Abstract
Background
The existence of circulating tumor cells (CTCs) in peripheral blood as an indicator of tumor recurrence has not been clearly established, particularly for gastric cancer patients. We conducted a retrospective analysis of the relationship between CTCs in peripheral blood at initial diagnosis and clinicopathologic findings in patients with gastric carcinoma.
Methods
Blood samples were obtained from 123 gastric carcinoma patients at initial diagnosis. mRNA was extracted and amplified for carcinoembryonic antigen (CEA) mRNA detection using real-time RT-PCR. Periodic 3-month follow-up examinations included serum CEA measurements and imaging.
Results
The minimum threshold for corrected CEA mRNA score [(CEA mRNA/GAPDH mRNA) × 106] was set at 100. Forty-five of 123 patients (36.6%) were positive for CEA mRNA expression. CEA mRNA expression significantly correlated with T stage and postoperative recurrence status (P = 0.001). Recurrent disease was found in 44 of 123 cases (35.8%), and 25 of these (56.8%) were positive for CEA mRNA. Of these patients, CEA mRNA was more sensitive than serum CEA in indicating recurrence. Three-year disease-free survival of patients positive for CEA mRNA was significantly poorer than of patients negative for CEA mRNA (P < 0.001). Only histological grade and CEA mRNA positivity were independent factors for disease-free survival using multivariate analysis.
Conclusions
CEA mRNA copy number in peripheral blood at initial diagnosis was significantly associated with disease recurrence in gastric adenocarcinoma patients. Real-time RT-PCR detection of CEA mRNA levels at initial diagnosis appears to be a promising predictor for disease recurrence in gastric adenocarcinoma patients.
Background
Gastric cancer remained the leading cause of cancer mortality worldwide throughout the 20th century. The only proven curative treatment is surgical resection of all gross and microscopic lesions. However, despite undergoing curative gastrectomy, including extended lymph node dissection and adjuvant chemotherapy, cancer recurs in both regional as well as distant sites in majority of the patients [1]. Diagnosis of recurrence with common follow-up protocols usually is made at a late stage, which, to an extent, precludes the possibility of effective treatment [2]. Surveillance of circulating tumor cells (CTCs) seems to offer greater possibility for earlier diagnosis of recurrent disease.
The concept of investigating the metastatic process in peripheral blood originated in the 19th century when T.R. Ashworth first described the phenomenon of CTCs, and S. Paget hypothesized a non-random pattern of cancer metastasization (the 'seed and soil' theory) [3,4]. Subsequently, the malignant nature of CTCs was confirmed by demonstrating that they possess tumor-specific chromosomal aberrations [5,6] and that they grow ex vivo as cell lines with a malignant phenotype [7]. Several approaches to detect CTCs have been described and can be classified into PCR-based methods and cytometric methods [8].
With the advent of quantitative real-time PCR techniques [9], precise quantification of a target sequence has become possible. Quantitative PCR provides investigators not only with technical advantages, but also with applicative advantages, such as the definition of cutoff values indicating mRNA expression levels of clinical relevance in cancer patients compared with healthy subjects. Real-time PCR also affords the possibilities of correlating target-sequence load with clinical outcome [10] or response to therapy [11].
CEA, originally described as a tumor-associated colon cancer antigen, was cloned in 1987 and is now recognized as a member of the immunoglobulin protein superfamily [12]. Many studies have reported detection of gastric cells in blood [13], bone marrow [14], and peritoneal washing [15] of gastric cancer patients by using real-time PCR for CEA mRNA.
The goal of this study was to evaluate the effectiveness of the CEA mRNA real-time PCR technique for the early detection of tumor recurrence. To meet this goal, the relationship between clinical recurrence and blood levels of CEA mRNA preoperatively was examined in gastric adenocarcinoma patients.
Methods
Patients
Written informed consent was obtained from every patient on the use of blood samples for research in accordance with the institutional guidelines of our hospital. Between February 2002 and December 2006, a total of 123 consecutive patients with gastric adenocarcinoma at Cancer Center of Sun Yat-sen University were enrolled into this study. All patients received radical resection and D2 lymphadenectomy. At lease 15 lymph nodes were available for the detection. No peritoneal dissemination was found. Clear records of serum CEA change and imaging evaluation before the operation and every three months after the operation were required. Patients who had positive lymph node were recommended to receive adjuvant chemotherapy but finally only eighty-three patients underwent adjuvant chemotherapy. The regimens included CAPOX (Capecitabine + Oxaliplatin, 16 cases, with a median cycle of 4), folfox6 (56 cases, with a median cycle of 6), taxol + cisplatin (4 cases, with a median cycle of 4), taxol + 5FU/CF (Fluorouracil/Leucovorin, 7 cases, with a median cycle of 6). Recurrent disease, including local relapse and distant metastases, was detected by computed tomography examination. New lesions detected by imaging examination in follow-up appointments were regarded as recurrence. Biopsy was not done routinely to determine histological recurrence. All imaging was evaluated by at least two independent observers, including radiologists. The median follow-up period was 37.0 months (range, 3.0-73.6 months).
