipitates with CENP-A nucleosomes. Consequently, CENP-A depletion alters CENP-N localization. In vitro, CENP-N directly recognizes CENP-A nucleosomes through the latter’s CATD domain [69]. In conventional CENP-A octamers, the two CATD domains are supposed to be inaccessible [10] (Figure 3), making it difficult to envision two molecules of CENP-N binding CATD efficiently. However, CATD domains should be accessible in both non-canonical tetramer models (Figure 3). Thus, future experiments to dissect precisely which CENP-A domain is bound by CENP-N in vivo will be very informative.
Figure 3.
Kinetochore access to CATD in CENH3 nucleosomal models. A hypothetical ~40 kD inner kinetochore protein (encapsulated in circle) has easy access to CENH3’s exposed CATD (depicted in yellow) in the homo-tetrameric or hetero-tetrameric nucleosomal configuration. Color Legend: magenta/dark blue: CenH3; orange/light blue: H2A; tan/terra cotta: H2B; grey/green: H4; red: DNA.
Other kinetochore proteins like CENP-W and CENP-T, originally identified as part of the human CAN proteins, also disrupt CENH3 chromatin when depleted [54], but these two proteins associate with H3 and DNA, not CENP-A, [60]. Thus, their interactions present a new mechanism by which kinetochore proteins bind centromeres. Over 40 kinetochore proteins have been identified in multiple species [1]. Because of the large size of the kinetochore complex, and the surprising fragility of CENH3 domains, teasing apart function by biochemical or genetic approaches seems a Sisyphean endeavor. Two new methods, lifetime-FRET and biFC, offer the distinct advantage of coupling fluorescently tagged proteins with live imaging to track kinetochore dynamics in situ. Such experiments independently confirm interactions between CENP-A and CENP-B/C/N, support the view that CENP-W/T bind H3 not CENP-A [58, 70–71], and unveil a new role for H1 at the centromere [72]. Thus, these kinds of studies will allow visualization of kinetochore protein binding to centromeric domains in situ.
Functional and structural dichotomy in pericentric domains
Over the past two years, pericentric heterochromatin has been found to be essential for centromere establishment [12]. A study in fission yeast has found that RNAi and pericentric repeats can be bypassed by tethering the histone methyltransferase Clr4 to create heterochromatin in an ectopic plasmid, which in turn, recruits CENH3/CENP-C [39]. Surprisingly, in fission yeast, native pericentric regions are also permissive to RNA polymerase III, while centric regions contain both RNA polymerase II and the active chromatin mark, H3K4Me2 [73–74]. In the human X centromere, repressive histone modifications such as H3K9Me/K20Me/K27Me co-exist with permissive states like H3K4Me2, indicating a bivalent state [65], correlating with the finding that pericentric gamma satellite DNA has an open chromatin structure [75]. Open chromatin structure is also supported by the observation that the kinetochore complex CENP-H recruits the chromatin remodeler FACT (FAcilitates Chromatin Transcription), which in turn promotes CENP-A localization [52]. Several groups have also reported non-coding RNA originating from centromeres, whose role is currently under-appreciated [77–80]. Furthermore, open chromatin at centromeres has strong implications for centromere higher order structure.
3D view of the centromere fiber at interphase and mitosis
Scanning electron microscopy (SEM) studies of intact chromosomes show that parallel fibers encompass the primary constriction, leading to an intuitive model [91] where CENH3 domains are presented to the outside, while intervening H3 domains are buried within. However, recent immuno-SEM data from HeLa chromosomes suggest that CENH3 chromatin occupies 2/3rd the length, but only 1/3rd the height and width of adjacent compacted H3 domains. These data have been interpreted to support 30nm fibers at the centromere [81]. 30nm chromatin fibers have a controversial history, and recent cryo-EM studies of unfixed mitotic chromosomes have challenged their very existence [82–83]. Thus, while these latest results present a serious challenge in envisioning how the kinetochore interacts with a buried centromeric fiber, they await validation by native-imaging techniques.
