Summary
Besides industrially produced gibberellins (GAs), Fusarium fujikuroi is able to produce additional secondary metabolites such as the pigments bikaverin and neurosporaxanthin and the mycotoxins fumonisins and fusarin C. The global regulation of these biosynthetic pathways is only poorly understood. Recently, the velvet complex containing VeA and several other regulatory proteins was shown to be involved in global regulation of secondary metabolism and differentiation in Aspergillus nidulans. Here we report on the characterization of two components of the F. fujikuroi velvet-like complex, FfVel1 and FfLae1. The gene encoding this first reported LaeA ortholog outside the class of Eurotiomycetidae is upregulated in ΔFfvel1 microarray-studies and FfLae1 interacts with FfVel1 in the nucleus. Deletion of Ffvel1 and Fflae1 revealed for the first time that velvet can simultaneously act as positive (GAs, fumonisins and fusarin C) and negative (bikaverin) regulator of secondary metabolism, and that both components affect conidiation and virulence of F. fujikuroi. Furthermore, the velvet-like protein FfVel2 revealed similar functions regarding conidiation, secondary metabolism and virulence as FfVel1. Cross genus complementation studies of velvet complex component mutants between Fusarium, Aspergillus and Penicillium support an ancient origin for this complex which has undergone a divergence in specific functions mediating development and secondary metabolism.
Keywords: Fusarium fujikuroi, velvet complex, LaeA, gibberellin, bikaverin
Introduction
Fusarium fujikuroi Nirenberg (teleomorph: Gibberella fujikuroi mating population C [MP-C]) belongs to the G. fujikuroi (Sawada) Wollenweber species complex. It predominantly occurs on rice (Oryza sativa Linné) where it causes the bakanae (e.g. foolish seedling) disease, resulting in a variety of symptoms such as abnormal elongation of plants, yellowish leaves, reduced tillering and sterile or empty grains at late infection (Desjardins et al., 2000; Sun and Snyder 1981). Many of these symptoms are due to exposure to gibberellins (GAs), a group of diterpenoid compounds originally characterized as plant hormones, which are produced by the fungus (Tudzynski and Hölter 1998; Yabuta, 1935). The growth promoting function of GAs has been exploited extensively by the agri- and horticulture biotechnology industries and GAs are routinely produced in mass quantities by F. fujikuroi fermentation (Rademacher 1997).
In addition to GAs, F. fujikuroi can produce a variety of other secondary metabolites including the mycotoxins fumonisins (Proctor et al., 2004) and fusarin C (Barrero et al., 1991), as well as the pigment bikaverin (Kjær et al., 1971). The genes involved in the production of secondary metabolite are typically clustered in the fungal genome (reviewed in Hoffmeister and Keller 2007). For example, the seven genes involved in GA synthesis (reviewed in Tudzynski 2005), the six genes involved in bikaverin synthesis (Wiemann et al., 2009) and the ~10 genes involved in fusarin C synthesis (D. Brown, unpublished results) are located adjacent to each other at separate loci. Although not examined in F. fujikuroi, a cluster of 21 genes in F. verticillioides has been associated with fumonisin synthesis (Brown et al., 2007; Proctor et al., 2003).
Little is known about environmental conditions that affect the transcriptional regulation of most secondary metabolite gene clusters in Fusarium. We do know that the GA cluster genes in F. fujikuroi are repressed by high amounts of nitrogen in an AreA-dependent manner (Mihlan et al., 2003; Tudzynski et al., 1999). We also know that fumonisin gene expression in F. verticillioides is repressed in a similar AreA-dependent manner (Kim and Woloshuk 2008) as well as repressed by PacC at alkaline ambient pH (Flaherty et al., 2003). Bikaverin cluster genes in F. fujikuroi are also repressed by nitrogen but independently of AreA, are repressed by PacC at alkaline ambient pH and are only expressed in liquid culture at early time points (Wiemann et al., 2009).
In addition to these environmental attuned systems, recent work has described tremendous progress towards understanding the role global regulators play in controlling secondary metabolism pathways and/or developmental processes. The best characterized global regulator to date is velvet (VeA), originally shown in Aspergillus spp. to activate production of a variety of secondary metabolites including sterigmatocystin (ST) in A. nidulans (Kato et al., 2003), aflatoxin (AF), cyclopiazonic acid and aflatrem in A. flavus (Amaike and Keller, 2009; Duran et al., 2007) as well as AF in A. parasiticus (Calvo et al., 2004). In other fungal genera, orthologs of VeA have a similar influence on species specific metabolites including positive regulation of both cephalosporin in Acremonium chrysogenum (Dreyer et al., 2007) and fumonisins and fusarins in F. verticillioides (Myung et al., 2009). So far there is no secondary metabolite that is clearly repressed by VeA or one of its orthologs although the influence of VeA on penicillin (PN) production in A. nidulans is ambiguous (Kato et al., 2003; Spröte and Brakhage 2007).
In addition to its effect on secondary metabolism, VeA also influences development and morphology. In A. nidulans the light mediated localization of VeA to the nucleus initiates the positive regulation of sexual development (Kim et al., 2002; Stinnett et al., 2007), most likely by activating the early sexual development gene esdC (Han et al., 2008). In contrast, VeA negatively affects asexual development by limiting transcription of brlA the central regulator of conidiophore development (Kato et al., 2003). In Neurospora crassa, deletion of the veA ortholog ve-1 leads to a similar increase in conidiation (Bayram et al., 2008a). In other fungi, the effect of veA deletion on development varies. In A. parasiticus and A. fumigatus sporulation is nutrient-dependent in ΔveA strains (Calvo et al., 2004; Krappmann et al., 2005), in A. flavus conidiation is decreased in veA deletions (Amaike and Keller, 2009) while in F. verticillioides, the ratio of microconidia-to-macroconidia produced is altered in the Fvve1 deletion mutant (Li et al., 2006).
Recent work in A. nidulans indicates that the positive regulation of secondary metabolite production observed is most likely achieved through the physical interaction of VeA with the velvet-like protein VelB and LaeA in the nucleus (Bayram et al., 2008b). LaeA, so far only identified in the fungal class of Eurotiomycetidae, is a putative methyltransferase that modulates heterochromatin structure thereby regulating transcription of specific DNA regions (Bok and Keller, 2004; Bok et al., 2006a; b; Reyes-Dominguez et al., 2010). Deletion of laeA in A. nidulans results in repression of transcription of the terrequinone A, lovastatin, ST and PN gene clusters with a concomitant loss of their respective products (Bok and Keller 2004; Bouhired et al., 2006). A similar effect was observed in A. flavus on AF (Amaike and Keller, 2009; Kale et al. 2008) and in A. fumigatus on helvolic acid and gliotoxin production (Bok et al., 2005; Lodeiro et al., 2009). In contrast, in Penicillium chrysogenum, although a decrease in PN production was seen, roquefortin C synthesis was not affected in the ΔlaeA strain (Kosalková et al., 2009). Microarray analysis of wild-type and ΔlaeA strains of A. fumigatus indicated that LaeA controls up to 9.5 % of the transcriptome and up to 13 of its 22 secondary metabolite gene clusters (Perrin et al., 2007).
In this study, we explore the influence of velvet on secondary metabolites produced by the biotechnologically important fungus F. fujikuroi. We show for the first time that FfVel1 can act as a positive (GAs, fumonisins and fusarin C) as well as a negative (bikaverin) regulator. In addition, FfVel1 dramatically affects asexual and sexual development and morphogenesis. The homolog of the A. nidulans velvet-like protein VelB, FfVel2, seems to execute similar functions as FfVel1 regarding GA and bikaverin production as well as sporulation in F. fujikuroi. Differential expression studies by microarray with the ΔFfvel1 mutant led to the first identification of a LaeA ortholog outside the class of Eurotiomycetidae. We show that both velvet family proteins, FfVel1 and FfVel2, as well as FfLae1 affect virulence of F. fujikuroi on rice. Bimolecular fluorescence complementation (BiFC) and yeast two hybrid (Y2H) analysis show that FfVel1 and FfLae1 interact with each other as well as with an orthologous component from A. nidulans. Furthermore, VeA and LaeA orthologs from P. chrysogenum can restore certain wild-type phenotypes of knock-out mutants in F. fujikuroi, and FfLae1 can rescue secondary metabolism in a ΔlaeA strain of A. nidulans.
Results
F. fujikuroi velvet displays common and distinct features to other velvet proteins
F. fujikuroi veA (Ffvel1) was obtained by heterologous PCR. Primers specific to the F. verticillioides ve1 (Fvve1) sequence (Li et al., 2006) were designed and used with F. fujikuroi genomic DNA as a template. The resulting amplicon was cloned and sequence analysis indicated that it shared significant identity with Fvve1. RT-PCR analysis revealed an ORF of 1593 bp interrupted by one intron of 94 bp. The gene encodes a predicted protein of 531 amino acids and was designated FfVel1 (FN548142). BLASTp analysis indicates that FfVel1 shares identity to velvet proteins from different ascomycetes: 98 % to FvVe1 (ABC02879) from F. verticillioides, 55 % to AcVeA (CAL68582) from A. chrysogenum, 42 % to Ve-1 (XP_957154) from N. crassa, 38 % to PcVeA (CAP92389) from P. chrysogenum and 36 % to VeA (AAD42946) from A. nidulans.
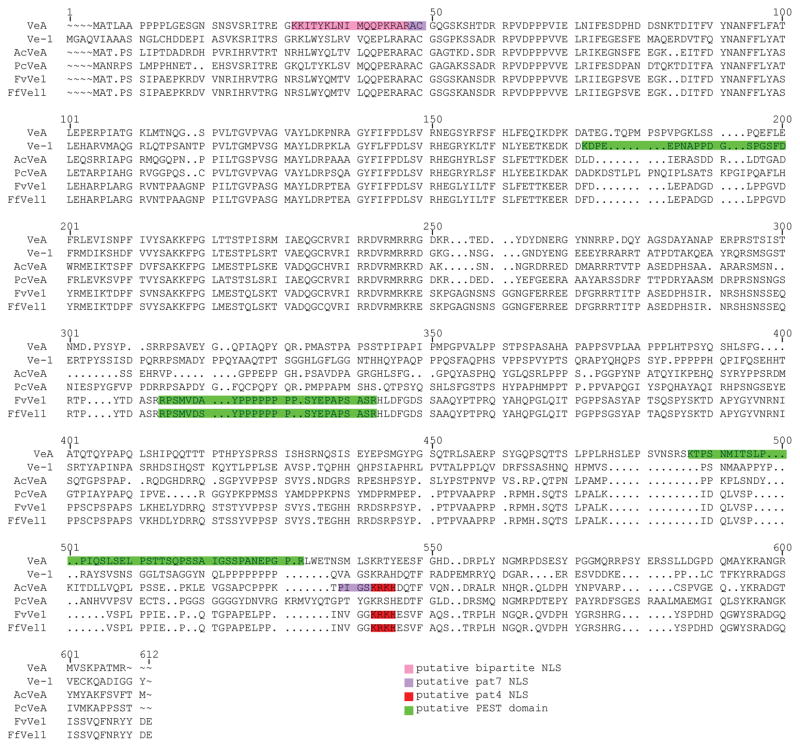
Analysis of the predicted protein and comparison to other velvet proteins identified a number of interesting characteristics. First, a pairwise alignment using the algorithm ClustalW of the above mentioned sequences identified several conserved regions between the proteins (Fig. 1). Using WolF PSORT (www.wolfpsort.org; Horton et al., 2007) to predict protein subcellular localization, VeA from A. nidulans contains a putative pat7 nuclear localization signal (NLS) and a second, overlaid bipartite NLS, at the N-terminus (Kim et al., 2002; Stinnett et al., 2007). In contrast, FfVel1 contains a pat4 NLS (K471RKH474) at the C-terminus which is highly conserved in FvVe1 and AcVeA but not present in the other velvet proteins (Fig. 1). The EMBOSS program “epestfind” (http://emboss.bioinformatics.nl/cgi-bin/emboss/epestfind) identified several proline, glutamate, serine and threonine (PEST) rich regions. PEST motifs are associated with proteins having a short intracellular half-life, where the PEST motif is thought to act as a signal peptide for protein degradation (Rogers et al., 1986). While the PEST region in FfVel1 (R288PSMVDAYPPPPPPPPSYEPAPSASR312) is highly conserved in FvVe1, the PEST motifs in the A. nidulans (VeA) and N. crassa (Ve-1) proteins are predicted at different positions, respectively (Fig. 1).
Figure 1. Multiple sequence alignment of FfVel1 with other characterized VeA homologous sequences.
The alignment was generated using ClustalW. Putative nuclear localization signals (NLS) were predicted using WolF PSORT (Horton et al., 2007) and are marked in shades of red. Putative PEST domains were predicted using the EMBOSS program “epestfind” and marked in green. Sequences aligned are: VeA (AAD42946) from A. nidulans, Ve-1 (XP_957154) from N. crassa, AcVeA (CAL68582) from A. chrysogenum, PcVeA (CAP92389) from P. chrysogenum, FvVe1 (ABC02879) from F. verticillioides and FfVel1 (FN548142) from F. fujikuroi.
FfVel1 regulates morphology and development
In order to examine the role FfVel1 plays in F. fujikuroi, Ffvel1 knock-out and Ffvel1 addback strains were created. In the first case, a DNA construct containing a hygromycin resistance cassette flanked by 1 kb upstream of the predicted Ffvel1 start codon and 1 kb downstream from the predicted Ffvel1 stop codon was transformed into F. fujikuroi strain IMI58289. Transformants whereby a homologous gene replacement event had occurred were identified by PCR (data not shown) and ectopic integration events were identified by Southern hybridization (Fig. S1A). Transformants ΔFfvel1-T3, -T4 and -T11 were found to have lost the target Ffvel1 gene and did not contain an ectopic integration of the gene replacement cassette. As all three transformants exhibited the same phenotype (data not shown), ΔFfvel1-T3 was arbitrarily chosen for further work. Two Ffvel1 addback strains were created by transforming ΔFfvel1-T3 with constructs containing a nourseothricin resistance cassette and a wild-type copy of Ffvel1 or a copy where the putative pat4 NLS was changed to A471AAH474 creating Ffvel1C and Ffvel1CΔpat4, respectively.