Blood samples
Blood samples were collected at initial diagnosis one or two days before surgery. The first 3 mL of blood was discarded to prevent epidermal contamination, and then a 5-mL blood sample was obtained from the peripheral vein. Peripheral blood samples obtained from 30 non-cancer patient volunteers were used as negative controls.
All patients provided written informed consent; we obtained separate consent for use of blood sample. Study approval was obtained from independent ethics committees at Cancer Center of Sun Yat-Sen University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Pre-processing of blood samples
Blood samples were collected in EDTA-containing tubes. Sample processing was performed within 2 hours after blood withdrawal. Blood was transferred into a 30-mL falcon tube and centrifuged at 1,800 rpm at room temperature for 20 minutes. Serum was removed, and cells were resuspended in 5 mL saline and 0.3 mL RNA later solution. After mixing well, the blood cell mixture was kept overnight at 4°C and stored at -80°C until used.
RNA extraction and cDNA synthesis
Total RNA of peripheral blood samples was extracted using RNAprep Cell Kit (Tiangen, Beijing, China) following the protocol provided by the manufacturer. RNA integrity was checked by electrophoresis and quantified by absorption at 260 and 280 nm using a UV-visible spectrophotometer (Beckman Coulter Du® 800, Fullerton, CA). For reverse transcription, 1 μg of total RNA, 1 μL Oligo(dT)15 and 1 μL dNTP were diluted in 10 μL RNase-free water, incubated 10 minutes at 37°C and 1 μL of 25 mmol/L EDTA was added. An 11 μL aliquot of reaction mixture was incubated for 10 minutes at 65°C and quickly chilled on ice for 2 minutes. cDNA was stored at -80°C until used. cDNA synthesis was performed using the TIANScript M-MLV method (Tiangen Biotech, Beijing, China).
Cell lines
To prepare for CEA-specific RT-PCR, two cell lines, SW-480 (colon cancer cell line) and SC-7901 (gastric cancer cell line) were used. Lymphocytes were collected from healthy volunteers without epithelial malignancy. After lymphocytes were isolated from peripheral blood by gradient centrifugation, the mononuclear cell layer was collected. Cell lines were serially diluted (10-fold) in 2 × 107 to 5 × 107 lymphocytes to give carcinoma cell: lymphocyte ratios ranging from 1:10 to 1:107.
CEA mRNA Analysis by Real-Time Quantitative PCR
Quantitative PCR was performed using the Sequence Detector System, ABI PRISM 7500 (Applied Biosystems 7500 Fast Real-Time PCR System). PCR primers and the TaqMan probes were designed using the Primer Express 1.0 software program. In this assay, the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize variations in integrity and total amount of RNA extracted. The real-time PCR assays for GAPDH and CEA were done in separate tubes. CEA mRNA values were adjusted against GAPDH mRNA values, and the relative CEA mRNA scores were presented as (CEA mRNA/GAPDH mRNA) × 106 for each sample.
5 μL of the sample cDNA was used for real-time PCR in a 20 μL reaction mixture consisting of 10 pmol appropriate primers (Invitrogen Cooperation, Japan) and 5 pmol TaqMan probe (Invitrogen Cooperation, Japan). The reporter dye (6-carboxy-fluorescein: FAM) was covalently attached to the 5' end of the probe, and the quencher dye (6-carboxy-tetramethyl-rhodamine: TAMRA) was attached to the 3' end of the probe. The temperature profile used for amplification was as follows: denaturation for 1 cycle at 95°C for 10 minutes, followed by 40 cycles at 95°C for 10 seconds, 60°C for 15 seconds, and 72°C for 5 seconds. Quantification was done by the ABI Prism 7500 Sequence detector system. Each set of samples and serially-diluted external controls were amplified in duplicate. The average value of the duplicates was used as the quantitative value.
A CEA-specific oligonucleotide primer was designed based on the report by Gerhard et al. [16]. The sequences were: 5'-TGTCGGCATCATGATTGG-3' (sense) and 5'-GCAAATGCTTTAAGGAAGAAGC-3' (antisense). Fluorescent and LC-Red probe sequences used for CEA identification were: 5'-CCTGAAATGAAGAAACTACACCAGGGC-fluorescein and 5'-LC-Red 640-GCTATATCAGAGCAACCCCAACCAGC-phosphate.
Real-time PCR monitoring was achieved by measuring the fluorescence signal at the end of annealing phase of each cycle. The primer sequences used for GAPDH amplification were: 5'-TGAACGGGAAGCTCACTGG-3'(sense) and 5'-TCCACCACCCTGTTGCTGTA-3' (antisense). The probe sequences used for GAPDH identification were: 5'-TCAACAGCGACACCCACTCCT-fluorescein and 5'-LC-Red 640-CACCTTTGACGCTGGGGCT-phosphate.
Determination of CEA in serum samples
Pre-operative serum samples were also used for assaying tumor marker CEA using a commercially available enzyme immunoassay kit (Cobas Core EIA, Roche, Basel, Switzerland). Pathological cutoff level was established at 5 ng/mL for serum CEA.