In situ imaging advances like super high-resolution light microscopy uses point-spread functions to observe pairs of fluorescently tagged proteins, achieving ~20nm resolution in vivo [84–85]. Applying this revolutionary approach to mitotic chromosomes physically attached to microtubules, CENP-A and CENP-C were reported to be most compliant of 16 inner kinetochore proteins studied, while cohesins form a ring around the centric domain. These data suggest that CENP-A/CENP-C chromatin adopts a spring-like state encircled by cohesin (Figure 4). Another study has found that condensin regulates the spring constant of the human CENP-A fiber, and, in condensin mutants, weak centromeric fibers fail to facilitate mitosis [85]. Indeed, spring-like behavior in centromeres is readily apparent in immuno-SEM images of human mitotic chromosomes, which reveal an extended CENP-A array when the lowest concentration of fixative is used, and conversely, which collapse into a coiled state deep within the primary constriction when exposed to high concentration of fixative [81, 87] (Figure 4).
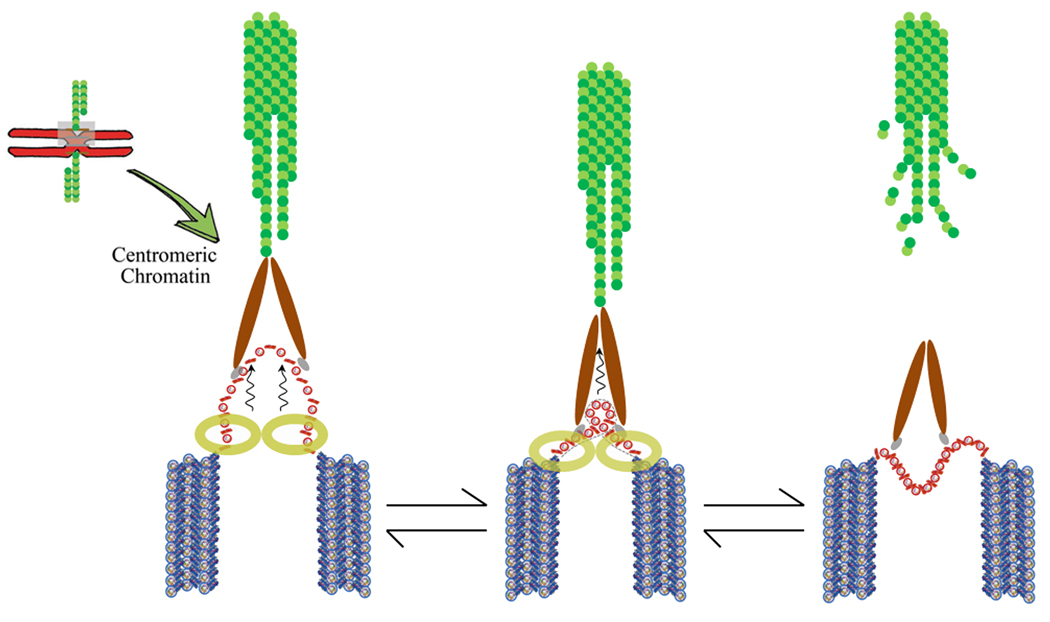
Figure 4.
Model for how centromeric chromatin behaves as a compliant spring. Left depicts tension-mediated spring behavior of centromeric domains which flex outward, tethered by cohesin. Middle depicts mitotic torque on the centromeric chromatin during chromosome movement. Right, collapse of the centric fiber upon microtubule loss, or cohesin/condensin depletion. Synthesized from [40, 45, 85, 86, 87].
Could features within individual CENH3 nucleosomes promote this intriguing spring-like behavior? Indeed, structural implications of arrays of right-handed tetramers are profound. Topological changes at the level of toroidal wrapping should also induce change in intervening linker DNA, thus disrupting the chromatin fiber’s plectonemic packing. A speculative outcome of altered wrap of DNA driven by CENH3 tetramers is entire CENH3 domains springing out from the flanking quasi-crystalline H3 chromatin, with each pliable loop tethered by cohesins at its base (Figure 4). Together, these features may afford resilience to the CENH3 fiber, thus reducing chromatin breakage upon application of mitotic torque.