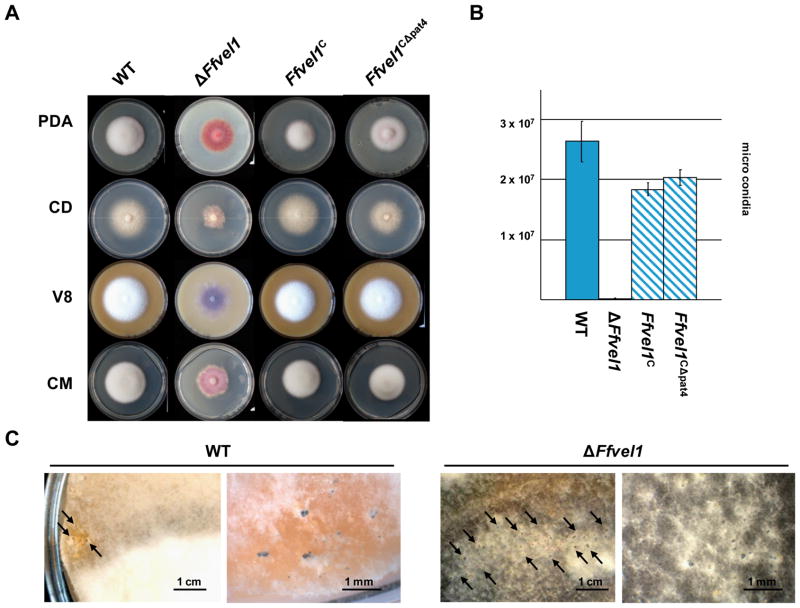
The ΔFfvel1, Ffvel1C, Ffvel1CΔpat4 and the wild-type strains were grown on different solidified media under constantly dark conditions and photographed after ten days. The ΔFfvel1 mutant exhibited several growths defects including reduced aerial hyphae and increased pigmentation. In contrast, the addback strains (Ffvel1C and Ffvel1CΔpat4) displayed wild-type-like fluffy growth with white aerial hyphae (Fig. 2A). The similar growth of the Ffvel1CΔpat4 and Ffvel1C strains suggest that the putative pat4 NLS does not play a major role in the nuclear localization of FfVel1. When grown in submerged cultures, there was no visible difference in hyphal morphology between the different strains (data not shown). When the strains were grown on solidified V8 medium under constant light conditions for 14 days, the wild type and both Ffvel1 addback strains produced almost identical amounts of microconidia, while the ΔFfvel1 strain generated significantly fewer microconidia (approximately 0.5% of the wild type) (Fig. 2B). To determine if FfVel1 plays a role in perithecial formation, the ΔFfvel1 mutant and its progenitor strain IMI58289 were crossed with the MAT-2 standard tester strain F. fujikuroi C-1995 and the MAT-1 standard tester strain C-1993. The observation that perithecia were only formed by the IMI58289/C-1995 cross indicated that IMI58289 contains the MAT-1 locus. It was interesting to note that the ΔFfvel1/C-1995 cross generated significantly more perithecia as compared to the wild type (IMI58289/C-1995 cross) (Fig. 2C).
Figure 2. Phenotypical analysis of Ffvel1 knock-out and addback strains.
A Photographs of the F. fujikuroi wild-type strain IMI58289, the Ffvel1 knock-out strain and the addback strains Ffvel1C and Ffel1CΔpat4 grown on different solidified media for 10 days under constant dark conditions. V8 = vegetable juice; CM = complete medium; PDA = Potatoe Dextrose Agar; CD = Czapek Dox (for details see Experimental Procedures).
B Spores produced by the F. fujikuroi wild type strain IMI58289, the Ffvel1 knock-out strain and the addback strains Ffvel1C and Ffel1CΔpat4 grown for 10 days on V8 solidified media under constant light conditions. Experiment was carried out in triplicate; bars show standard deviations.
C Photographs of the F. fujikuroi wild type strain IMI58289 and the Ffvel1 knock-out strain crossed with strain MRC1995. Strains were grown for 6 weeks on V8 solidified media as described in Experimental Procedures.
Microarray analysis reveals distinct functional sets of FfVel1 target genes
To gain broader insight into the function of FfVel1 as well as identify genes that it may regulate, a heterologous microarray experiment was performed. The F. fujikuroi wild-type and Ffvel1 deletion strains were grown under nitrogen-limiting conditions in liquid media, in duplicate, and sampled after 24 h, 72 h and 120 h. Isolated RNA was labelled and hybridized to F. verticillioides microarrays (Roche-NimbleGen) containing oligos representing over 12,500 genes or about 88% of the current predicted gene models.
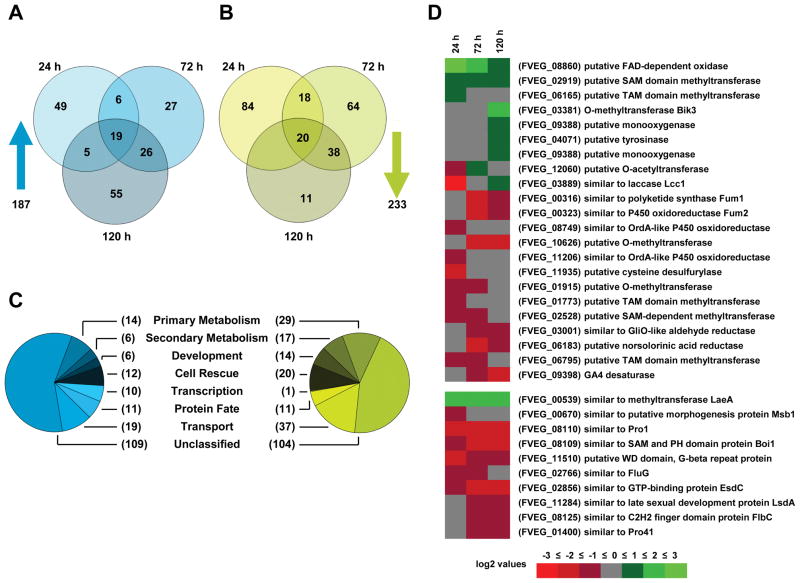
We found that 233 genes were downregulated while 187 genes were upregulated in ΔFfvel1 compared to the wild type. We then subdivided these two sets according to the time-point of up- and downregulation (Fig. 3A and B) and grouped them into several functional categories using the Munich Information Center for Protein Sequences (MIPS) functional database catalogue (http://mips.gsf.de) (Fig. 3C).
Figure 3. Microarray analysis of the F. fujikuroi wild-type strain IMI58289 compared to a Ffvel1 knock-out strain.
A Venn Diagram of genes upregulated in a Ffvel1 knock-out mutant compared to the WT at 24 h, 72 h and 120 h. Only genes with at least 2 fold upregulation in both replicate hybridizations as compared between strains or time points, were considered to be differentially regulated.
B Venn Diagram of genes downregulated in a Ffvel1 knock-out mutant compared to the WT at 24 h, 72 h and 120 h. Only genes with at least 2 fold downregulation in both replicate hybridizations as compared between strains or time points, were considered to be differentially regulated.
C Pie charts representing classification of protein sequences corresponding to differentially regulated oligo sets using the MIPS functional database catalogue (for details see Experimental Procedures).
D Heat map of genes encoding for proteins involved in secondary metabolism or development as classified by the MIPS functional database catalogue. Each colored square represents the mean log2-fold change of both replicate hybridizations at the given time point (green: up-regulation in the Ffvel1 knock-out mutant compared to the wild type; red: down-regulation in the Ffvel1 knock-out mutant compared to the wild type).
Among the 233 genes downregulated in the ΔFfvel1 mutant, 16 % (37 genes) grouped into the functional category of transport, comprising the largest group apart from the unclassified genes (Fig. 3C). Of these transport-associated genes 65 % (24 genes) were exclusively found to be downregulated at the 24 h time-point and include the L-α-amino acid transporter Dip5p homolog (Regenberg et al., 1998) and the peptide transporter Ptr2p homolog (Perry et al., 1994) (Table. S1). Another category of interest was the group of 14 genes (6 %) assigned to the developmental function group. Seven of the identified genes have been previously identified as regulators of development in different ascomycetes. These include a homolog of the Sordaria macrospora transcriptional regulatory protein Pro1 (Masloff et al., 2002) and the A. nidulans dioxygenase PpoC which affects the ratio of asexual to sexual spores (Tsitsigiannis et al., 2004), FluG which is responsible for production of an extracellular diffusible conidiation factor, and the C2H2-type transcriptional activator FlbC (Fig. 3D and Table S1). FluG functions most upstream of FlbC which has been shown to be essential for brlA expression and subsequent conidiation in A. nidulans (reviewed in Adams et al., 1998).
Consistent with findings in other fungi, we found 11 secondary metabolite genes exclusively downregulated at 120 h. These include Fffum1 and Fffum2, encoding a polyketide synthase and P450 monooxygenase, respectively, required for fumonisin production (Proctor et al., 2003) and des, required for gibberellin synthesis (Tudzynski et al., 2003). des is the only gene of the F. fujikuroi GA cluster present in F. verticillioides (Bömke et al., 2008) (Table S1).
Of the 187 upregulated genes, we were surprised to note 6 (3 %) that are likely associated with secondary metabolism including bik3 encoding the O-methyltransferase needed for bikaverin production (Wiemann et al., 2009) as well as two other genes encoding putative methyltransferases (FVEG_02919 and FVEG_06165), and three genes encoding putative oxygenases (FVEG_09388, FVEG_04071 and FVEG_08860) (Fig. 3D and Table S1). This group also contains a set of 19 genes (10 %) that were upregulated at all time-points sampled (Fig. 3C). The presence of several genes from the heat shock protein (Hsp) families (Table S1) suggests that stress perception is more pronounced in the ΔFfvel1 strain. We were most intrigued by the presence of a putative laeA homolog (FVEG_00539 and designated Fflae1) which shared 32% identity to LaeA from A. nidulans (AAQ95166; Bok and Keller 2004) (Table S1). Notably, laeA is also upregulated in the A. nidulans veA knock-out strain (Bayram et al., 2008b).
The differential expression of some FfVel1 target genes from different functional groups was confirmed by Northern blot analysis (Fig. S2). Additionally, expression of the flbC homolog (FfflbC) was also analyzed under conidiation-inducing conditions (solidified V8 medium) and also found to be significantly downregulated in the ΔFfvel1 mutant. These expression data fit very well with the significant reduction of microconidia formation by the mutant.
FfVel1 plays both positive and negative roles in regulating secondary metabolism
As genes from several secondary metabolite gene clusters (GA, bikaverin and fumonisin) were found to be deregulated in the ΔFfvel1 mutant, a more thorough chemical analysis of secondary metabolite production by the Ffvel1 knock-out strain was performed. At this time, we also created an Ffvel1 over-expressing mutant (designated OE::Ffvel1) by transforming a vector containing Ffvel1 fused to the F. fujikuroi glnA promoter (from the glutamine synthase gene glnA; Teichert et al., 2004) into the wild-type IMI58289 strain. The glnA promoter provides constitutive high expression under nitrogen limiting conditions. Therefore, all strains were grown under the same nitrogen limiting conditions used for the microarray analysis.
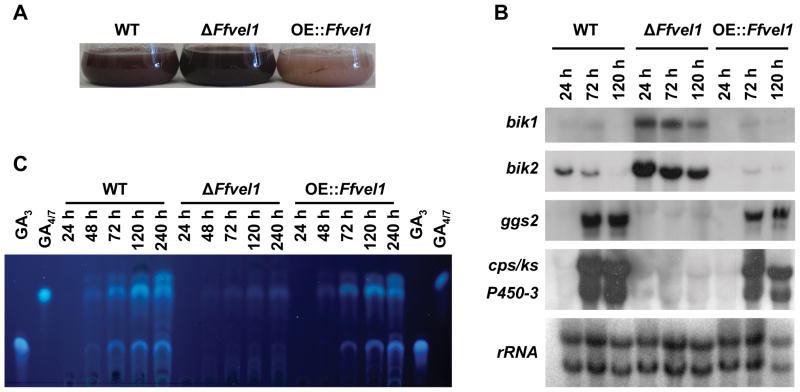
The culture broth of the ΔFfvel1 mutant appeared significantly more red-pigmented at all time-points than the wild type, while the OE::Ffvel1 strain showed significantly less coloration (Fig. 4A). Previously, we determined that the pigment responsible for this red color is the polyketide bikaverin (Wiemann et al., 2009). The deregulation of bikaverin production in the Ffvel1 knock-out and over-expressing strains was also detected in studies of the bikaverin cluster genes (shown for bik1 and bik2) which were significantly upregulated at all time-points in the ΔFfvel1 mutant and downregulated in the OE::Ffvel1 strain (Fig. 4B). In contrast to the bikaverin biosynthesis genes, expression of the GA cluster genes (shown for ggs2, cps/ks, and P450-3; Tudzynski, 2005) was not detected in the ΔFfvel1 mutant compared to the wild type (Fig. 4B) with a concomitant loss of GA4/7 and GA3 production (Fig. 4C).
Figure 4. Gibberellin and bikaverin analysis in the Ffvel1 knock-out and overexpression strain OE::Ffvel1 compared to the F. fujikuroi wild type IMI58289.