Statistical analyses
The Kaplan-Meier statistical method was used for analyzing clinical features and recurrence; differences were estimated with the log-rank test. Prognostic factors were examined by univariate and multivariate analyses (log-rank test for univariate analysis and Cox proportional hazards regression model, backward stepwise (conditional LR) for multivariate analysis). The chi-squared and Fisher exact tests were used for statistical analysis. All statistical analyses were done with SPSS16.0. All P values were 2-tailed, and the level of significance was set at 0.05.
Results
Clinical features
The 123 patients enrolled in the study aged 28 to 84 years (mean, 57.11 years; median, 59 years), and the ratio of males to females was 82:41 (Table 1). Staging was performed according to the Tumor-Node-Metastasis (TNM) classification of the American Joint Committee on Cancer (AJCC, revised 1997). Twenty-four tumors were located in the cardia, 3 in the gastric fundus, 44 in the gastric corpus, 45 in the gastric antrum, 5 involved the whole stomach, and 2 belonged to the remnant gastric carcinoma (Table 1).
Table 1.
Clinicopathologic features and CEA* mRNA expression detected by real-time RT-PCR
| Characteristics | Total number (N = 123) |
CEA mRNA | P value | |
|---|---|---|---|---|
| Positive (n = 45) |
Negative (n = 78) |
|||
| Age | ||||
| ≤40 | 13 | 3 | 10 | |
| 41-50 | 20 | 9 | 11 | |
| 51-60 | 39 | 9 | 30 | |
| 61-70 | 42 | 18 | 24 | |
| >70 | 9 | 6 | 3 | 0.063 |
| Sex | ||||
| Female | 41 | 12 | 29 | |
| Male | 82 | 33 | 49 | 0.234 |
| Tumor | ||||
| T1 | 10 | 6 | 4 | |
| T2 | 16 | 3 | 13 | |
| T3 | 73 | 26 | 47 | |
| T4 | 24 | 10 | 14 | 0.001 |
| Lymph node | ||||
| N0 | 31 | 11 | 20 | |
| N1 | 43 | 15 | 28 | |
| N2 | 29 | 8 | 21 | |
| N3 | 20 | 11 | 9 | 0.261 |
| Pathologic TNM# stage | ||||
| I | 16 | 7 | 9 | |
| II | 21 | 6 | 15 | |
| III | 51 | 17 | 34 | |
| IV | 35 | 15 | 20 | 0.623 |
| Histology subtype | ||||
| Well differentiated adenocarcinoma | 3 | 1 | 2 | |
| Moderately differentiated adenocarcinoma | 27 | 7 | 20 | |
| Poorly differentiated adenocarcinoma | 93 | 37 | 56 | 0.418 |
| Serum CEA condition | ||||
| Positive | 30 | 11 | 19 | |
| Negative | 93 | 34 | 59 | 0.992 |
| Recurrence | ||||
| Yes | 44 | 25 | 19 | |
| No | 79 | 20 | 59 | 0.001 |
| Modes of recurrence | ||||
| Local recurrence | 8 | 4 | 4 | |
| Abdominal cavity | 9 | 6 | 3 | |
| Liver | 8 | 5 | 3 | |
| pelvic | 6 | 3 | 3 | |
| Multiple sites | 10 | 5 | 5 | |
| others | 3 | 2 | 1 | 0.959 |
* Carcinoembryonic antigen
# TNM denotes tumor-node-metastasis
Detection sensitivity of CEA mRNA by real-time RT-PCR
CEA mRNA was detected in SW-480 and SC-7901 cell lines. The lower limit of detection was a concentration of 10 tumor cells per 107 lymphocytes. Conventional nested RT-PCR was employed to confirm the sensitivity of the RT-PCR product.
CEA mRNA expression in blood
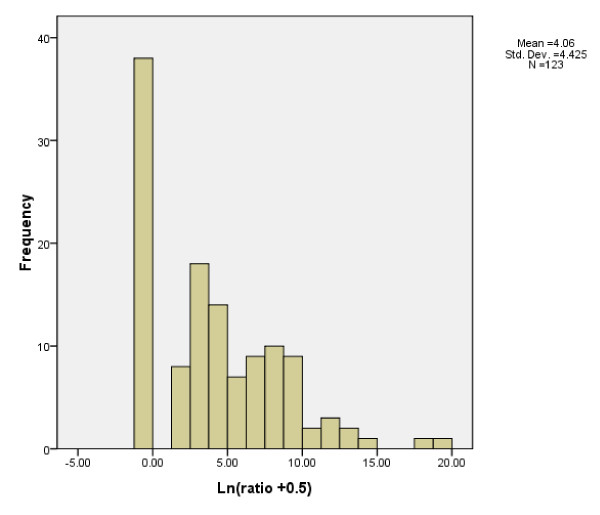
CEA mRNA expression was detected in 9 of 30 (30.0%) non-cancer patients, and the mean corrected CEA mRNA score was 7.5 (range, 0-92.5). The maximum value of corrected CEA mRNA score in patients without malignancy was 92.5, so a cutoff value of 100 was used in the present study. Using this cutoff value, 45 patients (36.6%) were diagnosed as CEA mRNA-positive. The mean corrected CEA mRNA score [(CEA mRNA/GAPDH mRNA) × 106] of the 123 patients was 37,510.0 (range, 0-3,695,652.1) copies Figure 1 showed the distribution of (CEA mRNA/GAPDH mRNA) × 106 in this group of patients.