Future prospects
The data discussed above have exposed surprising adaptability in CENH3 chromatin, strongly dependent upon location, timing, transcription, and interacting partners. These factors may assist in demarcating CENH3 centric domains from ectopic CENH3 nucleosomes during mitosis. Biochemical experiments designed to uncouple these forms will provide much-needed insight into the very dynamic centromeric nucleosome. In vivo imaging approaches, combined with classical genetic analyses, will address outstanding questions such as how stepwise centromeric assembly occurs, how remodeling events target centromeres in a cell-cycle dependent manner, and how these events reinforce variant occupancy. Deep sequencing technologies applied to regional centromeres will close the current genomic information gap to assess whether all CENH3s are indeed able to occupy two distinct nuclear domains.
New findings reviewed here might lead an observer, much like Alice in Wonderland [88], to exclaim that centromeres get “curiouser and curiouser”. However, an overarching theme that emerges is that centromeres, long cloaked in Bohemianism, are actually subject to same plebian forces shaping much of the genome’s epigenetic landscape. It will be exciting to decipher how CENH3 dynamic instability, in assembly and in structure, drive centromere function and dysfunction. While a singular theme that applies to all centromeres is not quite within our grasp, ongoing studies signal that escape from the black hole [90] is indisputably on the horizon.
Acknowledgements
YD thanks Kerry Bloom for sharing the concept of CENH3 dynamic instability, Ali Hamiche, Ariel Prunell, Carl Wu, Gary Karpen, Stephan Deikmann, and Steve Henikoff for sharing unpublished observations, Nathan Morris for graphical input, and Sam John for critical comments on the manuscript. We apologize to colleagues whose important findings could not be cited due to space constraints.
Funding
YD and MB are supported by the NCI intramural research program at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* Of special interest
** Of exceptional interest
- 1.Santaguida S, Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28(17):2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amor DJ, Kalitsis P, Sumer H, Choo KH. Building the centromere: from foundation proteins to 3D organization. Trends Cell Biol. 2004;14(7):359–368. doi: 10.1016/j.tcb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Fritz B, Hasson D, Abrusan G, Cheung F, Yoda K, Radlwimmer B, Ladurner AG, Warburton PE. Co-localization of CENP-C and CENP-H to discontinuous domains of CENP-A chromatin at human neocentromeres. Genome Biol. 2007;8(7):R148. doi: 10.1186/gb-2007-8-7-r148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam AL, Boivin CD, Bonney CF, Rudd MK, Sullivan BA. Human centromeric chromatin is a dynamic chromosomal domain that can spread over noncentromeric DNA. Proc Natl Acad Sci U S A. 2006;103(11):4186–4191. doi: 10.1073/pnas.0507947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell. 2006;10(3):303–315. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Biol. 2003;10(11):882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- 7.Henikoff S, Ahmad K, Platero JS, van Steensel B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc Natl Acad Sci U S A. 2000;97(2):716–721. doi: 10.1073/pnas.97.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieland G, Orthaus S, Ohndorf S, Diekmann S, Hemmerich P. Functional complementation of human centromere protein A (CENP-A) by Cse4p from Saccharomyces cerevisiae. Mol Cell Biol. 2004;24(15):6620–6630. doi: 10.1128/MCB.24.15.6620-6630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermaak D, Hayden HS, Henikoff S. Centromere targeting element within the histone fold domain of Cid. Mol Cell Biol. 2002;22(21):7553–7561. doi: 10.1128/MCB.22.21.7553-7561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black BE, Foltz DR, Chakravarthy S, Luger K, Woods VL, Jr, Cleveland DW. Structural determinants for generating centromeric chromatin. Nature. 2004;430(6999):578–582. doi: 10.1038/nature02766. [DOI] [PubMed] [Google Scholar]