A Photographs of the F. fujikuroi wild-type strain IMI58289, the Ffvel1 knock-out strain and the overexpression strain OE::Ffvel1 grown in 10 % ICI cultures for 3 days.
B Expression of selected gibberellin and bikaverin genes in the wild type, the Ffvel1 knockout and the OE::Ffvel1 overexpression mutant. The strains were grown for the indicated time in 10 % ICI medium. The northern blot was hybridized with the indicated probes. 28S and 18S rRNA was visualized by EtBr staining as control.
C Thin layer chromatogram of the F. fujikuroi wild-type strain IMI58289, the Ffvel1 knockout strain and the overexpression strain OE::Ffvel1 grown in 10 % ICI cultures for the indicated time. GA4/7 and GA3 were used as standards (for details see Experimental procedures).
In comparison to GA and bikaverin biosynthesis, little is known about fusarin C and fumonisin biosynthesis in F. fujikuroi. In order to elucidate the influence of FfVel1 on fumonisin production, the wild type and the ΔFfvel1 mutant were grown on cracked corn media for 10 days, extracted and analyzed by liquid chromatography-mass spectrometry (LC-MS). Consistent with the microarray data, the ΔFfvel1 mutant accumulated significantly less (2 %) fumonisin B2 (FB2) as compared to the wild type and did not produce any detectable fumonisin B1 (FB1) (Table 1). For fusarin C analysis, the wild type and the ΔFfvel1 mutant were cultivated under restrictive (10 % ICI) and excessive (100 % ICI) nitrogen conditions, before extraction and LC-MS/MS analysis. Like the major effect of FfVel1 on GAs, bikaverin and fumonisin, there was a significant difference between relative amounts of fusarin C produced by the wild type and the ΔFfvel1 mutant under nitrogen sufficient conditions (100 % ICI medium) that paralleled with the expression patterns of three putative fusarin C cluster genes (FVEG_11079, FVEG_11080 and FVEG_11082) (Table 1 and Fig. S3). We noted, with interest, that fusarin C production was drastically reduced when the fungus was grown under nitrogen-limiting (10 % ICI) conditions (Table 1). This is in contrast to the production of GAs, bikaverin and fumonisin for which nitrogen-limiting conditions are essential for biosynthesis (reviewed in Tudzynski 1999; Wiemann et al., 2009; Kim and Woloshuk, 2008).
Table 1.
Production of fumonisins and fusarin C by the F. fujikuroi wild-type strain IMI58289 and the ΔFfvel1 mutant
| strain | fumonisins [ng/μl] | fusarin C [%] | ||
|---|---|---|---|---|
| FB1 | FB2 | − Na | + Nb | |
| WT | 0.17 (± 0.06) | 1.19 (± 0.60) | 1.50 (± 0.2) | 100 (± 10.9) |
| ΔFfvel1 | n.d. | 0.02 (± 0.01) | 4.71(± 2.6) | 43.96 (± 12.9) |
10 % ICI;
100 % ICI;
n.d. = not detectable
Experiments were carried out in duplicate; values in brackets show standard deviations.
Does FfVel1 interfere with environmentally attuned regulation mechanisms?
For GA gene expression, the GATA-type transcription factor AreA is known to be a key activator upon nitrogen starvation in F. fujikuroi (Mihlan et al., 2003) whereas bikaverin gene expression is dependent on the positively acting pathway-specific Zn(II)2Cys6 transcription factor Bik5 (Wiemann et al., 2009). Bikaverin gene expression is repressed under nitrogen sufficient conditions by an enigmatic mechanism which is different from the canonical AreA-dependent nitrogen regulation and by the C2H2 transcription factor PacC in response to ambient pH (Wiemann et al., 2009). To test whether deletion of Ffvel1 would affect the environmentally attuned specific regulation of both secondary metabolite gene clusters in F. fujikuroi, expression studies varying nitrogen concentration and ambient pH were performed.
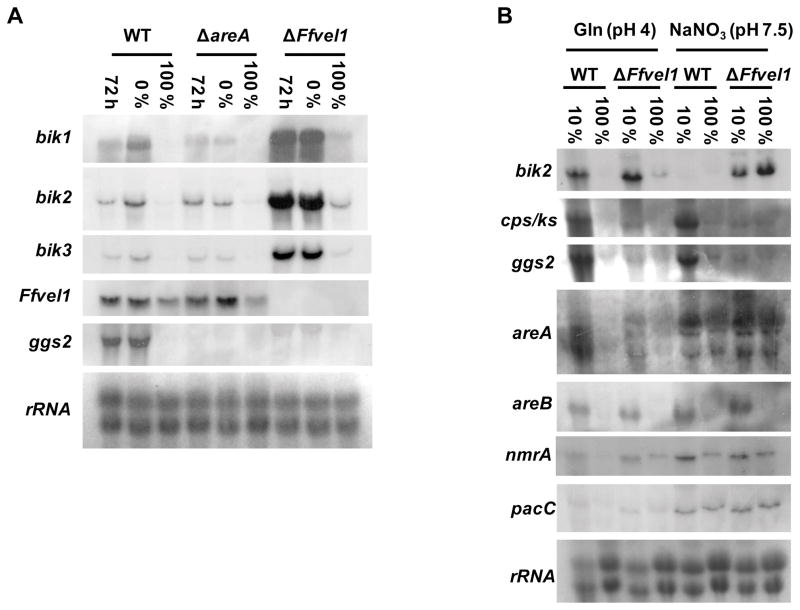
First, we tested whether expression of Ffvel1 was affected in the areA deletion mutant. We found that Ffvel1 transcript levels did not differ significantly between the wild type and the areA mutant and was dependent on nitrogen concentration. A significantly weaker signal for Ffvel1 was observed when both strains were shifted into excessive nitrogen conditions (100 % ICI) compared to nitrogen-free conditions (0 % ICI) indicating that Ffvel1 itself is subject to nitrogen regulation in an AreA-independent manner (Fig. 5A). The loss of Ffvel1 or areA affected GA and bikaverin synthesis differently. As expected, expression of the GA biosynthetic gene ggs2 was significantly downregulated in both the Ffvel1 and areA deletion mutants compared to the wild type (Fig. 5A and B). Interestingly, no significant alteration in gene expression of key components of the nitrogen regulation network (areA, areB and nmrA) were observed in the ΔFfvel1 mutant (Fig. 5B). In contrast to GA gene expression, signal intensities of the bikaverin cluster genes bik1-3 were significantly stronger in the ΔFfvel1 mutant compared to the wild type and the areA deletion strain (Fig. 5A). It is worth noting that the expression of these bik genes was still detected in the ΔFfvel1 mutant with excessive nitrogen (100 % ICI) in contrast to the wild type where no signal was observed (Fig. 5A and B). Even more surprising, in the ΔFfvel1 mutant, bik2 expression was detected in neutral to alkaline media containing NaNO3, although expression of pacC was not changed compared to the wild type (Fig. 5B). Taken together, these data indicate that FfVel1 may partially overcome nitrogen and pH repression of bikaverin genes and modulate the AreA-dependent activation of GA genes.
Figure 5. Influence of nitrogen availability and ambient pH on gene expression in a Ffvel1 knock-out strain compared to the F. fujikuroi wild type IMI58289.
A Expression of bik1-3, cps/ks, ggs2 and Ffvel1 in the wild type, the Ffvel1 and areA knockout mutants. The strains were grown for 72 h in 10 % ICI medium, washed and shifted into 0 % ICI or 100 % ICI medium, respectively, for 2 h. 28S and 18S rRNA was visualized by EtBr staining as control.
B Expression of bik2, cps/ks, ggs2, areA, areB, nmrA and pacC in the wild type and the Ffvel1 knock-out mutant. The strains were grown for 72 h in 10 % ICI or 100 % ICI medium containing glutamine or NaNO3, respectively. 28S and 18S rRNA was visualized by EtBr staining as control.
Expression of the F. fujikuroi laeA-like gene is regulated by FfVel1
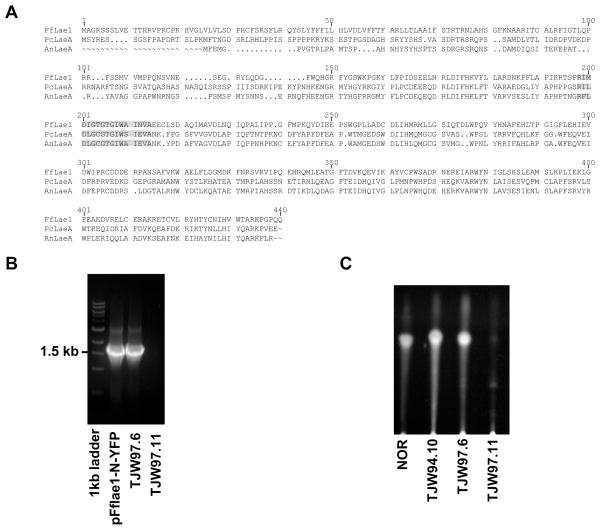
One of the most interesting differentially expressed genes found by microarray analysis shares significant similarity to the A. nidulans laeA gene, encoding a key regulator of secondary metabolism (Bok and Keller, 2004). So far, no laeA homolog has been identified for fungi outside the class of Eurotiomycetidae. The genomic fragment of the F. fujikuroi laeA homolog was cloned and sequenced. RT-PCR analysis revealed an ORF of 1266 bp. The ORF is interrupted by eight introns of 34 bp, 60 bp, 51 bp, 49 bp, 56 bp, 50 bp, 47 bp and 57 bp, respectively. The gene encodes a predicted protein of 421 amino acids and was designated FfLael1 (FN548141). BLASTp analysis indicates that FfLael1 shares 33 % amino acid identity to both LaeA (AAQ95166) from A. nidulans and PcLaeA (ACD50375) from P. chrysogenum. A pairwise alignment using ClustalW of these three LaeA homologs showed a highly conserved region which has been described as a HemK motif and is associated with proteins of the S-adenosyl-L-methionine (SAM)-dependent methyltransferase superfamily (Fig. 6A).
Figure 6. Multiple sequence alignment of FfLae1 with other characterized LaeA homologous sequences and restoration of secondary metabolism in A. nidulans ΔlaeA by Fflae1.
A The alignment was generated using ClustalW. Putative HemK domains are marked in gray. Sequences aligned are: LaeA (AAQ95166) from A. nidulans, PcLaeA (ACD50375) from P. chrysogenum, and FfLae1 (FN548141) from F. fujikuroi.
B Confirmation of heterologous complementation in A. nidulans by PCR. pFflae1-N-YFP: a plasmid containing the F. fujikuroi lae1 gene used for complementation of A. nidulans ΔlaeA strain RJW33.2, TJW97.6: RJW33.2 transformed with F. fujikuroi lae1, TJW97.11: RJW33.2 transformed with trpC marker gene.
C Confirmation of heterologous complementation in A. nidulans by TLC. NOR was used as a standard for TLC. TJW97.6 produces similar amounts of NOR as TJW94.10, an A. nidulans laeA over-expressed strain. Negative control TJW97.11 (ΔlaeA) can produce a small amount of NOR.
In order to confirm that FfLae1 is an ortholog of A. nidulans LaeA we complemented an A. nidulans laeA deletion mutant with a Fflae1 gene copy. Transformants containing the entire F. fujikuroi gene were confirmed by PCR (Fig. 6B) and checked for restoration of norsolorinic acid (NOR, a visible orange precursor of sterigmatocystin) biosynthesis. As shown in Figure 6C, NOR production was clearly restored in the Fflae1 transformant.
FfVel1 and FfLae1 are localized in the nucleus and physically interact
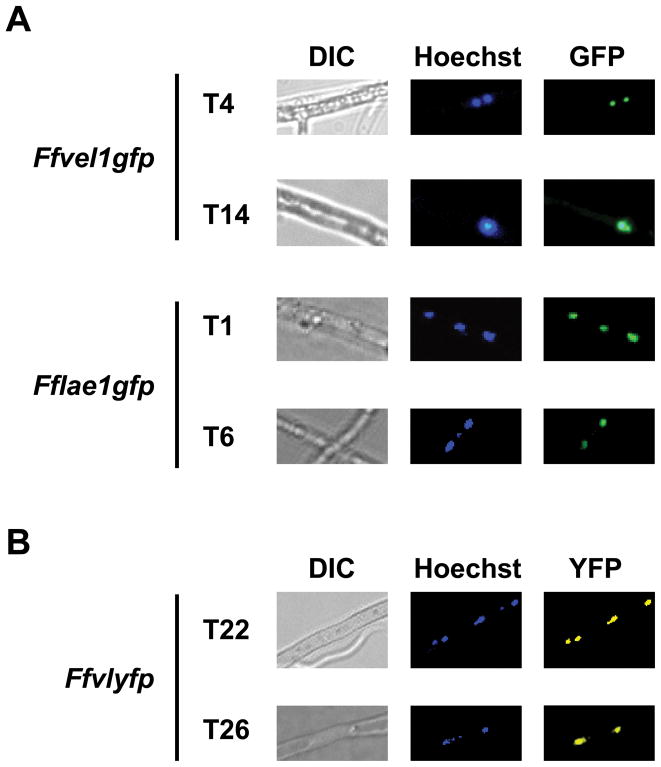
In order to investigate subcellular localization of FfLae1 and FfVel1, we constructed strains Ffvel1gfp and Fflae1gfp, harbouring a wild-type copy of Ffvel1 or Fflae1 fused to gfp, respectively. Three independent transformants containing either construct were verified by PCR (data not shown) and termed Ffvel1gfp-T4, -T14 and -T15 and Fflae1gfp-T1, -T6 and -T8. By using an epifluorescence microscope we showed that FfVel1 and FfLae1 were localized in the nucleus (Fig. 7A). We looked for evidence of a physical interaction between FfVel1 and FfLae1 using BiFC, recently established for filamentous fungi (Hoff and Kück, 2005). Our microscopic analysis in three independent transformants (e.g. Ffvlyfp-T22, -T26 and -T33), carrying FfVel1 tagged with C-terminal YFP and FfLae1 tagged with N-terminal YFP, showed that the two proteins interact in the nucleus in F. fujikuroi (Fig. 7B). Complementation of the Ffvel1 and Fflae1 deletion mutants with the plasmids containing the respective gene fusions restored wild-type features regarding sporulation (data not shown), indicating that the tagged gene products remain functional.