Figure 1.
The distribution of CEA expression level. The ratio means (CEA mRNA/GAPDH mRNA) × 106. Considering the ratio of some patients were zero, we added 0.5 to the ratio.
Relationship between CEA mRNA expression and clinicopathologic features
CEA mRNA expression did not correlate with age, gender, N stage, TNM stage, histological subtype and serum CEA condition (Table 1). However, patients with postoperative recurrence had significantly higher percentage of CEA mRNA positive than those without tumor recurrence (P = 0.001) (Table 1). Besides, tumor depth also positively correlated with CEA mRNA expression (P = 0.001).
Relationship between recurrence and CEA mRNA expression as well as serum CEA
The mode of recurrence includes 8 local recurrence, 9 abdominal dissemination except liver, 8 liver metastasis, 6 pelvic metastasis, 3 other sites metastasis and 10 multiple sites metastasis. There is no significant difference between the CEA mRNA expression and the mode of recurrence (Table 1).
Recurrent disease was found in 44 of 123 cases (35.8%). Twenty-five of these patients (56.8%) were CEA mRNA-positive. By contrast, only 14 patients with recurrent disease (31.8%) were positive for preoperative serum CEA. The specificities of CEA mRNA and serum CEA to indicate recurrence were 74.7% and 79.9%, respectively. (Table 2).
Table 2.
Comparison between CEA* mRNA and serum CEA in predicting recurrence
| CEA mRNA | Serum CEA | ||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Recurrence | Yes | 25 | 19 | 14 | 30 |
| No | 20 | 59 | 16 | 63 | |
| P | 0.001 | 0.152 | |||
| X2 | 12.088 | 2.050 | |||
| Sensitivity (%) | 56.8 | 31.8 | |||
| Specificity (%) | 74.7 | 79.7 | |||
* Carcinoembryonic antigen
Univariate and multivariate analyses of 3-year disease-free survival
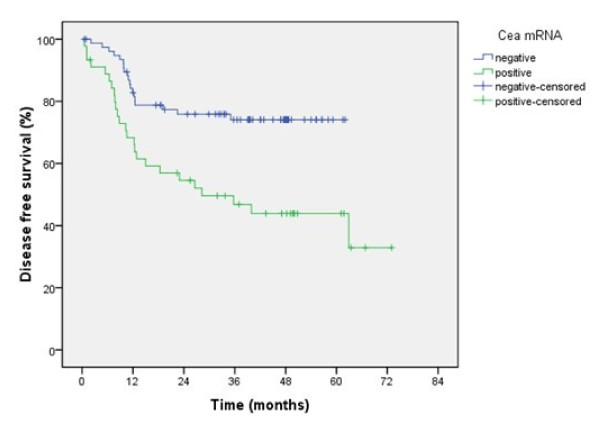
The 5-year overall survival was 58.9% and 3-year disease free survival was 63.9%. Both univariate and multivariate analyses were used to evaluate factors relating to disease-free survival. According to univariate analysis, age, tumor depth, nodal metastasis, histological grade, TNM stage, CEA mRNA positivity and serum CEA positivity were significantly related to disease-free survival (P = 0.031, <0.001, 0.001, 0.022, <0.001, 0.001, 0.045 respectively) (Table 3). Multivariate regression analysis showed that only histological grade and CEA mRNA positivity were independent factors for disease-free survival (P = 0.047 and 0.020, respectively, Table 4). Three-year disease-free survival rates for CEA mRNA-positive patients were significantly lower than for CEA mRNA negative patients (43.9% versus 74.1%, respectively, P = 0.001, Figure 2).
Table 3.
Univariate analysis of disease-free survival in Gastric carcinoma
| Parameter | Disease-free survival, P value a |
|---|---|
| Age | |
| <59 vs. ≥59 | 0.031 |
| Gender | |
| Male vs. female | 0.433 |
| CEA mRNA | |
| (+) vs. (-) | 0.001 |
| Serum CEA | |
| (+) vs. (-) | 0.045 |
| Histological grade | |
| Well/moderately vs. poorly | 0.022 |
| pT | |
| pTis/pT1 vs. pT2/pT3/pT4 | <0.001 |
| pN | |
| (+) vs. (-) | 0.001 |
| Stage | |
| 1/2 vs. 3/4 | <0.001 |
| Adjuvant chemotherapy agents | |
| CAPOX vs. mFOLFOX6 vs. Taxol+DDP vs. Taxol+5Fu/CF | 0.850 |
a Log-rank test
Table 4.