- 11.Regnier V, Novelli J, Fukagawa T, Vagnarelli P, Brown W. Characterization of chicken CENP-A and comparative sequence analysis of vertebrate centromere-specific histone H3-like proteins. Gene. 2003;316:39–46. doi: 10.1016/s0378-1119(03)00768-6. [DOI] [PubMed] [Google Scholar]
- 12.Bernad R, Sanchez P, Losada A. Epigenetic specification of centromeres by CENP-A. Exp Cell Res. 2009;315(19):3233–3241. doi: 10.1016/j.yexcr.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Silva MC, Jansen LE. At the right place at the right time: novel CENP-A binding proteins shed light on centromere assembly. Chromosoma. 2009;118(5):567–574. doi: 10.1007/s00412-009-0227-3. [DOI] [PubMed] [Google Scholar]
- 14.Black BE, Jansen LE, Maddox PS, Foltz DR, Desai AB, Shah JV, Cleveland DW. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol Cell. 2007;25(2):309–322. doi: 10.1016/j.molcel.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad K, Henikoff S. Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci U S A. 2002;99 Suppl 4:16477–16484. doi: 10.1073/pnas.172403699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118(6):715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Pidoux AL, Choi ES, Abbott JK, Liu X, Kagansky A, Castillo AG, Hamilton GL, Richardson W, Rappsilber J, He X, Allshire RC. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell. 2009;33(3):299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell. 2007;12(1):17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Maddox PS, Oegema K, Desai A, Cheeseman IM. "Holo"er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res. 2004;12(6):641–653. doi: 10.1023/B:CHRO.0000036588.42225.2f. [DOI] [PubMed] [Google Scholar]
- 20.Furuyama T, Dalal Y, Henikoff S. Chaperone-mediated assembly of centromeric chromatin in vitro. Proc Natl Acad Sci U S A. 2006;103(16):6172–6177. doi: 10.1073/pnas.0601686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lefrancois P, Euskirchen GM, Auerbach RK, Rozowsky J, Gibson T, Yellman CM, Gerstein M, Snyder M. Efficient yeast ChIP-Seq using multiplex short-read DNA sequencing. BMC Genomics. 2009;10:37. doi: 10.1186/1471-2164-10-37.. ** First evidence of endogenous CENH3 occupying genomic locations with high turnover.
- 22.Pietrasanta LI, Thrower D, Hsieh W, Rao S, Stemmann O, Lechner J, Carbon J, Hansma H. Probing the Saccharomyces cerevisiae centromeric DNA (CEN DNA)-binding factor 3 (CBF3) kinetochore complex by using atomic force microscopy. Proc Natl Acad Sci U S A. 1999;96(7):3757–3762. doi: 10.1073/pnas.96.7.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen LE, Black BE, Foltz DR, Cleveland DW. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol. 2007;176(6):795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuh M, Lehner CF, Heidmann S. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol. 2007;17(3):237–243. doi: 10.1016/j.cub.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 25.Erhardt S, Mellone BG, Betts CM, Zhang W, Karpen GH, Straight AF. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol. 2008;183(5):805–818. doi: 10.1083/jcb.200806038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lermontova I, Schubert V, Fuchs J, Klatte S, Macas J, Schubert I. Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain. Plant Cell. 2006;18(10):2443–2451. doi: 10.1105/tpc.106.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han F, Gao Z, Birchler JA. Reactivation of an inactive centromere reveals epigenetic and structural components for centromere specification in maize. Plant Cell. 2009;21(7):1929–1939. doi: 10.1105/tpc.109.066662.. * Shows that inactivation and reactivation of centromeres can reveal order in which centromeres are established.
- 28. Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137(3):485–497. doi: 10.1016/j.cell.2009.02.040.. ** Along with Foltz et al (2009) and Shuaib et al (2009), shows that HJURP is a CENP-A specific chaperone.