Figure 7. Localization and interaction studies of FfVel1 and FfLae1 in F. fujikuroi.
A Microscopic analysis of strains Ffvel1gfp and Fflae1gfp. The strains were grown for 3 days in 10 % ICI in the dark. Samples were stained with Hoechst 33342 and analyzed using an epifluorescence microscope as described in Experimental Procedures.
B Microscopic analysis strains Ffvlyfp carrying Ffvel1 fused to the C-terminal part of YFP and Fflae1 fused to the N-terminal part of YFP. The strains were grown for 3 days in 10 % ICI in the dark. Samples were stained with Hoechst 33342 and analyzed using an epifluorescence microscope as described in Experimental Procedures.
Does the laeA-like target gene of FfVel1 have similar functions as LaeA in A. nidulans?
To examine if the newly identified F. fujikuroi laeA-like gene is similar in function to LaeA in A. nidulans, we generated a knock-out mutant by transforming a double stranded fragment of DNA, containing a hygromycin (hyg) resistance cassette flanked by 1 kb non-coding regions of Fflae1, into F. fujikuroi strain IMI58289. Double homologous gene replacement events were identified by PCR (data not shown) and ectopic integration events were excluded by Southern hybridization (Fig. S1B) leaving strains ΔFflae1-T48, -T53 and -T55. As all mutants exhibited the same phenotype, strain ΔFflae1-T48 was arbitrarily chosen for further work.
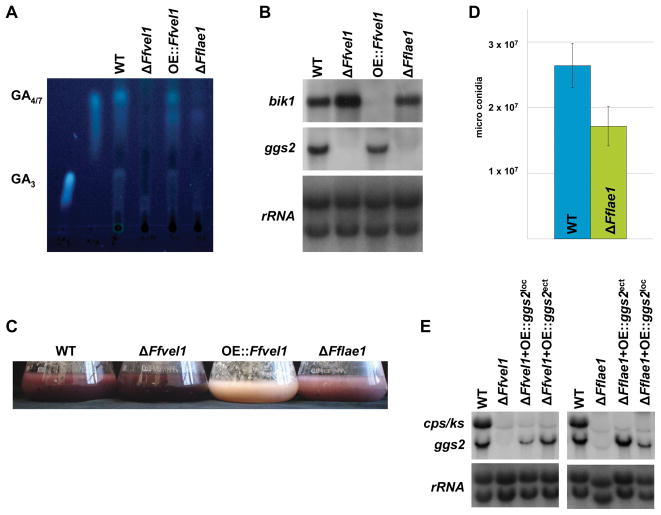
GA and bikaverin production by the ΔFflae1 and ΔFfvel1 mutants and the wild type were examined after growth in 10 % ICI media for 3 days. GA3 and GA4/7 production by the Δ Fflae1 mutant was significantly decreased compared to the wild type and was similar to the ΔFfvel1 mutant (Fig. 8A). The quantity of GAs produced by each strain roughly paralleled the expression levels of the GA cluster genes examined (Fig. 8B) suggesting that FfLae1 and FfVel1 are activators of GA production. In contrast, the pigmentation of the ΔFflae1 mutant was slightly reduced compared to the wild type while it was significantly increased in the ΔFfvel1 mutant (Fig. 8C). Expression analysis of bik1 paralleled pigmentation and further confirmed that FfLae1 does not affect bikaverin production as severely as GA production and in opposite manner to FfVel1. We also observed a reduction in microconidia formation by the Fflae1 knock-out mutant compared to wild type of about 30 % (Fig. 8D).
Figure 8. Analysis of the Fflae1 knock-out strains compared to the F. fujikuroi wild type IMI58289 in regard to secondary metabolism and conidiation.
A Thin layer chromatogram of the F. fujikuroi wild-type strain IMI58289, the Ffvel1 and Fflae1 knock-out strains as well as the overexpression strain OE::Ffvel1 grown in 10 % ICI cultures for 7 days. GA4/7 and GA3 were used as standards (for details see Experimental procedures).
B Expression of bik1 and ggs2 in the wild type, the Ffvel1 and Fflae1 knock-out strains as well as the OE::Ffvel1 overexpression mutant. The strains were grown for 3 days in 10 % ICI medium. 28S and 18S rRNA was visualized by EtBr staining as control.
C Photographs of the F. fujikuroi wild-type strain IMI58289, the Ffvel1 and Fflae1 knockout strains as well as the overexpression strain OE::Ffvel1 grown in 10 % ICI cultures for 3 days.
D Spores produced by the F. fujikuroi wild-type strain IMI58289 and the Fflae1 knock-out strain grown for 10 days on V8 solidified media under constant light conditions. Experiment was carried out on triplicate; bars show standard deviations.
E Expression of ggs2 and cps/ks in the wild type, the Ffvel1 and Fflae1 knock-out strains as well as in strains expressing ggs2 in the GA gene locus (ΔFfvel1+OE::ggs2loc and ΔFflae1+OE::ggs2loc) or ectopically (ΔFfvel1+OE::ggs2ect and ΔFflae1+OE::ggs2ect), respectively. The strains were grown for 3 days in 10 % ICI medium. 28S and 18S rRNA was visualized by EtBr staining as control.
In order to investigate if FfLae1 affects chromatin modification as suggested for A. nidulans (Reyes-Dominguez et al., 2010), a plasmid containing the GA cluster gene ggs2 under the control of the strong glnA promoter (pglnAprom::ggs2) was integrated into the Fflae1 deletion strain either ectopically or at the GA cluster locus (confirmed by PCR, data not shown). As FfLae1 and FfVel1 were shown to interact in the nucleus and similarly affect GA production, plasmid pglnAprom::ggs2 was also integrated into the ΔFfvel1 mutant in a similar manner. Northern blot analyses revealed that integration of the plasmid into the original GA cluster locus resulted in a much weaker expression of ggs2 compared to the signal obtained from ectopically integrated plasmids (Fig. 8E). Taken together, our data support a mechanism in F. fujikuroi where the velvet-like complex controls gene expression of defined regions in the fungal genome.
Are velvet and LaeA proteins of different ascomycetes functionally conserved?
In order to further investigate whether the distinct roles of FfVel1 and FfLae1 on secondary metabolism and fungal morphology in F. fujikuroi can also be executed by homologs from other ascomycetes, we performed heterologous complementation of Ffvel1 and Fflae1 knock-out strains with constructs containing PcvelA and PclaeA from P. chrysogenum, respectively. Transformants were selected using nourseothricin and integration of the heterologous genes was confirmed by PCR yielding strains Ffvel1CPcvelA-T1 and -T2 as well as FflaeACPclaeA-T1, -T2 and -T3 (data not shown).
Transformants containing the P. chrysogenum genes were grown on solidified V8 medium. PcVelA almost fully restored the ability to produce aerial hyphae in the Ffvel1 mutant (Fig. S4A). In order to investigate if the drastic reduction of microconidia formation observed in Ffvel1 and Fflae1 knock-out mutants could be rescued by the P. chrysogenum velA and laeA constructs, the strains were grown for 10 days under microconidia inducing conditions. Interestingly, PcvelA enabled the Ffvel1 mutants to form approximately 50% of the amount of microconidia produced by the wild type (Fig. S4B), while PclaeA restored microcondia formation to wild-type level in the FflaeA mutants (Fig. S4B). Despite the morphological rescue achieved by PcVelA and the restoration of microconidia formation achieved by PcVelA and PcLaeA, the production of GAs could not be restored, neither by PcVelA nor by PcLaeA (Fig. S4C).
Finally, a Y2H experiment was executed to assess the velvet complex formation of heterologous proteins. Results from β-galactosidase tests showed a strong interaction of FfVel1 with AnLaeA (Fig. S5).
The velvet-like protein FfVel2 controls processes of development and secondary metabolism similar to FfVel1
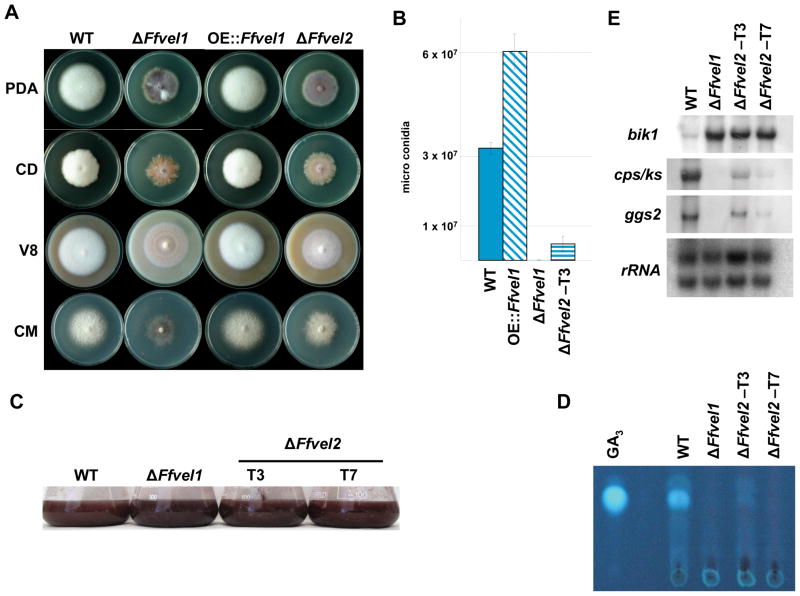
In A. nidulans a second velvet-like protein, VelB, is also part of the velvet complex (Bayram et al., 2008b). Therefore, we identified the F. fujikuroi homolog and characterized the FfvelB mutants with respect to morphology and secondary metabolism. Sequencing of the genomic fragment and cDNA clones of the F. fujikuroi velB homolog revealed an ORF of 1389 bp. The ORF is interrupted by three introns of 61 bp, 57 bp and 69 bp, respectively. The gene encodes a predicted protein of 482 amino acids and was designated FfVel2 (FN675836). BLASTp analysis indicates that FfVel2 shares 49 % amino acid identity to VelB (ABQ17967) from A. nidulans. Knock-out mutants were obtained by transforming a fragment of DNA, containing a hygromycin (hyg) resistance cassette flanked by 1 kb non-coding regions of Ffvel2, into F. fujikuroi strain IMI58289. Homologous integration events were identified by PCR (data not shown) and additional ectopic integration of the replacement cassette was excluded by Southern hybridization (Fig. S6) leaving strains ΔFfvel2-T3, and –T7.
Comparing growth of Ffvel1 and Ffvel2 deletion strains with the wild type and the Ffvel1 over-expressing mutant OE:Ffvel1 on different media, both deletion mutants exhibited similar defects of aerial hyphae formation (Fig. 9A) and significantly reduced microconidia formation compared to the wild type, while OE::Ffvel1 produced significantly more microconidia (Fig. 9B). Northern analyses revealed similar effects on bikaverin and GA gene expression in the Ffvel1 and Ffvel2 deletion mutants compared to the wild type. In the ΔFfvel2 mutants, the first gene of the bikaverin pathway, bik1, was upregulated similar to the situation in the Ffvel1 mutant with concomitant darker coloration of the culture fluid (Fig. 9C and D). Regarding GA production, deletion of Ffvel2 resembled the restricted GA3 production and gene expression of the ΔFfvel1 mutant (Fig. 9C and E). These data suggest that FfVel1 and FfVel2 coordinate similar processes regarding sporulation and secondary metabolite production in F. fujikuroi.
Figure 9. Phenotypical and secondary metabolite analysis in the Ffvel2 knock-out strain compared to the F. fujikuroi wild type IMI58289.
A Photographs of the F. fujikuroi wild type strain IMI58289, the Ffvel1 and Ffvel2 knockout strain and the overexpression strain OE::Ffvel1 grown on different solidified media for 10 days under constant dark conditions. V8 = vegetable juice; CM = complete medium; PDA = Potatoe Dextrose Agar; CD = Czapek Dox (for details see Experimental Procedures).
B Spores produced by the F. fujikuroi wild type strain IMI58289, the Ffvel1 and Ffvel2 knock-out strains and the overexpression strain OE::Ffvel1 grown for 10 days on V8 solidified media under constant light conditions. Experiment was carried out in triplicate; bars show standard deviations.
C Expression of selected gibberellin and bikaverin genes in the wild type and the Ffvel1 and Ffvel2 knock-out strains. The strains were grown for the indicated time in 10 % ICI medium. The northern blot was hybridized with the indicated probes. 28S and 18S rRNA was visualized by EtBr staining as control.
D Photographs of the F. fujikuroi wild type strain IMI58289, the Ffvel1 and Ffvel2 knock-out strains grown in 10 % ICI cultures for 3 days.
E Thin layer chromatogram of the F. fujikuroi wild type strain IMI58289 and the Ffvel1 and Ffvel2 knock-out strains grown in 10 % ICI cultures for the indicated time. GA3 was used as standard (for details see Experimental procedures).