Multivariate analysis of disease-free survival in gastric carcinoma
| Factors | Characteristics | Hazard ratio | 95%CI | P value | |
|---|---|---|---|---|---|
| Unfavorable | Favorable | ||||
| Age | ≥59 | <59 | 1.018 | 0.986-1.050 | 0.273 |
| Histological grade | Poorly | Well/moderately | 0.412 | 0.171-0.990 | 0.047 |
| pT | 2/3/4 | Tis/1 | 1.673 | 0.100-8.690 | 0.947 |
| pN | (+) | (-) | 2.030 | 0.264-15.611 | 0.497 |
| Stage | 3/4 | ½ | 1.437 | 0.124-16.613 | 0.771 |
| CEA mRNA | (+) | (-) | 2.243 | 1.138-4.424 | 0.020 |
| Serum CEA | (+) | (-) | 1.130 | 0.582-2.194 | 0.717 |
CI, confidence interval; pT, depth of tumor invasion
Figure 2.
Disease-free survival of patients according to CEA mRNA expression. Three-year disease-free survival of CEA mRNA-positive patients was significantly lower than that of CEA mRNA-negative patients (43.9% versus 74.1%, respectively; P = 0.001).
Discussion
The semi-quantitative nature of traditional PCR technology has made it difficult to differentiate baseline gene expression levels in normal tissues from increased gene expression levels in cancer, thereby increasing the concern for false-positive results [17]. In our study, real-time PCR of CEA mRNA was used to investigate the possibility of peripheral blood as a source for CTC detection and prediction of cancer recurrence in gastric carcinoma patients. Real-time quantitative CEA mRNA analysis in cancer patients is often performed based on CEA mRNA positivity, which is determined using a cutoff level [13]. CEA mRNA can be detected in patients with benign disease as well as healthy volunteers, so the cutoff levels are usually determined by maximum expression in non-malignant patients [18,19]. Setoyama T et al. found that the maximum value of CEA mRNA in patients without malignancy was 8.6, they therefore set the cutoff value as 9.0 [20]. Schuster R et al.[21] also used the maximum value of healthy volunteer background as the cut-off value for the CEA mRNA detection in colorectal cancer patients. In our study, we also used the maximum value of corrected CEA mRNA score in patients without malignancy as the cutoff value. By establishing a cutoff value of 100 for normalized CEA mRNA levels, we can distinguish cancer patients from non-cancer patients and, therefore, more confidently consider the expression of CEA mRNA as a marker of circulating tumor cells.
We found that 10 patients with T1 tumor, 6 patients had positive CEA-mRNA expression. But no record of recurrence was found in the 10 patients. It seems that there is no relationship between the CEA mRNA expression and recurrence in T1 tumor. It is hard to explain the high positive rate of CEA-mRNA in T1 patients, but we found that the CEA mRNA expression was low in the 6 T1 patients, ranging from 4320 copies to 44 600 copies. Ikeguchi M [22] reported that 12.5% of the stage I gastric carcinoma patients expressed CK20 mRNA and they considered that it was induced by a small CK20 expression in peripheral white blood cells.
Few reports have assessed the condition of CTCs in gastric carcinoma patients before treatment. Ikeguchi and Kaibara reported [23] that they could not find any cancer cells in peripheral blood from untreated gastric carcinoma patients. The authors speculated that cancer cells did not appear to easily migrate to the peripheral blood from primary tumors in patients with untreated gastric carcinoma. By contrast, Miyazono et al. [24] showed that the positive rate for CEA mRNA of gastric carcinoma patients was 8.8% before operation. The presence of CTCs before treatment and its relationship with clinical outcome thus remains controversial. In this study, we evaluated the clinical significance of CTCs in blood before operation by using real-time RT-PCR to detect expression of CEA mRNA. The positive rate of CEA mRNA before any treatment is 36.6%. Additional file 1 shows the positive rate of mRNA markers from literature for detection of tumor cells by real-time RT-PCR.
O'Sullivan et al. [25] suggested that preoperative detection of micrometastasis may reflect either transient shedding of cells, metastatic potential, or residual disease. In the present study, we found that CTCs were detected in blood before treatment in relation to recurrence. Several reports have demonstrated that preoperative detection of circulating cancer cells was a clear marker of poor patient survival, because many cases with circulating cancer cells preoperatively showed either extended lymph node metastasis or distant metastasis, thus the prognosis of such patients was poor [26-28]. In current study, we found that the expression of CEA mRNA was significantly related to disease recurrence. Furthermore, patients with positive CEA mRNA had shorter 3-year disease-free survival outcome. The incidence of recurrence was significantly higher in patients positive for CEA mRNA than in those negative.