- 29. Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137(3):472–484. doi: 10.1016/j.cell.2009.02.039.. ** see Dunleavy et al (2009).
- 30. Shuaib MA, Ouararhni K, Dimitrov S, Hamiche A. HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0913709107. Epub ahead of time Dec 2009: p.. ** See Dunleavy et al (2009)
- 31. Sanchez-Pulido L, Pidoux AL, Ponting CP, Allshire RC. Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell. 2009;137:1173–1174. doi: 10.1016/j.cell.2009.06.010.. *Exciting evidence linking assembly complexes in fungi and mammals.
- 32.Williams JS, Hayashi T, Yanagida M, Russell P. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell. 2009;33(3):287–298. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 2007;129(6):1153–1164. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 34. Camahort R, Shivaraju M, Mattingly M, Li B, Nakanishi S, Zhu D, Shilatifard A, Workman JL, Gerton JL. Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell. 2009;35(6):794–805. doi: 10.1016/j.molcel.2009.07.022.. * Challenges non-canonical models of CENH3 structure proposed by Mizuguchi et al (2007) and Dalal et al (2007).
- 35.Kato T, Sato N, Hayama S, Yamabuki T, Ito T, Miyamoto M, Kondo S, Nakamura Y, Daigo Y. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67(18):8544–8553. doi: 10.1158/0008-5472.CAN-07-1307. [DOI] [PubMed] [Google Scholar]
- 36. Zeitlin SG, Baker NM, Chapados BR, Soutoglou E, Wang JY, Berns MW, Cleveland DW. Double-strand DNA breaks recruit the centromeric histone CENP-A. Proc Natl Acad Sci U S A. 2009;106(37):15762–15767. doi: 10.1073/pnas.0908233106.. * Shows that GFP-CENP-A enriches at artificial breakpoints in the human genome.
- 37.Stoler S, Rogers K, Weitze S, Morey L, Fitzgerald-Hayes M, Baker RE. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci U S A. 2007;104(25):10571–10576. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westermann S, Cheeseman IM, Anderson S, Yates JR, 3rd, Drubin DG, Barnes G. Architecture of the budding yeast kinetochore reveals a conserved molecular core. J Cell Biol. 2003;163(2):215–222. doi: 10.1083/jcb.200305100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer T, Cui B, Dhakshnamoorthy J, Zhou M, Rubin C, Zofall M, Veenstra TD, Grewal SIS. Diverse roles of HP1 proteins in heterochromatin assembly and functions in fission yeast. Proc Natl Acad Sci U S A. 2009;106(22):8998–9003. doi: 10.1073/pnas.0813063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalal Y, Wang H, Lindsay S, Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5(8):e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Dalal Y, Henikoff S, Lindsay S. Single-epitope recognition imaging of native chromatin. Epigenetics Chromatin. 2008;1(1):10. doi: 10.1186/1756-8935-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalal Y, Furuyama T, Vermaak D, Henikoff S. Structure, dynamics, and evolution of centromeric nucleosomes. Proc Natl Acad Sci U S A. 2007;104(41):15974–15981. doi: 10.1073/pnas.0707648104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 44. Visnapuu ML, Greene EC. Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition. Nat Struct Mol Biol. 2009;16(10):1056–1062. doi: 10.1038/nsmb.1655.. ** First evidence of bistability in CENH3 nucleosome in vitro.
- 45. Furuyama T, Henikoff S. Centromeric nucleosomes induce positive DNA supercoils. Cell. 2009;138(1):104–113. doi: 10.1016/j.cell.2009.04.049.. ** Shows that CENH3 nucleosomes are wrapped right-handed in vivo in yeast.