FfVel1, Ffvel2 and FfLae1 are virulence factors
To test whether the deletions of Ffvel1 and/or Fflae1 have an influence on virulence of F. fujikuroi on rice, virulence assays were performed with 7 day-old seedlings. The seedlings, grown in glass tubes filled with Vermiculite, were inoculated with agar plugs from plates containing the wild-type, ΔFfvel1, Ffvel1C or ΔFflae1 strains. Seedlings inoculated with the GA-deficient strain Δcps/ks or with sterile water served as negative controls while seedlings inoculated with water containing GA3 served as a positive control. After two weeks, those plants infected with the wild-type and the Ffvel1C strains showed the typical bakanae symptoms like etiolation of the whole plant with chlorotic stems and leaves similar to the positive control (Fig. S7). In contrast, the water control and the plants infected with Δcps/ks, ΔFfvel1, ΔFfvel2 and ΔFflae1 looked similar to each other with normal height and dark green stems and leaves (Table 2 and Fig. S7). These findings clearly show that FfVel1, FfVel2 and FfLael1 are virulence factors of F. fujikuroi during infection, most likely due to their role as activators for GA biosynthesis.
Table 2.
Length of rice seedlings (Oryza sativa, L.) infected with agar plugs of different F. fujikuroi strains
| strain | length [cm] |
|---|---|
| H2O | 21 (± 0.82) |
| WT | 28.55 (± 0.67) |
| Δcps/ks | 16.9 (± 0.14) |
| ΔFfvel1 | 17.48 (± 1.65) |
| Ffvel1C | 23.63 (± 1.11) |
| ΔFfvel2 | 21.5 (± 0.99) |
| ΔFflae1 | 19.4 (± 1.82) |
| GA3 | 37.95 (± 1.91) |
Experiments were carried out in triplicate; values in brackets show standard deviations.
Discussion
Recent studies in several fungi have conclusively shown that the conserved VeA protein plays a major role in fungal growth, hyphal and colony morphology, development and secondary metabolism. However, the mode of action of VeA may differ in different fungi. Thus, it can act as positive or negative regulator of sexual and asexual differentiation and mainly as positive regulator of secondary metabolism (reviewed in Calvo, 2008). In A. nidulans, VeA is part of a nuclear protein complex together with LaeA, a putative methyltransferase, which is proposed to affect secondary metabolism by chromatin remodelling (Bok and Keller, 2004; Bok et al., 2006a; b; Bayram et al., 2008b; Reyes-Dominguez et al., 2010). In addition, this complex can include the blue light (LreA and LreB) and red light (phytochrome FphA) receptor proteins leading to the hypothesis that VeA serves as a scaffold protein that senses external light by interaction with light-sensing proteins (Purschwitz et al., 2009) and integrates the light signals with a nuclear response by an orchestrated action with other proteins such as LaeA (Calvo, 2008).
F. fujikuroi is an industrially important fungus used for the production of GAs in large scale with a yearly market value exceeding $100 million (Rademacher, 1997). Understanding the regulatory mechanisms affecting GA production as well as co-occurring and devaluing other secondary metabolites is therefore of great importance. To clarify the involvement of FfVel1 in regulation of secondary metabolism, growth and development, we generated Ffvel1 knock-out and over-expression mutants and searched for velvet target genes by microarray analysis.
Ffvel1 expression is affected by nitrogen availability
A general tenant of veA is that it is constitutively expressed. For example, in A. nidulans, veA transcripts were detected in all growth stages tested (Kim et al., 2002), and in N. crassa, the veA transcripts were detected in all different developmental stages and light conditions tested (Bayram et al., 2008a). Initial evidence in F. fujikuroi was consistent with this tenant. We found that the expression of Ffvel1 was not altered over a time course of 5 days (Table 1). However, we did find that expression of Ffvel1 is significantly affected by nitrogen. Its expression was repressed in media containing high amounts of nitrogen (60 mM glutamine). It will be interesting to determine if veA expression is similarly affected by nitrogen in A. nidulans and N. crassa. A link between nitrogen source, conidiation and veA has been described in A. fumigatus. In this case, deletion of veA led to a more pronounced decrease of asexual spore formation when the fungus was grown on nitrate instead of ammonium as the sole nitrogen source (Krappmann et al., 2005). Taken together, these results suggest that these fungi share many aspects of the veA regulatory mechanism.
FfVel1 affects sexual and asexual development
Although the velvet proteins are highly conserved throughout the ascomycetes, the effects on sporulation seem to vary significantly. In A. nidulans and N. crassa, deletion of veA led to a significant increase of conidia formation (Kim et al., 2002; Kato et al., 2003; Bayram et al., 2008a). In contrast, in P. chrysogenum (Hoff et al., 2010), A. parasiticus (Calvo et al., 2004), and A. fumigatus (Krappmann et al., 2005) deletion of veA led to a decrease in conidia formation. Similarly, a decrease of microconidia formation on solid V8 medium was observed in vel1 mutants of F. verticillioides (Li et al., 2006) and F. fujikuroi (this paper, Fig. 2C and 9B). The link between microconidia production and vel1 in F. fujikuroi may be via the F. fujikuroi homologs of the A. nidulans genes fluG and flbC which are known to be important for conidiation in A. nidulans (reviewed in Adams et al., 1998) and in Podospora anserina (Coppin et al., 2002). Both F. fujikuroi genes (designated FffluG and FfflbC) are significantly downregulated in the Ffvel1 knock-out strain (Figs. 3D, S2 and Table S1). In A. nidulans flbC encodes a C2H2-type transcriptional activator that has been shown to be a central regulator of conidiophore development prior to BrlA (Adams et al., 1998). Since no obvious homologs of BrlA appear to exist in the genomes of the four sequenced Fusarium species, it would be interesting to further investigate the role of FfFluG and FfFlbC in conidiogenesis in F. fujikuroi. Interestingly, deletion of Ffvel2 in F. fujikuroi resulted in a similar reduction of microconidia formation, suggesting that both velvet proteins feed into similar pathways regarding sporulation. The fact that overexpression of Ffvel1 led to a significant higher production of microconidia (Fig. 9) indicates that FfVel1 is an activator of asexual development in F. fujikuroi in contrast to its function in A. nidulans and N. crassa (Kim et al., 2002; Kato et al., 2003; Bayram et al., 2008a). Furthermore, deletion of Ffvel1 increased perithecia formation significantly when the mutant strain was crossed with a corresponding mating partner (Fig. 2C), suggesting that FfVel1 acts as repressor of sexual development. Sexual crosses with both mating types bearing a Ffvel1 deletion would verify this observation. Deletion of veA abolishes cleistothecial production in the homothallic genus A. nidulans but the effect of loss of this gene in N. crassa is unclear (Kim et al., 2002; Bayram et al., 2008a). Future work to understand better the role of velvet-family VeA proteins in asexual sporulation and sexual development may explain how VeA differentially affects those fundamental aspect processes of fungal development.
FfVel1and FfVel2 can act as positive regulators of secondary metabolism
Secondary metabolism throughout fungal genera has been shown to be positively regulated by velvet proteins. Here we show that the velvet homolog in F. fujikuroi positively regulates GA gene expression and product synthesis. Expression analysis, initially by microarray and confirmed by Northern blot, of a velvet deletion strain (ΔFfvel1) showed a drastic downregulation of GA cluster genes compared to the wild type. As expected, low levels of GA3 and GA4/7 were detected by TLC (Figs. 3, 4, 5, 9, S2 and Table S1). Deletion of the second velvet-like gene, Ffvel2, paralleled the deficiency in GA3 production of the Ffvel1 deletion mutant (Fig. 9). The dramatic downregulation of secondary metabolite gene clusters is consistent with reports from Aspergillus spp. where VeA has been shown to activate production of ST in A. nidulans (Kato et al., 2003), AF, cyclopiazonic acid and aflatrem in A. flavus (Amaike and Keller, 2009; Duran et al., 2007) as well as AF in A. parasiticus (Calvo et al., 2004). Similar to our results, cephalosporin and PN production is impaired in velvet knock-out strains in A. chrysogenum and P. chrysogenum, respectively (Dreyer et al., 2007; Hoff et al., 2010).
Similar to GA production, formation of the polyketides FB1 and FB2 is reduced in ΔFfvel1 strains (Table 1). The limited fumonisin production was also observed recently by velvet knock-out strains in the related fungus F. verticillioides (Myung et al., 2009). This is the first report of a velvet protein to positively affect production of a terpene and a polyketide-derived secondary metabolite in one organism.
Myung et al., (2009) also reported that biosynthesis of fusarin C, a PKS/NRPS-derived metabolite in F. verticillioides, is completely blocked when grown on cracked corn. In F. fujikuroi, we detected a significant reduction (about 50 %) of fusarin C as well as a slight downregulation of four putative fusarin C biosynthesis genes under high nitrogen conditions in submerged medium (Table 1, Fig. S3).
FfVel1and FfVel2 can act as negative regulators of secondary metabolism
We were most intrigued to observe an increase in expression of the bikaverin biosynthesis genes and bikaverin production by the ΔFfvel1 strain compared to the wild type (Figs. 3 4, 5 and 9). Bikaverin formation and bik gene expression is known to be subject to a complex regulatory network and is affected by nitrogen source, pH and age of culture (Wiemann et al., 2009). Deletion of Ffvel1 not only led to an upregulation of bikaverin genes at early time points, but also to a much longer duration of expression as compared to the wild type (Table 1, Figs. 3 and 4B). Previously, we found that nitrogen and a neutral to alkaline ambient pH represses bikaverin gene expression. Interestingly, in ΔFfvel1 mutants bik gene expression is still detectable under unfavored pH conditions despite an unchanged expression of pacC (Fig. 5), which was shown to encode for a repressor of bik genes (Wiemann et al., 2009). Furthermore, bik gene expression was also observed under nitrogen repressing conditions in the ΔFfvel1 mutant although the expression of genes coding for key components of the nitrogen regulation network (areA, areB, and nmr) was unchanged (Fig. 5). However, the partial de-repression of bikaverin gene expression in the Ffvel1 knock-out is much weaker in cultures that are amply supplied with nitrogen, indicating that nitrogen repression on one hand, and overexpression of bik genes in the Ffvel1 mutant on the other hand, act independently of each other (Fig. 5). Interestingly, overexpression of Ffvel1 does not lead to an enhanced GA gene expression (Fig. 4).
The complexity of the bikaverin regulation network regarding external signals suggests that de-repression of bikaverin genes is a secondary effect of Ffvel1 deletion, due to a defective perception of environmental signals. The large group of FfVel1 target genes whose products are probably involved in transport supports this possibility. Furthermore, the evidence that FfVel1 seems to be involved in regulating only certain chromosomal regions (Fig. 8E), suggests a role of the velvet-like complex in chromatin remodelling as recently shown in A. nidulans (Bayram et al., 2008b; Reyes-Dominguez et al., 2010). Apart from global regulators, environmentally attuned regulators like AreA in A. nidulans were found to be involved in chromatin restructuring as well (Berger et al., 2008). In the future, we will elucidate the interconnection of the global regulator FfVel1 and repressors of bikaverin genes (e.g. PacC and a yet unknown nitrogen regulator) in order to see if restricted accessibility of repressors to the bikaverin gene cluster due to Ffvel1 deletion might be a reason for the observed de-repression in unfavored conditions.
Beside bikaverin biosynthesis in F. fujikuroi, a repressing effect of velvet on putative secondary metabolite gene clusters is also reported in P. chrysogenum (Hoff et al., 2010) as well as in A. nidulans, where veA deletion strains exhibit an increased production of a yet uncharacterized brown pigment (Bayram et al., 2008a) and therefore might be a common phenomenon in fungi.
Identification of a LaeA homolog in F. fujikuroi
Initial analysis of the microarray data identified an upregulated gene in the Ffvel1 knockout strain with significant similarity to LaeA, a global transcriptional regulator of secondary metabolism in A. nidulans. Recent reports that expression of laeA in A. nidulans, A. flavus and P. chrysogenum is negatively affected by velvet (Amaike and Keller, 2009; Bayram et al., 2008b; Hoff et al., 2009) support our hypothesis that we have identified the F. fujikuroi laeA homolog. Conclusive evidence came from complementation experiments, where the A. nidulans laeA deletion mutant was restored in NOR production by transformation with F. fujikuroi Fflae1 (Fig. 6C). Localization studies in F. fujikuroi also revealed a nuclear localization of a FfLae1-GFP fusion protein (Fig. 7A) and, even more interesting, evidence for a physical interaction with FfVel1 in the nucleus (Fig. 7B). This is consistent with the report that VeA and LaeA can form a complex in both A. nidulans and in P. chrysogenum (Bayram et al., 2008b; Hoff et al., 2009).