The sensitivity of CEA mRNA expression to predict recurrence is only 56.8%. Nineteen of seventy-eight patients (24.4%) with negative CEA mRNA expression had tumor recurrence. Setoyama et al. [20] showed that 8 of 69 (11.6%) esophageal carcinoma patients with negative CEA mRNA expression had tumor relapse, and 6 patients had lymph node recurrence. One frequently used explanation of detection failure is that circulating cells are not homogeneously distributed and non-continuously shed into circulation [29,30]. Furthermore, the ideal marker (no illegitimate expression in blood, high expression in tumor cells) has not yet been found. Beyond CEA mRNA, other transcripts, including cytokeratin (CA) 18 [31], matrix metalloproteinase (MMP)-7 [32], CK 20 [33], Urokinase-type plasminogen activator receptor (uPAR), CK 19 and CK 7 [34], have been tried as potential markers of CTCs. However, the tumor cell shed should be a relatively rare event. Thus, whether peripheral blood is a suitable compartment for early detection of micrometastases is still controversial. Other compartments such as bone marrow or abdominal cavity are known to provide higher detection rates, probably due to a larger number of tumor cells present [35-37].
Another important issue is false positive expression of CEA mRNA. Twenty patients (44.4%) who had positive CEA mRNA expression did not record recurrence in the follow-up. One reason may be the relatively short follow-up period.
Alternatively, this may be quite reasonable because few carcinoma cells shed into the bloodstream succeed in establishing metastatic disease [25]. These circulating cancer cells might not attach to distant organs and might not grow. Recently, Méhes et al. [38] investigated the morphology of circulating cancer cells and found that the majority of circulating breast cancer cells was in a state of apoptosis. In the peripheral blood of cancer patients, the existence of the tumor cell-lymphocyte complex was observed [39]. These findings indicate that macrophages or lymphocytes could play an important role in the induction of circulating tumor cell apoptosis and the antitumor immune response of the host. These immunized macrophages may sensitize the cytotoxic T lymphocytes of the host, and the sensitized T lymphocytes may attack the residual micrometastatic cancer lesions of the patients [23]. To clarify this hypothesis, further investigations regarding the correlation between the existence of circulating cancer cells and the host immune response are necessary.
The current study was retrospective analysis, and patients who should receive adjuvant chemotherapy were not set ahead of time. Generally, patients who had positive lymph node were recommended to receive adjuvant chemotherapy. Till now, there are no standard criteria for adjuvant chemotherapy of gastric cancer in China. Our study showed that the CEA mRNA copy number in peripheral blood at initial diagnosis was significantly associated with disease recurrence in gastric adenocarcinoma patients. In the viewpoint of recurrence, we therefore suggest that patients who have positive CEA mRNA expression preoperatively receive adjuvant chemotherapy after radical resection.
Conclusions
In this study, the sensitivity of CEA mRNA was higher than that of serum CEA. Moreover, according to multivariate regression analysis, CEA mRNA positivity was an independent factor for recurrence. The current study confirmed that such a method was promising for the early detection of CTCs in patients with gastric carcinoma before treatment; patients with positive CEA mRNA may have a higher risk of recurrence even after curative resection. However, a large randomized prospective study is warranted to define the role of CEA mRNA detection in blood.
Abbreviations
RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction; CTCs: Circulating Tumor Cells; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; TNM: Tumor-Node-Metastasis; AJCC: American Joint Committee on Cancer; GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; MMP-7: Matrix Metalloproteinase-7; uPAR: urokinase-type Plasminogen Activator Receptor.
Competing interests
We have no financial or personal relationships with other people or organizations that would bias our work. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of our article.
Authors' contributions
QMZ carried out the real-time RT-PCR, participated in the clinical data collecting of the gastric carcinoma patients and drafted the manuscript. LZH carried out the real-time RT-PCR. ZZW participated in the blood sample collecting. LYH performed the statistical analysis. WZQ and WFH participated in the design of the study. FA and WDY drafted the manuscript and participated in the statistical analysis. HP and XRH conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Data from literature for detection of tumor cells by real-time RT-PCR of mRNA markers. It shows the positive rate of mRNA markers from literature for detection of tumor cells by real-time RT-PCR.
Contributor Information
Miao-zhen Qiu, Email: qiumzh@sysucc.org.cn.
Zhuang-hua Li, Email: lizhh@sysucc.org.cn.
Zhi-wei Zhou, Email: zhouzhw@sysucc.org.cn.
Yu-hong Li, Email: liyh@sysucc.org.cn.
Zhi-qiang Wang, Email: wangzhq@sysucc.org.cn.
Feng-hua Wang, Email: wangfh@sysucc.org.cn.
Peng Huang, Email: phuang@mdanderson.org.
Fahad Aziz, Email: fahadaziz.md@gmail.com.
Dao-yuan Wang, Email: wangdy@vip.163.com.
Rui-hua Xu, Email: xurh@sysucc.org.cn.
Acknowledgements
These work was funded by National Natural Science Foundation of China grant 30672408, Guangzhou Bureau of Science and Technology grant 2006Z3-E0041 and Sun Yat-sen University 985 Program Initiation Fund (China). We gratefully thank the staff members in the Department of Medical Oncology and GI Surgery Oncology at Sun Yat-sen University Cancer Center for their suggestion and assistance.