- 46.Bloom K, Amaya E, Yeh E. Centromeric DNA structure in yeast. In: Borisy GG, Cleveland DW, Murphy DB, editors. Molecular biology of the cytoskeleton. Cold Spring Harbor N.Y.: 1984. pp. 175–184. [Google Scholar]
- 47.Dechassa ML, D'Arcy S, Luger K. A positive spin on the centromere. Cell. 2009;138(1):22–24. doi: 10.1016/j.cell.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan KF. A solid foundation: functional specialization of centromeric chromatin. Curr Opin Genet Dev. 2001;11(2):182–188. doi: 10.1016/s0959-437x(00)00177-5. [DOI] [PubMed] [Google Scholar]
- 49. Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K. Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol. 2009;185(3):397–407. doi: 10.1083/jcb.200903088.. * Along with Okada et al, first evidence of chromatin remodelers at human centromeres.
- 50.Hanai K, Furuhashi H, Yamamoto T, Akasaka K, Hirose S. RSF governs silent chromatin formation via histone H2Av replacement. PLoS Genet. 2008;4(2):e1000011. doi: 10.1371/journal.pgen.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark 'nucleosome-free regions' of active promoters and other regulatory regions. Nat Genet. 2009;41(8):941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okada M, Okawa K, Isobe T, Fukagawa T. CENP-H-containing complex facilitates centromere deposition of CENP-A in cooperation with FACT and CHD1. Mol Biol Cell. 2009;20(18):3986–3995. doi: 10.1091/mbc.E09-01-0065.. * See Perpelescu et al (2009).
- 53. Meraldi P, McAinsh AD, Rheinbay E, Sorger PK. Phylogenetic and structural analysis of centromeric deposition of CENP-A in cooperation with FACT and CHD1. Mol Biol Cell. 2009;20(18):3986–3995. doi: 10.1091/mbc.E09-01-0065.. *See Ref. [49].
- 54.Foltz DR, Jansen LE, Black BE, Bailey AO, Yates JR, 3rd, Cleveland DW. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8(5):458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- 55.Kalitsis P, Fowler KJ, Earle E, Hill J, Choo KH. Targeted disruption of mouse centromere protein C gene leads to mitotic disarray and early embryo death. Proc Natl Acad Sci U S A. 1998;95(3):1136–1141. doi: 10.1073/pnas.95.3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardinale S, Bergmann JH, Kelly D, Nakano M, Valdivia MM, Kimura H, Masumoto H, Larionov V, Earnshaw WC. Hierarchical inactivation of a synthetic human kinetochore by a chromatin modifier. Mol Biol Cell. 2009;20(19):4194–4204. doi: 10.1091/mbc.E09-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izuta H, Ikeno M, Suzuki N, Tomonaga T, Nozaki N, Obuse C, Kisu Y, Goshima N, Nomura F, Nomura N, Yoda K. Comprehensive analysis of the ICEN (Interphase Centromere Complex) components enriched in the CENP-A chromatin of human cells. Genes Cells. 2006;11(6):673–684. doi: 10.1111/j.1365-2443.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- 58. Trazzi S, Perini G, Bernardoni R, Zoli M, Reese JC, Musacchio A, Della Valle G. The C-terminal domain of CENP-C displays multiple and critical functions for mammalian centromere formation. PLoS One. 2009;4(6):e5832. doi: 10.1371/journal.pone.0005832.. * See Hori et al (2009).
- 59.Yoda K, Morishita S, Hashimoto K. Histone variant CENP-A purification, nucleosome reconstitution. Methods Enzymol. 2004;375:253–269. doi: 10.1016/s0076-6879(03)75017-4. [DOI] [PubMed] [Google Scholar]
- 60.Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang WH, Suzuki E, Okawa K, Cheeseman IM, Fukagawa T. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008;135(6):1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Conde e Silva N, Black BE, Sivolob A, Filipski J, Cleveland DW, Prunell A. CENP-A-containing nucleosomes: easier disassembly versus exclusive centromeric localization. J Mol Biol. 2007;370(3):555–573. doi: 10.1016/j.jmb.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 62.Noll M, Thomas JO, Kornberg RD. Preparation of Native Chromatin and Damage Caused by Shearing. Science. 1975;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- 63. Gopalakrishnan S, Sullivan BA, Trazzi S, Della Valle G, Robertson KD. DNMT3B interacts with constitutive centromere protein CENP-C to modulate DNA methylation and the histone code at centromeric regions. Hum Mol Genet. 2009;18(17):3178–3193. doi: 10.1093/hmg/ddp256.. *New data showing CENP-C is able to recruit de novo DNA methylation to pericentric regions.