Deletion of Fflae1 resulted in significant reduction of GA and bikaverin gene expression and product formation (Fig. 8). This is consistent with reports from Aspergillus spp. where numerous secondary metabolic pathways are negatively affected in laeA knock-out mutants (Bok and Keller, 2004; Bouhired et al., 2006; Amaike and Keller, 2009; Kale et al. 2008; Lodeiro et al., 2009). The same effect on PN production was observed in the P. chrysogenum laeA deletion mutant (Hoff et al., 2009). In the future, it will be interesting to see if the velvet-like complex in Fusarium spp. is involved in chromatin remodelling of secondary metabolite cluster regions as recently shown in A. nidulans (Reyes-Dominguez et al., 2010).
velvet and LaeA proteins have common and distinct functions in heterologous systems
Homologs of veA and laeA in different ascomycetes generally control development and secondary metabolism (reviewed in Calvo, 2008; Hoff et al., 2010). This study presents evidence for a velvet-like complex in F. fujikuroi having influence on morphogenesis, virulence and secondary metabolism. To investigate whether these features are functionally conserved in ascomycetes, we performed complementation experiments transforming heterologous genes from P. chrysogenum into the F. fujikuroi mutants, and the F. fujikuroi lae1 gene into the A. nidulans mutant. The PcvelA gene was able to restore aerial hyphae formation and microconidia production in F. fujikuroi vel1 knock-out strains. Additionally, addback of laeA orthologs of P. chrysogenum into the F. fujikuroi lae1 deletion mutant and vice versa restored microconida production in F. fujikuroi and partial conidiation in P. chrysogenum (Hoff et al., 2010), proving a conservation of function in this aspect. Remarkably, F. fujikuroi Lae1 could also rescue production of PN when transformed into the P. chrysogenum laeA knock-out mutant (Hoff et al., 2010) as well as NOR production in the A. nidulans ΔlaeA (Fig. 6C). Our data also suggest that P. chrysogenum and F. fujikuroi homologs of VeA and LaeA are able to form heterologous complexes with each other similar to the complexes formed between VeA and LaeA homologs from A. nidulans and F. fujikuroi as shown by Y2H (Fig. S5). These heterologous complexes are able to restore morphological features in P. chrysogenum, A. nidulans and F. fujikuroi (Hoff et al., 2010 and this study). Additionally, F. fujikuroi Lae1 and Vel1 are able to restore secondary metabolism in P. chrysogenum (Hoff et al., 2010) and F. fujikuroi Lae1 can rescue NOR production in A. nidulans (Fig. 6C). However, restoration of secondary metabolism was not achieved when PcvelA and PclaeA were used to complement the F. fujikuroi deletion mutants, respectively. Therefore, it is likely that velvet complex homologs included species specific features that allow full functionality only in certain ascomycetes. This specificity is also observed for the VeA homolog Ve1 from F. verticillioides, which is not able to rescue the A. nidulans veA knock-out strain’s phenotype (Calvo, 2008), while Ve-1 from N. crassa can restore wild type sporulation processes (Bayram et al., 2008a).
In summary, we studied the influence of the velvet protein on secondary metabolism and development in the biotechnologically important fungus F. fujikuroi by microarray and chemical analytical methods and show an eclectic effect of FfVel1 on important secondary metabolites produced. Furthermore, the array data enabled us to identify an important component of the velvet-like complex, the putative methyltransferase FfLae1. Like FfVel1, FfLae1 has a major influence on both secondary metabolism and conidiation in F. fujikuroi. This is the first evidence of a velvet-like complex in the genus Fusarium that establishes novel features of its role in secondary metabolism, differentiation and virulence that will aid to the understanding of this fungal-specific protein complex in general. Significantly, the cross species complementation experiments described in this work argue for an ancient ancestry of the velvet complex in the fungal kingdom which has undergone a divergence in specific functions mediating development and secondary metabolism.
Experimental Procedures
Fungal strains and culture conditions
The wild-type strain F. fujikuroi IMI58289 (Commonwealth Mycological Institute, Kew, UK) was used as the parent strain for all knock-out experiments. The GA deficient strain Δcps/ks (Tudzynski et al., 1998) was used for rice infection experiments. F. fujikuroi strains C-1993 and C-1995 were obtained from J.F. Leslie (Kansas State University). For all cultures, F. fujikuroi was preincubated at 28°C for 48 h in 300 ml Erlenmeyer flasks with 100 ml Darken medium (DVK) (Darken et al., 1959) on a rotary shaker at 190 rpm. For RNA isolation, gibberellin bikaverin and fusarin C analysis, 0.5 ml DVK were used to inoculate ICI (Imperial Chemical Industries Ltd., UK) media (Geissman et al., 1966) containing 6 mM glutamine (10 % ICI medium) or 60 mM glutamine (100 % ICI medium). These cultures were incubated at 28°C on a rotary shaker at 190 rpm for 1, 2, 3, 5 or 10 days. For protoplasting, 0.5 ml of the starter culture was transferred into Erlenmeyer flasks with 100 ml complete medium (CM) (Pontecorvo et al., 1953) and incubated at 28°C on a rotary shaker at 190 rpm for 18 h. For DNA extraction, fungal strains were grown for 3 days at 28°C on cellophane sheets (Alba Gewürze, Bielefeld, Germany) placed on solidified CM. Additional solidified media used for plate assays of fungal strains were 20 % (v/v) vegetable juice (V8) (Campbell Foods, Puurs, Belgium) containing 30 mM CaCO3, 3.9 % (w/v), Potato Dextrose Agar (PDA) and 5 % (w/v) Czapek Dox (CD) Agar (both Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Sexual crossings were performed on carrot agar as described by Klittich and Leslie (1988). For fumonisin analysis, the fungal strains were grown on cracked maize medium as previously described (Brown et al. 2007). A. nidulans strains were grown on solid glucose minimal media (GMM) at 37°C as previously described (Bok and Keller, 2004).
Standard molecular methods
DNA and RNA analysis used standard techniques (Sambrook et al., 1989). Fungal DNA or RNA was prepared by first grinding lyophilized mycelium into a fine powder with a mortar and pestle and then dispersing it in extraction buffer as described by Cenis (1992). DNA for Southern hybridization experiments was prepared following the protocol of Doyle and Doyle (1990). For Southern blot analysis, genomic DNA was digested with the indicated restriction enzymes (Fermentas GmbH, St. Leon-Rot, Germany), fractionated in 1 % (w/v) agarose gels, and transferred to Nytran® nylon transfer membranes (Whatman Inc., Sanford, ME, USA) by downward blotting (Asubel et al., 1987). 32P-labelled probes were prepared using the random oligomer-primer method and membranes were hybridized according to the protocol of Sambrook et al., 1989.
Total F. fujikuroi RNA was isolated using the RNAgents total RNA isolation kit (Promega GmbH, Mannheim, Germany). Samples of 15 μg of total RNA were transferred to Hybond-N+ membranes after electrophoresis on a 1 % (w/v) agarose gel containing formaldehyde, according to Sambrook et al. (1989). Northern blot hybridizations were accomplished by the method of Church and Gilbert (1984). cDNA was synthesized from 1 μg of total RNA and the SuperScript II reverse transcriptase (Invitrogen, Groningen, The Netherlands) according to the manufacturer’s instructions.
All primers used for PCR were obtained from Eurofins GmbH (Ebersberg, Germany) (Table S1). PCR reactions contained 25 ng DNA, 5 pmol of each primer, 200 nM dNTPs, and 1 unit of BioTherm™ DNA polymerase (GeneCraft GmbH, Lüdinghausen, Germany) and were initiated with a 4 min soak at 94 °C followed by 35 cycles of 1 min at 94 °C, 1 min at 56 to 65 °C, 1–3 min at 70 °C, and a final soak for 10 min at 70 °C. PCR products were cloned into pCR®2.1-TOPO® vector using the TOPO TA Cloning® kit (Invitrogen, Groningen, The Netherlands) and transformed into Escherichia coli (Invitrogen).
Plasmid DNA from E. coli was extracted using the GeneJET™ Plasmid Miniprep Kit (Fermentas GmbH, St. Leon-Rot, Germany) and sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit and the ABI Prism® 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according the manufacture’s instructions. DNA and protein sequence alignments were done with DNA STAR (Madison, WI, USA). Sequence homology searches were performed using the NCBI database server. Protein homology was based on BlastX searches (Altschul et al., 1990).
Plasmid construction
The putative F. fujikuroi veA, velB and laeA genes were amplified using the primer pairs veA-F1/-R1 velB-F1/-R1 and laeA-F1/-R1 which were based on the putative F. verticillioides veA, velB and laeA sequences, respectively. The F. fujikuroi veA knock-out plasmid pΔveA was created by sequentially cloning the 0.8 kb 5′ Ffvel1 flank (generated with primers veA-5′-F1/-R1) and the 0.5 kb 3′ Ffvel1 flank (generated with primers veA-3′-F1/-R1) into pUCH2-8 (Alexander et al., 1999) such that the hygromycin resistance cassette was flanked by F. fujikuroi genomic sequence. The Ffvel1 over-expression plasmid pOE::veA was created by cloning, via a triple ligation, a 2.2 kb NotI/SacI fragment containing the Ffvel1 coding sequence and 0.5 kb terminator region (generated with primers veA-OE-F1 and veA-3′-R1 and digested with NotI and SacI) and the 0.5 XbaI/NotI fragment containing the F. fujikuroi glnA promoter (Teichert et al. 2004) (generated with primers glnA-XbaI-F1/glnA-NotI-R1 and digested with XbaI and NotI) into XbaI/SacI restricted pUCH2-8.
The plasmids pΔlaeA and pΔvelB were assembled using yeast recombinational cloning essentially as described (Colot et al., 2006). Briefly, the 5′ and 3′ flanks of Fflae1 and Ffvel2 were amplified using primer pairs “gene”-5′-F1/-R1 and “gene”-3′-F1/-R1, respectively. The hygromycin resistance cassette, consisting of the hygromycin B phosphotransferase gene hph (Gritz and Davies, 1983) driven by the trpC promoter, was amplified using the primer pair hph-F/-R and the plasmid pCSN44 (Staben et al., 1989) as a template. All three PCR fragments were cloned into Sacharomyces cerevisiae strain FY834 (Winston et al., 1995) together with the EcoRI/XbaI restricted plasmid pRS426 (Christianson et al., 1992) as essentially described (Gietz and Schiestl, 2007). Plasmid DNA from S. cerevisiae was extracted using the GeneJET™ Plasmid Miniprep Kit (Fermentas GmbH, St. Leon-Rot, Germany) with slight modifications: cells were resuspended in 300 μl Resuspension Solution plus 100 μl glass beads, lysed by addition of 600 μl Lysis Solution and neutralized with 450 μl Neutralization Solution. Transforming DNA to delete Fflae1 and Ffvel2 were prepared by PCR using primers “gene”-5′-F1 and laeA-3′-R1 and 1μl of pΔlaeA or pΔvelB, respectively, as template.
The plasmids pveAC and pveAΔpat4C were constructed using pNglnAProm (C. Bömke, personal communication) which is a derivate of pNR1 (Malonek et al. 2004). The plasmids pveAgfp and plaeAgfp were constructed using pIGI1783 which contains an egfp coding region driven by the A. nidulans gpd promoter region (Pöggeler et al., 2003). Using the two primer pairs veA-NcoI-F1/-R1 and Pgpd-laeA-F1/laeA-GFP-R1, the coding regions of Ffvel1 and Fflae1 were amplified, respectively, thereby changing the stop codons TAG and TAA, respectively, to TCC. The Ffvel1 NcoI fragment was cloned into NcoI restricted pNglnAProm, pIGI1783 and pEYFPC (Hoff and Kück, 2005) yielding pveAC, pveAgfp and pFfvel1-C-YFP, respectively. For creating plaeAgfp the Fflae1 fragment was cloned into NcoI restricted pIGI1783 using the In-Fusion™ CF Dry-Down PCR Cloning Kit (Clontech, Mountain View, CA, USA) after the manufacturer’s instructions. For creating pFflae1-N-YFP, the coding region of Fflae1 was amplified using the primers Pgpd-laeA-F1 and laeA-YFP-R1 (thereby exchanging the stop codon TAA for TCC) and ligated into NcoI restricted pEYFPN (Hoff and Kück, 2005) using In-Fusion™ cloning as described above. For creating a Ffvel1 sequence which did not include the pat4 NLS motif, two primer pairs (veA-NcoI-F1/veA-NotI-R1 and veA-NotI-F1/veA-Nco-R1) were used to create two NcoI/NotI fragments that were simultaneously cloned into NcoI restricted pNglnAProm, yielding pveAΔpat4C. The predicted VeA protein, encoded on pveAΔpat4C has a modified sequence where the endogenous Lys471Arg472Lys473His474 is changed to Ala471Ala472Ala473His474.
The overexpression plasmid pglnAprom::ggs2 was constructed using pUCH-N-glnA (Wiemann et al., 2009). The F. fujikuroi ggs2 gene was amplified using the primer pair ggs2-F1-Sal/ggs2-R1-Apa and subsequently ligated into Sal/Apa digested pUCH-N-glnA.
Fungal transformations
Preparation of protoplasts from F. fujikuroi mycelium was carried out as described (Tudzynski et al., 1999). Briefly, 107 protoplasts of strains IMI58289 were transformed with 10 μg of the replacement cassettes of the vectors pΔveA, pΔvelB and pΔlaeA, respectively, or with 10 μg of the over expression plasmid pOE::veA, or with 10 μg of the GFP fusion plasmids pveAgfp and plaeAgfp, respectively. For gene replacement of Ffvel1, Ffvel2 and Fflae1, over expression of Ffvel1, Ffvel1- and Fflae1-gfp fusion strains, transformed protoplasts were regenerated at 28°C in a complete regeneration agar (0.7 M sucrose, 0.05 % yeast extract, 0.1 % casamino acids) containing 120 μg/ml hygromycin B (Calbiochem, Darmstadt, Germany) for 6–7 days. For complementation of ΔFfvel1 strains, 10 μg of the plasmids pveAC and pveAΔpat4C were used for transformation as described above, but with 100 μg/ml nourseothricin (Werner-Bioagents, Jena, Germany) instead of hygromycin B. For BiFC strain construction, 107 protoplasts from the wild-type strain IMI58289 were transformed with pFfvel1-C-YFP/pFflae1-N-YFP or pFfvel1-C-YFP/pEYFPN and pFflae1-N-YFP/pEYFPC as negative controls and selected on 120 μg/ml hygromycin B and 100 μg/ml nourseothricin as described above. For complementation of ΔFfvel1 and ΔFflae1 strains with the corresponding genes from P. chrysogenum, 10 μg of pYNvelA and pYClaeA (Hoff et al., 2010) or with the F. fujikuroi gene containing plasmids pFfvel1-C-YFP or pFflae1-N-YFP were used, respectively, following the transformation described above using 100 μg/ml nourseothricin or 120 μg/ml hygromycin B, respectively, for selection. For over-expressing ggs2 in the ΔFfvel1 and ΔFflae1 strains, respectively, pglnAprom::ggs2 was used for transformation following the procedure described above using 100 μg/ml nourseothricin as selection marker.