References
- Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002;235:499–506. doi: 10.1097/00000658-200204000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viste A, Rygh AB, Søreide O. Cancer of the stomach: is a follow-up program of any clinical importance for the patient? Clin Oncol. 1984;10:325–332. [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Ulmer A, Schmidt-Kittler O, Fischer J, Ellwanger U, Rassner G, Riethmüller G, Fierlbeck G, Klein CA. Immunomagnetic enrichment, genomic characterization, and prognostic impact of circulating melanoma cells. Clin Cancer Res. 2004;10:531–537. doi: 10.1158/1078-0432.CCR-0424-03. [DOI] [PubMed] [Google Scholar]
- Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng S, Morrison L, Tucker T, Lane N, Ghadimi BM, Heselmeyer-Haddad K, Ried T, Rao C, Uhr J. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8:2073–2084. [PubMed] [Google Scholar]
- Reinhold U, Lüdtke-Handjery HC, Schnautz S, Kreysel HW, Abken H. The analysis of tyrosinase-specific mRNA in blood samples of melanoma patients by RT-PCR is not a useful test for metastatic tumor progression. J Invest Dermatol. 1997;108:166–169. doi: 10.1111/1523-1747.ep12333341. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Keilholz U, Rossi CR, Nitti D. Circulating tumor cells: the 'leukemic phase' of solid cancers. Trends Mol Med. 2006;12:130–139. doi: 10.1016/j.molmed.2006.01.006. Epub 2006 Feb 20. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Rossi CR, Pilati P, Nitti D, Marincola FM. Quantitative real time PCR: a powerful ally in cancer research. Trends Mol Med. 2003;9:189–195. doi: 10.1016/S1471-4914(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Bosma AJ, Hart AA, Rodenhuis S, van 't Veer LJ. Marker genes for circulating tumour cells predict survival in metastasized breast cancer patients. Br J Cancer. 2003;88:1091–1094. doi: 10.1038/sj.bjc.6600868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Slade MJ, English J, Graham H, Lüchtenborg M, Sinnett HD, Cross NC, Coombes RC. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J Clin Oncol. 2000;18:1432–1439. doi: 10.1200/JCO.2000.18.7.1432. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Takemoto Y, Nakashima Y, Kinugasa S, Kotoh T, Dhar DK, Kohno H, Nagasue N. Serum Carcinoembryonic Antigen as a Prognostic Factor in Resectable Gastric Cancer. J Am Coll Surg. 1998;187:64–68. doi: 10.1016/S1072-7515(98)00133-1. [DOI] [PubMed] [Google Scholar]
- Ishigami S, Sakamoto A, Uenosono Y, Nakajo A, Okumura H, Matsumoto M, Setoyama T, Arigami T, Uchikado Y, Arima H, Natsugoe S, Aikou T. Carcinoembryonic Antigen Messenger RNA Expression in Blood Can Predict Relapse in Gastric Cancer. J Surg Res. 2008;148:205–209. doi: 10.1016/j.jss.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Terashima M, Hoshino Y, Ohtani S, Kashimura S, Kanzaki N, Osuka F, Kogure M, Gotoh M. Detection of cancer cells disseminated in bone marrow using real-time quantitative RT-PCR of CEA, CK19, and CK20 mRNA in patients with gastric cancer. Gastric Cancer. 2006;9:308–314. doi: 10.1007/s10120-006-0398-z. Epub 2006 Nov 24. [DOI] [PubMed] [Google Scholar]
- To EM, Chan WY, Chow C, Ng EK, Chung SC. Gastric Cancer Cell Detection in Peritoneal Washing: Cytology Versus RT-PCR for CEA Transcripts. Diagn Mol Pathol. 2003;12:88–95. doi: 10.1097/00019606-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Juhl H, Kalthoff H, Schreiber HW, Wagener C, Neumaier M. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol. 1994;12:725–729. doi: 10.1200/JCO.1994.12.4.725. [DOI] [PubMed] [Google Scholar]
- Ge MJ, Shi D, Wu QC, Wang M, Li LB. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol. 2006;132:248–256. doi: 10.1007/s00432-005-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmler A, Gerhards R, Betz C, Günther K, Reingruber B, Horbach T, Baumann I, Kirchner T, Hohenberger W, Papadopoulos T. Transcription of cytokeratins 8, 18, and 19 in bone marrow and limited expression of cytokeratins 7 and 20 by carcinoma cells: inherent limitations for RT-PCR in the detection of isolated tumor cells. Lab Invest. 2001;81:1351–1361. doi: 10.1038/labinvest.3780349. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakanishi H, Hirai T, Kato T, Kodera Y, Feng Z, Kasai Y, Ito K, Akiyama S, Nakao A, Tatematsu M. Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer Lett. 2002;183:195–203. doi: 10.1016/S0304-3835(02)00157-X. [DOI] [PubMed] [Google Scholar]
- Setoyama T, Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Ishigami S, Owaki T, Takao S, Aikou T. Carcinoembryonic AntigenMessenger RNA Expression in Blood Predicts Recurrence in Esophageal Cancer. Clin Cancer Res. 2006;12:5972–5977. doi: 10.1158/1078-0432.CCR-06-0637. [DOI] [PubMed] [Google Scholar]