- 64.Zhang W, Lee HR, Koo DH, Jiang J. Epigenetic modification of centromeric chromatin: hypomethylation of DNA sequences in the CENH3-associated chromatin in Arabidopsis thaliana and maize. Plant Cell. 2008;20(1):25–34. doi: 10.1105/tpc.107.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mravinac B, Sullivan LL, Reeves JW, Yan CM, Kopf KS, Farr CJ, Schueler MG, Sullivan BA. Histone modifications within the human X centromere region. PLoS One. 2009;4(8):e6602. doi: 10.1371/journal.pone.0006602.. *Show biphasic nature of centromeric chromatin.
- 66. Hou H, Wang Y, Kallgren SP, Thompson J, Yates JR, 3rd, Jia S. Histone variant H2A.Z regulates centromere silencing and chromosome segregation in fission yeast. J Biol Chem. 2010;285(3):1909–1918. doi: 10.1074/jbc.M109.058487.. * See Buchanan et al (2009).
- 67. Buchanan L, Durand-Dubief M, Roguev A, Sakalar C, Wilhelm B, Stralfors A, Shevchenko A, Aasland R, Ekwall K, Francis Stewart A. The Schizosaccharomyces pombe JmjC-protein, Msc1, prevents H2A.Z localization in centromeric and subtelomeric chromatin domains. PLoS Genet. 2009;5(11):e1000726. doi: 10.1371/journal.pgen.1000726.. * Along with Hou et al (2009), shows that H2A.Z and DNA methylation are involved in centromere integrity.
- 68.Tanaka K, Chang HL, Kagami A, Watanabe Y. CENP-C functions as a scaffold for effectors with essential kinetochore functions in mitosis and meiosis. Dev Cell. 2009;17(3):334–343. doi: 10.1016/j.devcel.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 69. Carroll CW, Silva MC, Godek KM, Jansen LE, Straight AF. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11(7):896–902. doi: 10.1038/ncb1899.. * Shows an inner kinetochore protein CENP-N directly recognizes CENP-A nucleosomes in vitro.
- 70.Orthaus S, Biskup C, Hoffmann B, Hoischen C, Ohndorf S, Benndorf K, Diekmann S. Assembly of the inner kinetochore proteins CENP-A and CENP-B in living human cells. Chembiochem. 2008;9(1):77–92. doi: 10.1002/cbic.200700358. [DOI] [PubMed] [Google Scholar]
- 71.Hellwig D, Hoischen C, Ulbricht T, Diekmann S. Acceptor-photobleaching FRET analysis of core kinetochore and NAC proteins in living human cells. Eur Biophys J. 2009;38(6):781–791. doi: 10.1007/s00249-009-0498-x. [DOI] [PubMed] [Google Scholar]
- 72.Orthaus S, Klement K, Happel N, Hoischen C, Diekmann S. Linker histone H1 is present in centromeric chromatin of living human cells next to inner kinetochore proteins. Nucleic Acids Res. 2009;37(10):3391–3406. doi: 10.1093/nar/gkp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kagansky A, Folco HD, Almeida R, Pidoux AL, Boukaba A, Simmer F, Urano T, Hamilton GL, Allshire RC. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science. 2009;324(5935):1716–1719. doi: 10.1126/science.1172026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott KC, White CV, Willard HF. An RNA polymerase III-dependent heterochromatin barrier at fission yeast centromere 1. PLoS One. 2007;2(10):e1099. doi: 10.1371/journal.pone.0001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SI. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature. 2008;451(7179):734–737. doi: 10.1038/nature06561. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Ebersole T, Kouprina N, Noskov VN, Ohzeki J, Masumoto H, Mravinac B, Sullivan BA, Pavlicek A, Dovat S, Pack SD, Kwon YW, Flanagan PT, Loukinov D, Lobanenkov V, Larionov V. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19(4):533–544. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topp CN, Zhong CX, Dawe RK. Centromere-encoded RNAs are integral components of the maize kinetochore. Proc Natl Acad Sci U S A. 2004;101(45):15986–15991. doi: 10.1073/pnas.0407154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong LH, Brettingham-Moore KH, Chan L, Quach JM, Anderson MA, Northrop EL, Hannan R, Saffery R, Shaw ML, Williams E, Choo KH. Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res. 2007;17(8):1146–1160. doi: 10.1101/gr.6022807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferri F, Bouzinba-Segard H, Velasco G, Hube F, Francastel C. Non-coding murine centromeric transcripts associate with and potentiate Aurora B kinase. Nucleic Acids Res. 2009;37(15):5071–5080. doi: 10.1093/nar/gkp529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu J, Gilbert DM. Proliferation-dependent and cell cycle regulated transcription of mouse pericentric heterochromatin. J Cell Biol. 2007;179(3):411–421. doi: 10.1083/jcb.200706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marshall OJ, Marshall AT, Choo KH. Three-dimensional localization of CENP-A suggests a complex higher order structure of centromeric chromatin. J Cell Biol. 2008;183(7):1193–1202. doi: 10.1083/jcb.200804078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eltsov M, Maclellan KM, Maeshima K, Frangakis AS, Dubochet J. Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc Natl Acad Sci U S A. 2008;105(50):19732–19737. doi: 10.1073/pnas.0810057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kireev I, Lakonishok M, Liu W, Joshi VN, Powell R, Belmont AS. In vivo immunogold labeling confirms large-scale chromatin folding motifs. Nat Methods. 2008;5(4):311–313. doi: 10.1038/nmeth.1196. [DOI] [PubMed] [Google Scholar]
- 84. Wan X, O'Quinn RP, Pierce HL, Joglekar AP, Gall WE, DeLuca JG, Carroll CW, Liu ST, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137(4):672–684. doi: 10.1016/j.cell.2009.03.035.. ** See Anderson et al (2009).
- 85. Anderson M, Haase J, Yeh E, Bloom K. Function and assembly of DNA looping, clustering, and microtubule attachment complexes within a eukaryotic kinetochore. Mol Biol Cell. 2009 20;19:4131–4139. doi: 10.1091/mbc.E09-05-0359.. ** Along with Wan et al (2009) uses novel super resolution light microscopy to reveal 3D structure and behavior of centromeres attached to microtubules.
- 86.Ribeiro SA, Gatlin JC, Dong Y, Joglekar A, Cameron L, Hudson DF, Farr CJ, McEwen BF, Salmon ED, Earnshaw WC, Vagnarelli P. Condensin regulates the stiffness of vertebrate centromeres. Mol Biol Cell. 2009;20(9):2371–2380. doi: 10.1091/mbc.E08-11-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houben A, Schroeder-Reiter E, Nagaki K, Nasuda S, Wanner G, Murata M, Endo TR. CENH3 interacts with the centromeric retrotransposon cereba and GC-rich satellites and locates to centromeric substructures in barley. Chromosoma. 2007;116(3):275–283. doi: 10.1007/s00412-007-0102-z. [DOI] [PubMed] [Google Scholar]
- 88.Carroll L. Alice's Adventures in Wonderland. 1865 [Google Scholar]
- 89.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127(5):983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 90.Henikoff S. Near the edge of the chromosome's "black hole". Trends in Gen. 2002;18(4):165–167. doi: 10.1016/s0168-9525(01)02622-1. [DOI] [PubMed] [Google Scholar]
- 91.Blower MD, Sullivan BA, Karpen GH. Conserved organization of centromeric chromatin in flies and humans. Dev Cell. 2002;2(3):319–330. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]