Co-transformation with plasmids pFflae1-N-YFP and p14 (containing the pyroA allele, May et al., 1989) was performed to complement the A. nidulans laeA deletion strain RJW33.2 following previously described procedures (Bok and Keller, 2004).
The homologous integration events in transformants targeting replacement of Ffvel1, Ffvel2 or Fflae1 with a selectable marker were verified by PCR using a primer targeting the replaced coding region (“gene”-WT-F1/-R1) or outside the replacement fragment (veA-F1/pUCH-P and veA-R1/pUCH-T in case of ΔFfvel1 and “gene”-F1/pCSN44-trpC-T and “gene”-R1/pCSN44-trpC-P in case of ΔFflae1 and ΔFfvel2) and another primer located within the hygromycin resistance cassette. Homologous integration of pglnAprom::ggs2 in strains ΔFfvel1 and ΔFflae1, respectively, was verified by PCR using the primer pair GS-M/ggs2-D-F1 and were named ΔFfvel1+OE::ggs2loc and ΔFflae1+OE::ggs2loc, respectively. Mutants containing an ectopic integration of the plasmid did not yield a PCR signal using the primer pair GS-M/ggs2-D-F1 but revealed a signal using the primer pair GS-M/ggs2-R1-Apa and were named ΔFfvel1+OE::ggs2ect and ΔFflae1+OE::ggs2ect, respectively.
Random integration of pveAC and pveAΔpat4C, creating rescue strains Ffvel1C and Ffvel1CΔpat4, respectively, were verified using the primers veA-NcoI-F1 and mrfp-R1. To confirm integration of pveAgfp and plaeAgfp the primers gpd-F1 and gfp-R1 were used. Integration of pOE::veA was confirmed using primers GS-M and veA-3′-R1. The BiFC strains were screened using primers gdp-F1/EYFP-N-MCS and gdpd-F1/EYFP-C-MCS to confirm integration of both plasmids. Integration of pYNvelA and pYClaeA into the rescue strains Ffvel1CPcvelA and Fflae1CPclaeA, respectively, was confirmed using primers gpd-F1/EYFP-N-MCS- and gpd-F1/EYFP-C-MCS, respectively.
A. nidulans transformants, TJW97, were PCR screened for the presence of Fflae1 using the two primers Fwdfusar and Revfusar and simultaneously grown on oatmeal medium for a visual screen to look for restoration of NOR production.
Yeast two hybrid analysis
The yeast two hybrid plasmid pTLex3 (Cho et al., 2003) was modified by the Quick change technique (van den Ent and Löwe, 2006) using PfuUltra II fusion HS DNA polymerase (Stratagene). The bait plasmid with Ffvel1 cDNA was constructed using two Quick change primers (FveALexAFWD and FveALexAREV) to place Ffvel1 into pTLex3. Ffvel1-pTLex3 was co-transformed into the S. cerevisiae reporter strain L40 with either pGAD424 (empty prey vector) or with A. nidulans laeA cDNA in pGAD424 (Bayram et al., 2008b). Transformants were selected on -UTL (-leu, -trp, -ura) containing 2 % (w/v) glucose (SD) media. Ten transformants of each combination, YTJW7 (Ffvel1-pTlex3 plus pGAD424) and YTJW10 (Ffvel1-pTlex3 plus laeA prey), were tested for their coloration on -UTL medium containing X-Gal.
Expression microarrays and data analysis
Each Roche-NimbleGen (Madison, WI) array contained up to 13 unique 60 mer oligonucleotide probes per gene. The gene sequence dataset from which the oligos were designed included 12,514 F. verticillioides gene models derived from a gene call set obtained from the Broad Institute in January, 2008. Additional sequences unique to the F. verticillioides EST collection (Brown et al., 2005) were included bringing the total number of sequences represented on the array over 13,000. The microarray data is available from NCBI Gene Expression Omnibus (GEO) under the series accession number GSE21623.
F. fujikuroi mycelium was harvested from two individual cultures and RNA was extracted as described above. Cross species microarray hybridization, data acquisition and initial analysis were conducted by Roche NimbleGen, Iceland. Normalized data from the probe sets of the 12 arrays (two biological repetitions for each strain and time point) were compared using the Acuity 4.0 microarray analysis software package (Molecular Devices Corp, Sunnyvale, USA.) and the Excel® 15 macro collection FiRe (Garcion et al., 2006) essentially as described (Schönig et al., 2008). Briefly, only oligo sets with at least 2 fold up- or downregulation in both replicate hybridizations as compared between strains or time-points, were considered to be differentially regulated. The F. verticillioides genes corresponding to hybridizing oligo sets were analysed by BlastN against the EST database of a F. fujikuroi cDNA library (Teichert et al., 2004). BlastN matches of this cDNA library with e-value ≤1.00×10−100 were regarded as homologous. Both, F. verticillioides gene calls and F. fujikuroi ESTs were examined by BlastX against the non-redundant protein sequence NCBI database. BlastP searches against the MIPS functional database catalogue (http://mips.gsf.de) were performed in order to group the proteins into several functional categories.
Virulence assays
Seeds from a single plant of Oryza sativa spp. japonica c.v. Nipponbare were prepared and surface sterilized. Briefly, the husks of the seeds were removed and seeds were submerged in 70 % (v/v) ethanol for 1 min followed by three rinses with sterile H2O. Next, seeds were incubated in 4% (v/v) Ca(ClO)2 for 10 min and again rinsed in sterile H2O. The sterilized seeds were incubated on solidified H2O (15 g/l Agar) at 4 °C in the dark for 3 days and subsequently at 28°C at a 12 h light – 12 h dark cycle for 3 days. A 5 mm diameter mycelial plug of a V8 media plate with F. fujikuroi strains grown for 3 days on V8 media was transferred to a 3×20 cm test tube filled 25 % with Vermiculite (Deutsche Vermiculite Dämmstoff-GmbH, Sprockhövel, Germany) and covered with a 1 cm layer of Vermiculite. As negative control, an agar plug from solidified H2O was used instead of a fungal strain and 100 ppm GA3 were used as positive control. Germinated seeds were transferred to the prepared test tubes, covered with 1cm of Vermiculite and watered with 10 ml of a 3.16 g/l Gamborg B5 Medium (Duchefa Biochemie, Haarlem, The Netherlands) solution. The test tubes were then incubated at 28°C at a 12 h light – 12 h dark cycle for 7 days. Rice plants were cleaned from Vermiculite and measured from the base of the stem to the second nodule.
Gibberellic acid, fumonisin, fusarin C and norsolorinic acid analysis
The gibberellic acids GA3 and GA4/7 were extracted from 900 μl of F. fujikuroi culture filtrates, after acidification with 10 μl 25 % (v/v) HCl, with 910 μl ethyl acetate. The ethyl acetate fraction was then evaporated to dryness, dissolved in 20 μl 20 % (v/v) ethanol and applied to a 20×20 cm Silica gel 60 F254 TLC aluminium sheet (Merck KGaA, Darmstadt, Germany). Chloroform, ethyl acetate and acetic acid (57:38:5 %) (v/v/v) served as the mobile phase. The dried sheet was vaporized over 32 % (v/v) HCl, baked for 10 min at 110 °C and GAs were visualized using 254 nm UV light. For analysis of fumonisins, F. fujikuroi cultures on cracked maize medium were extracted as previously described (Brown et al., 2007) and quantified by liquid chromatography-mass spectrometry (LC-MS) as previously described (Plattner et al., 1996).
The fusarin C content in the wild type and the ΔFfvel1 mutant culture filtrates of ICI 100% N and ICI 10% N was analyzed by HPLC-MS/MS after purification on RP-18 material. After centrifugation with a Hettich-Universal 16 R centrifuge (Hettich AG, Bäch, Germany) at 3000 rpm for 10 min, 1 mL of the culture filtrate was passed through a Strata C18-E column (55 um, 70 A, 100 mg/1 mL) (Phenomenex, Aschaffenburg, Germany) and the column was washed with 3 mL H2O before fusarin C was eluted with 3 mL MeOH. The eluate was directly used for analysis using an Agilent 1200 series HPLC System (Agilent Technologies, Waldbronn, Germany) linked to a API 3200 mass spectrometer (Applied Biosystems, Darmstadt, Germany). Data acquisition was performed with the Analyst 1.4.2 software (Applied Biosystems).
Chromatographic separation was achieved on a 150×2.00 mm i.d., 3 μm Hyperclone 3u BDS C8 130 A column (Phenomenex) at 35 °C using a linear binary gradient from 45% MeOH and 55% H2O to 100 % MeOH in 25 min. The injection volume was 10 μL and the flow rate was set at 200 μL/min.
For electrospray ionization, the ion voltage was set at 5500 V in the positive mode. Zero-grade air served as nebulizer gas (35 psi) and turbo gas for solvent drying (50 psi) which was heated at 350 °C. Nitrogen served as curtain gas (20 psi) and as collision gas (4.5 × 10−5 Torr). The mass spectrometer was operated in multiple reaction monitoring mode (MRM) detecting the fragmentation of the [M+H]+ ions into specific product ions after collision with nitrogen.
Each transition reaction was monitored for duration of 150 ms with both quadrupols set at unit resolution. The declustering potential (DP) was set to 31 V and collision cell exit potential (CXP) set to 4 V. The collision energy (CE) for respective transition is given in brackets: m/z 432.26 – m/z 115.20 (CE 127 V). m/z 432.26 – m/z 141.20 (CE 93 V).
For quantitative comparison, cultivations and analysis were preformed in duplicates. The fusarin C levels are given as area percent relative to the peak area determined for the sum of the fusarin C peaks observed for the wild type IMI58289 grown under excessive nitrogen conditions. For quantification, the mass transition m/z 432.26 – m/z 115.20 was used. Further conformation of the identity of the fusarin C peaks was achieved by applying a second qualifier transition (m/z 432.26 – m/z 141.20).
For NOR (a sterigmatocystin precursor regulated identically to sterigmatocystin) extractions, all A. nidulans strains were point inoculated onto solid GMM in 10-cm-diameter petri dishes and incubated for 5 days at 37°C. For NOR visualization, dried organic extracts were resuspended in 100 μl of chloroform, and 10 μl was separated by TLC using chloroform:acetone (80:20 %) (v/v) as mobile phase.
Cytological techniques
F. fujikuroi strains grown in 10 % ICI were used for microscopy. Calcofluor White staining of hyphae was done by incubation of the whole slide for 20 min under dark conditions in Calcofluor White (0.1% in 0.1 M Tris-HCl pH 8.5). Slides were washed with distilled water twice and observed using epifluorescence microscopy. Nuclei were stained using Hoechst 33342 as described previously (Kangatharalingam and Ferguson, 1984). Epifluorescence was observed using a Leica DMRBE microscope (Leica, Wetzlar, Germany) equipped with a high-performance charge-coupled device (CDD) 12 bit SensiCam (PCO AG, Kehlheim, Germany) and filter set A (excitation band-pass filter 340–380, dichromatin mirror 400, suppression long-pass filter 425) for Hoechst 33342 fluorescence, or filter set L5 (excitation band-pass filter 480/40, dichromatin mirror 505, suppression band-pass filter 527/30) for GFP and YFP fluorescence.