- Schuster R, Max N, Mann B, Heufelder K, Thilo F, Gröne J, Rokos F, Buhr HJ, Thiel E, Keilholz U. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108:219–227. doi: 10.1002/ijc.11547. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Ohro S, Maeda Y, Fukuda K, Yamaguchi K, Shirai H, Kondo A, Tsujitani S, Kaibara N. Detection of cancer cells in the peripheral blood of gastric cancer patients. Int J Mol Med. 2003;11:217–221. [PubMed] [Google Scholar]
- Ikeguchi M, Kaibara N. Detection of Circulating Cancer Cells After a Gastrectomy for Gastric Cancer. Surg Today. 2005;35:436–441. doi: 10.1007/s00595-004-2978-z. [DOI] [PubMed] [Google Scholar]
- Miyazono F, Natsugoe S, Takao S, Tokuda K, Kijima F, Aridome K, Hokita S, Baba M, Eizuru Y, Aikou T. Surgical Maneuvers Enhance Molecular Detection of Circulating Tumor Cells During Gastric Cancer. Ann Surg. 2001;233:189–194. doi: 10.1097/00000658-200102000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan GC, Collins JK, Kelly J, Morgan J, Madden M, Shanahan F. Micrometastases: marker of metastatic potential or evidence of residual disease? Gut. 1997;40:512–515. doi: 10.1136/gut.40.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Chen KY, Wang WY, Jan JS, Liang WM, Wei YH. Evaluation of cytokeratin-19 mRNA as a tumor marker in the peripheral blood of nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Cancer. 2002;97:548–553. doi: 10.1002/ijc.10075. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Miyoshi Y, Motomura K, Inaji H, Koyama H, Noguchi S. Prognostic significance of occult bone marrow micrometastases of breast cancer detected by quantitative polymerase chain reaction for cytokeratin 19 mRNA. Jpn J Cancer Res. 2000;91:918–924. doi: 10.1111/j.1349-7006.2000.tb01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Mimori K, Ueo H, Tsuji K, Shiraishi T, Barnard GF, Sugimachi K, Akiyoshi T. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription-polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol. 1998;16:128–132. doi: 10.1200/JCO.1998.16.1.128. [DOI] [PubMed] [Google Scholar]
- Jonas S, Windeatt S, O-Boateng A, Fordy C, Allen-Mersh TG. Identification of carcinoembryonic antigen-producing cells circulating in the blood of patients with colorectal carcinoma by reverse transcriptase polymerase chain reaction. Gut. 1996;39:717–721. doi: 10.1136/gut.39.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells A, Boix L, Bessa X, Gargallo L, Piqué JM. Detection of colonic cells in peripheral blood of colorectal cancer patients by means of reverse transcriptase and polymerase chain reaction. Br J Cancer. 1998;78:1368–1372. doi: 10.1038/bjc.1998.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlimok G, Funke I, Pantel K, Strobel F, Lindemann F, Witte J, Riethmüller G. Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer. 1991;27:1461–1465. doi: 10.1016/0277-5379(91)90032-9. [DOI] [PubMed] [Google Scholar]
- Yonemura Y, Fujimura T, Ninomiya I, Kim BS, Bandou E, Sawa T, Kinoshita K, Endo Y, Sugiyama K, Sasaki T. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001;7:1647–1653. [PubMed] [Google Scholar]
- Soeth E, Vogel I, Röder C, Juhl H, Marxsen J, Krüger U, Henne-Bruns D, Kremer B, Kalthoff H. Comparative analysis of bone marrow and venous blood isolated from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 1997;15:3106–3110. [PubMed] [Google Scholar]
- Kita Y, Fukagawa T, Mimori K, Kosaka Y, Ishikawa K, Aikou T, Natsugoe S, Sasako M, Mori M. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer. 2009;100:153–159. doi: 10.1038/sj.bjc.6604806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmüller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. doi: 10.1016/0140-6736(92)92230-D. [DOI] [PubMed] [Google Scholar]
- Ueda T, Furui J, Komuta K, Yamaguchi J, Yamamoto M, Furukawa K, Kanematsu T. Detection of carcinoembryonic antigen mRNA in the mesenteric vein of patients with resectable colorectal cancer. Surg Today. 1998;28:701–706. doi: 10.1007/BF02484615. [DOI] [PubMed] [Google Scholar]
- Koch M, Weitz J, Kienle P, Benner A, Willeke F, Lehnert T, Herfarth C, von Knebel Doeberitz M. Comparative analysis of tumor cell dissemination in mesenteric, central, and peripheral venous blood in patients with colorectal cancer. Arch Surg. 2001;136:85–89. doi: 10.1001/archsurg.136.1.85. [DOI] [PubMed] [Google Scholar]
- Méhes G, Witt A, Kubista E, Ambros PF. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159:17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar B, Ladanyi A, Tanko L, Sréter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7:4080–4085. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data from literature for detection of tumor cells by real-time RT-PCR of mRNA markers. It shows the positive rate of mRNA markers from literature for detection of tumor cells by real-time RT-PCR.