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (DFG) (Tu1245/7) and work in Wisconsin was funded by NSF MCB-0236393 and NIH 1 R01 Al065728-01 to NPK. PW was holder of a research fellowship of the Graduiertenkolleg 1409 funded by the Deutsche Forschungsgemeinschaft (DFG). We thank Birgit Hoff and Ulrich Kück (Bochum University) for cross-species complementation of P. chrysogenum mutants with the Fflae1 and Ffvel1 genes. We thank Birgitt Oeser for her help with FiRe analysis, Mark Busman for analysis of fumonisins, Thomas Briel, Janina Post, Stefanie Traeger and Helena Klamm for their help with mutant screens, BiFC vector construction and fusarin C analysis as well as Sabine Huber for excellent technical support. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- Adams TH, Wieser JK, Yu JH. Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev. 1998;62:35–54. doi: 10.1128/mmbr.62.1.35-54.1998. Erratum in: Microbiol Mol Biol Rev62: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander NJ, McCormick SP, Hohn TM. TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl Environ Microbiol. 1999;64:221–225. doi: 10.1128/aem.64.1.221-225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers W, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;251:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amaike S, Keller NP. Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot Cell. 2009;8:1051–1060. doi: 10.1128/EC.00088-09. Epub 2009 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley and Sons; New York, NY: 1987. [Google Scholar]
- Barrero AF, Sánchez JF, Oltra JE, Tamayo N, Cerdá-Olmedo E, Candau R, Avalos J. Fusarin C and 8Z-Fusarin C from Gibberella fujikuroi. Phytochemistry. 1991;30:2259–2263. [Google Scholar]
- Bayram Ö, Krappmann S, Seiler S, Vogt N, Braus GH. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet Biol. 2008a;45:127–138. doi: 10.1016/j.fgb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Bayram Ö, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008b;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- Berger H, Basheer A, Böck S, Reyes-Dominguez Y, Dalik T, Altmann F, Strauss J. Dissecting individual steps of nitrogen transcription factor cooperation in the Aspergillus nidulans nitrate cluster. Mol Microbiol. 2008;69:1385–1398. doi: 10.1111/j.1365-2958.2008.06359.x. [DOI] [PubMed] [Google Scholar]
- Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Noordermeer D, Kale SP, Keller NP. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol. 2006a;61:1636–1645. doi: 10.1111/j.1365-2958.2006.05330.x. [DOI] [PubMed] [Google Scholar]
- Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. Genomic mining for Aspergillus natural products. Chem Biol. 2006b;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bömke C, Rojas MC, Hedden P, Tudzynski B. Loss of gibberellin production in Fusarium verticillioides (Gibberella fujikuroi MP-A) is due to a deletion in the gibberellic acid gene cluster. Appl Environ Microbiol. 2008;74:7790–7801. doi: 10.1128/AEM.01819-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhired S, Weber M, Kempf-Sontag A, Keller NP, Hoffmeister D. Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet Biol. 2007;44:1134–1145. doi: 10.1016/j.fgb.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Brown DW, Cheung F, Proctor RH, Butchko RAE, Zheng L, Lee Y, Utterback T, Smith S, Feldblyum T, Glenn AE, Plattner RD, Kendra DF, Town CD, Whitelaw CA. Comparative analysis of 87,000 expressed sequence tags from the fumonisin-producing fungus Fusarium verticillioides. Fungal Genet Biol. 2005;42:848–861. doi: 10.1016/j.fgb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Brown DW, Butchko RAE, Busman M, Proctor RH. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot Cell. 2007;6:1210–1218. doi: 10.1128/EC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AM, Bok J, Brooks W, Keller NP. VeA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Cenis JL. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992;20:2380. doi: 10.1093/nar/20.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Yun SS, Jang YK, Cha MJ, Kwon NJ, Chae SK. Identification and cloning of jipA encoding a polypeptide that interacts with a homolog of yeast Rad6, UVSJ in Aspergillus nidulans. J Microbiol. 2003;41:46–51. [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci USA. 2006;103:10352–7. doi: 10.1073/pnas.0601456103. Erratum in: Proc Natl Acad Sci USA103: 16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin E. The fle1 gene encoding a C2H2 zinc finger protein co-ordinates male and female sexual differentiation in Podospora anserina. Mol Microbiol. 2002;43:1255–1268. doi: 10.1046/j.1365-2958.2002.02819.x. [DOI] [PubMed] [Google Scholar]
- Darken MA, Jensen AL, Shu P. Production of gibberellic acid by fermentation. Appl Microbiol. 1959;7:301–303. doi: 10.1128/am.7.5.301-303.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins AE, Manandhar HK, Plattner RD, Manandhar GG, Poling SM, Maragos CM. Fusarium species from nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl Environ Microbiol. 2000;66:1020–1025. doi: 10.1128/aem.66.3.1020-1025.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Dreyer J, Eichhorn H, Friedlin E, Kürnsteiner H, Kück U. A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Appl Environ Microbiol. 2007;73:3412–3422. doi: 10.1128/AEM.00129-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RM, Cary JW, Calvo AM. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl Microbiol Biotechnol. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- van den Ent F, Löwe J. RF cloning: a restriction-free method for inserting target genes into plasmids. J Biochem Biophys Methods. 2006;67:67–74. doi: 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Estrada AF, Avalos J. The White Collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet Biol. 2008;45:705–718. doi: 10.1016/j.fgb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Flaherty JE, Pirttilä AM, Bluhm BH, Woloshuk CP. PAC1, a pH-regulatory gene from Fusarium verticillioides. Appl Environ Microbiol. 2003;69:5222–5227. doi: 10.1128/AEM.69.9.5222-5227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion C, Baltensperger R, Fournier T, Pasquier J, Schnetzer MA, Gabriel JP, Métraux JP. FiRe and microarrays: a fast answer to burning questions. Trends Plant Sci. 2006;11:320–322. doi: 10.1016/j.tplants.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Geissman TA, Verbiscar AJ, Phinney BO. Studies on the biosynthesis of gibberellins from (−)-kaurenoic acid in cultures of Gibberella fujikuroi. Phytochemistry. 1966;5:933–947. [Google Scholar]
- Gietz RD, Schiestl RH. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2:35–37. doi: 10.1038/nprot.2007.14. [DOI] [PubMed] [Google Scholar]
- Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983;25:179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Hoff B, Kück U. Use of bimolecular fluorescence complementation to demonstrate transcription factor interaction in nuclei of living cells from the filamentous fungus Acremonium chrysogenum. Curr Genet. 2005;47:132–138. doi: 10.1007/s00294-004-0546-0. [DOI] [PubMed] [Google Scholar]
- Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I, Kürnsteiner H, Kück U. VeA and LaeA homologues of the Aspergillus velvet complex control hyphal morphogenesis, conidiospore formation and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot Cell. 2010 doi: 10.1128/EC.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister D, Keller NP. Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep. 2007;24:393–416. doi: 10.1039/b603084j. Epub 2006 Dec 20. [DOI] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale SP, Milde L, Trapp MK, Frisvad JC, Keller NP, Bok JW. Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet Biol. 2008;45:1422–1429. doi: 10.1016/j.fgb.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangatharalingam N, Ferguson MW. A Simple and Rapid Technique for Fluorescence Staining of Fungal Nuclei. Current Microbiology. 1984;10:99–104. [Google Scholar]
- Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Han K, Kim K, Han D, Jahng K, Chae KS. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet Biol. 2002;37:72–80. doi: 10.1016/s1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- Kim H, Woloshuk CP. Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet Biol. 2008;45:947–953. doi: 10.1016/j.fgb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Kjær D, Kjær A, Pederson C, Bu’Lock JD, Smith JR. Bikaverin and nor-bikaverin, benzoxanthentrione pigments of Gibberella fujikuroi. J Chem Soc. 1971;1971:2792–2797. doi: 10.1039/j39710002792. [DOI] [PubMed] [Google Scholar]
- Klittich C, JR, Leslie JF. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi) Genetics. 1988;118:417–423. doi: 10.1093/genetics/118.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosalková K, García-Estrada C, Ullán RV, Godio RP, Feltrer R, Teijeira F, Mauriz E, Martín JF. The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie. 2009;91:214–225. doi: 10.1016/j.biochi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Krappmann S, Bayram Ö, Braus GH. Deletion and allelic exchange of the Aspergillus fumigatus veA locus via a novel recyclable marker module. Eukaryot Cell. 2005;4:1298–1307. doi: 10.1128/EC.4.7.1298-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Myung K, Guse D, Donkin B, Proctor RH, Grayburn WS, Calvo AM. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Mol Microbiol. 2006;62:1418–1432. doi: 10.1111/j.1365-2958.2006.05447.x. [DOI] [PubMed] [Google Scholar]
- Lodeiro S, Xiong Q, Wilson WK, Ivanova Y, Smith ML, May GS, Matsuda SP. Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus. Org Lett. 2009;11:1241–1244. doi: 10.1021/ol802696a. [DOI] [PubMed] [Google Scholar]
- Malonek S, Rojas MC, Hedden P, Gaskin P, Hopkins P, Tudzynski B. The NADPH-cytochrome P450 reductase gene from Gibberella fujikuroi is essential for gibberellin biosynthesis. J Biol Chem. 2004;279:25075–25084. doi: 10.1074/jbc.M308517200. [DOI] [PubMed] [Google Scholar]
- Masloff S, Jacobsen S, Pöggeler S, Kück U. Functional analysis of the C6 zinc finger gene pro1 involved in fungal sexual development. Fungal Genet Biol. 2002;36:107–16. doi: 10.1016/S1087-1845(02)00010-5. [DOI] [PubMed] [Google Scholar]
- May GS, Waring RB, Osmani SA, Morris NR, Denison SH. In: Proceedings of the EMBO-Alko Workshop on Molecular Biology of Filamentous Fungi. Nevalainen H, Pepperkok R, editors. Vol. 6. Foundation for Biotechnical and Industrial Fermentation Research; Helsinki, Finland: 1989. pp. 11–20. [Google Scholar]
- Mihlan M, Homann V, Liu TW, Tudzynski B. AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by Nmr. Mol Microbiol. 2003;47:975–991. doi: 10.1046/j.1365-2958.2003.03326.x. [DOI] [PubMed] [Google Scholar]
- Myung K, Li S, Butchko RAE, Busman M, Proctor RH, Abbas HK, Calvo AM. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J Agric Food Chem. 2009;57:5089–5094. doi: 10.1021/jf900783u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RM, Fedorova ND, Bok JW, Cramer RA, Wortman JR, Kim HS, Nierman WC, Keller NP. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3:e50. doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JR, Basrai MA, Steiner HY, Naider F, Becker JM. Isolation and characterization of a Saccharomyces cerevisiae peptide transport gene. Mol Cell Biol. 1994;14:104–115. doi: 10.1128/mcb.14.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner RD, Weisleder D, Poling SM. Analytical determination of fumonisins and other metabolites produced by Fusarium moniliforme and related species on corn. Adv Exp Med Biol. 1996;392:57–64. doi: 10.1007/978-1-4899-1379-1_5. [DOI] [PubMed] [Google Scholar]
- Pöggeler S, Masloff S, Hoff B, Mayrhofer S, Kück U. Versatile EGFP reporter plasmids for cellular localization of recombinant gene products in filamentous fungi. Curr Genet. 2003;43:54–61. doi: 10.1007/s00294-003-0370-y. [DOI] [PubMed] [Google Scholar]
- Pontecorvo GV, Roper JA, Hemmonns LM, Mac Donald KD, Buften AWJ. The genetics of Aspergillus nidulans. Adv Genet. 1953;141:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Brown DW, Plattner RD, Desjardins AE. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet Biol. 2003;38:237–249. doi: 10.1016/s1087-1845(02)00525-x. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Plattner RD, Brown DW, Seo JA, Lee YW. Discontinuous distribution of fumonisin biosynthetic genes in the Gibberella fujikuroi species complex. Mycol Res. 2004;108:815–822. doi: 10.1017/s0953756204000577. [DOI] [PubMed] [Google Scholar]
- Purschwitz J, Müller S, Fischer R. Mapping the interaction sites of Aspergillus nidulans phytochrome FphA with the global regulator VeA and the White Collar protein LreB. Mol Genet Genomics. 2009;281:35–42. doi: 10.1007/s00438-008-0390-x. [DOI] [PubMed] [Google Scholar]
- Rademacher W. Gibberellins. In: Anke T, editor. Fungal Biotechnology. Chapman and Hall; London: 1997. pp. 193–205. [Google Scholar]
- Regenberg B, Holmberg S, Olsen LD, Kielland-Brandt MC. Dip5p mediates high-affinity and high-capacity transport of L-glutamate and L-aspartate in Saccharomyces cerevisiae. Curr Genet. 1998;33:171–177. doi: 10.1007/s002940050324. [DOI] [PubMed] [Google Scholar]
- Reyes-Dominguez Y, Bok JW, Berger H, Shwab EK, Basheer A, Gallmetzer A, Scazzocchio C, Keller NP, Strauss J. Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Schönig B, Brown DW, Oeser B, Tudzynski B. Cross-species hybridization with Fusarium verticillioides microarrays reveals new insights into Fusarium fujikuroi nitrogen regulation and the role of AreA and NMR. Eukaryot Cell. 2008;7:1831–1846. doi: 10.1128/EC.00130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spröte P, Brakhage AA. The light-dependent regulator velvet A of Aspergillus nidulans acts as a repressor of the penicillin biosynthesis. Arch Microbiol. 2007;188:69–79. doi: 10.1007/s00203-007-0224-y. [DOI] [PubMed] [Google Scholar]
- Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl. 1989;36:79–81. [Google Scholar]
- Stinnett SM, Espeso EA, Cobeño L, Araújo-Bazán L, Calvo AM. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol Microbiol. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- Sun S-K, Snyder WC. The bakanae disease of the rice plant. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: diseases, biology and taxonomy. University Park: Pennsylvania State University Press; 1981. pp. 104–113. [Google Scholar]
- Teichert S, Schönig B, Richter S, Tudzynski B. Deletion of the Gibberella fujikuroi glutamine synthetase gene has significant impact on transcriptional control of primary and secondary metabolism. Mol Microbiol. 2004;53:1661–1675. doi: 10.1111/j.1365-2958.2004.04243.x. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot Cell. 2004;3:1398–411. doi: 10.1128/EC.3.6.1398-1411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudzynski B, Hölter K. Gibberellin biosynthetic pathway in Gibberella fujikuroi: evidence for a gene cluster. Fungal Genet Biol. 1998;25:157–170. doi: 10.1006/fgbi.1998.1095. [DOI] [PubMed] [Google Scholar]
- Tudzynski B, Kawaide H, Kamiya Y. Gibberellin biosynthesis in Gibberella fujikuroi: cloning and characterization of the copalyl diphosphate synthase gene. Curr Genet. 1998;34:234–240. doi: 10.1007/s002940050392. [DOI] [PubMed] [Google Scholar]
- Tudzynski B, Homann V, Feng B, Marzluf GA. Isolation, characterization and disruption of the areA nitrogen regulatory gene of Gibberella fujikuroi. Mol Gen Genet. 1999;261:106–114. doi: 10.1007/s004380050947. [DOI] [PubMed] [Google Scholar]
- Tudzynski B, Mihlan M, Rojas MC, Linnemannstöns P, Gaskin P, Hedden P. Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi: des and P450-3 encode GA4 desaturase and the 13-hydroxylase, respectively. J Biol Chem. 2003;278:28635–28643. doi: 10.1074/jbc.M301927200. [DOI] [PubMed] [Google Scholar]
- Tudzynski B. Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology. Appl Microbiol Biotechnol. 2005;66:597–611. doi: 10.1007/s00253-004-1805-1. [DOI] [PubMed] [Google Scholar]
- Wiemann P, Willmann A, Straeten M, Kleigrewe K, Beyer M, Humpf H-U, Tudzynski B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: genes, their function and regulation. Mol Microbiol. 2009;72:931–946. doi: 10.1111/j.1365-2958.2009.06695.x. [DOI] [PubMed] [Google Scholar]
- Winston F, Dollard C, Ricupero-Hovasse SL. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- Yabuta T. Biochemistry of the ‘bakanae’ fungus of rice. Agric Hortic. 1935;10:17–22. [Google Scholar]