Abstract
An analytical assay has been developed and validated for ultrafast and high-throughput mass spectrometric determination of pemetrexed concentrations in plasma using matrix assisted laser desorption/ionization–triple quadrupole–tandem mass spectrometry. Patient plasma samples spiked with the internal standard methotrexate were measured by multiple reaction monitoring. The detection limit was 0.4 fmol/μL, lower limit of quantification was 0.9 fmol/μL, and upper limit of quantification was 60 fmol/μL, respectively. Overall observed pemetrexed concentrations in patient samples ranged between 8.7 (1.4) and 142.7 (20.3) pmol/μL (SD). The newly developed mass spectrometric assay is applicable for (routine) therapeutic drug monitoring of pemetrexed concentrations in plasma from non-small cell lung cancer patients.
Keywords: Pemetrexed, NSCLC, MALDI-QqQ-MS/MS, Therapeutic drug monitoring, Alimta, Methotrexate
Introduction
Pemetrexed (PTX) (Alimta®; N-[4-[2-[2-Amino-4,7-dihydro-4-oxy-3H-pyrrolo[2,3-d]pyrimidine-5-yl ethyl] benzoyl]-l-glutamic acid]; Fig. 1a) is a multi-targeted anti-folate drug applied in the treatment of malignant pleural mesothelioma and non-small cell lung cancer (NSCLC) [1].
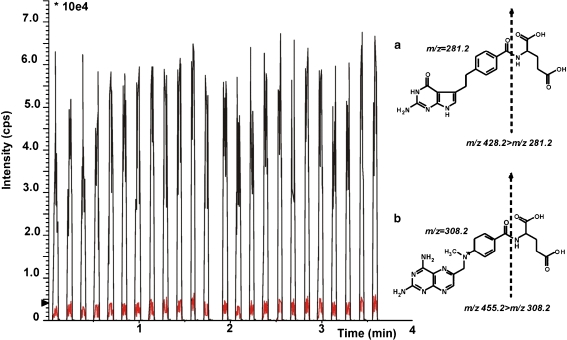
Fig. 1.
Example of ultrafast and high-throughput MALDI-QqQ-MS/MS analyses. Illustrated are reconstructed MRM traces of Pemetrexed (black) and internal standard methotrexate (red) from a QC samplecontaining pemetrexed at a concentration level of 30 fmol/μL. Chemical structure of a pemetrexed and b methotrexate and respective fragmentation and MRM transitions
Therapeutic drug plasma concentrations are defined for various types of drugs, offering a useful tool for monitoring drug dosage and avoiding drug toxicity and obtain therapeutic efficacy. PTX plasma concentrations can be determined by (high-performance) liquid chromatography with ultraviolet (HPLC-UV) [2–4] or mass spectrometric detection (LC-MS) [5]. HPLC-UV and LC-MS methods are time-consuming and less suitable for routine measurement of many samples.
To avoid long analysis time, we applied matrix assisted laser desorption/ionization–triple quadrupole–tandem mass spectrometry (MALDI-QqQ-MS/MS) for the development of a new assay. MALDI-QqQ-MS/MS does not require liquid chromatographic separation prior to analyses and thus can result in analysis times of approx. 10 s per sample and is therefore very suitable for the analysis of many samples in a short time. The MALDI-QqQ-MS/MS technology has been used previously for the determination of drug concentrations [6–9] and for enzyme inhibition studies [10].
Here, we report a new ultrafast and high-throughput MALDI-QqQ-MS/MS assay for therapeutic drug monitoring of pemetrexed concentrations in plasma from NSCLC patients.
Methods and materials
Chemicals
Pemetrexed (Alimta®; N-[4-[2-[2-Amino-4,7-dihydro-4-oxy-3H-pyrrolo[2,3-d]pyrimidine-5-yl ethyl]benzoyl]-l-glutamic acid disodium salt heptahydrate]) was obtained from the Department of Pharmacy, Erasmus MC and methotrexate (4-amino-10-methylpteroylglutamic acid, MTX) used as internal standard was from Schircks Laboratories (Jona, Switzerland). Solvents were of LC-MS grade (Biosolve, Valkenswaard, the Netherlands) and other chemicals were from ACS grade (Sigma Aldrich, Zwijndrecht, the Netherlands).
Preparation of plasma matrix-based calibrators and calibration curve
Standard stock solution (PTX, 600 nmol/L and MTX, 550 nmol/L) were prepared in LC-MS quality water. PTX plasma matrix-based calibrators were prepared by dilution with drug-free human control plasma (Sanquin Blood Supply Foundation, Rotterdam, the Netherlands) to yield following calibrators: 60, 30, 15, 7.5, 3.75, 1.88, and 0.94 pmol/μL. Ten microliter of the calibrators were pipetted into 10 mL volumetric flasks and simultaneously deproteinized/diluted (1:1,000) with an acetonitrile/water (60/40% v/v) mixture. Calibrators were centrifuged (5 min at 2,000×g) and 20 μL of supernatant were mixed with α-CHCA (10 μL, 6.2 mg/mL in methanol/acetonitrile/water 36/56/8% (v/v/v), pH = 2.5) matrix solution and 1.5 μL were spotted (n = 3, 10 spots per quality control (QC) sample) on a Opti-TOF 96-well stainless steel target plate (123 × 81 mm; MDS Analytical Technologies, Concord, Canada) and spots were dried at room temperature (5 min).
MALDI-QqQ-MS/MS instrumentation
Analyses were conducted by a Flashquant Workstation with a 4000 API mass analyzer (MDS Analytical Technologies, Concord, Canada) operating in the positive ionization mode. MS settings were: skimmer voltage 0 V, CAD gas 8 arbitrary units, source gas 10 arbitrary units, dwell time 20 ms, plate voltage 45 V, laser power 45% and laser raster speed 1 mm/s. Instrument control/data analyses were performed using Flashquant 1.0 and Analyst 1.4.2 application software. Different MALDI matrices such as 2,5-dihydroxy benzoic acid (2,5-DHB), 7-hydroxy-4-(trifluoromethyl)-coumarin (HFMC) [11] and α-cyano-hydroxy-cinnamic acid (α-CHCA) were used to determine the highest possible sensitivity. Therefore, 10 μL of a PTX/MTX solution (approx. 100 nmol/L) were mixed with all MALDI matrices (10 μL) and analyzed in positive full scan mode (m/z = 150 to m/z = 350).
Assay validation
Validated assay parameters according to FDA guidelines [12] were: limit of detection (LOD), lower limit of quantification (LLOQ), linearity, accuracy, precision, recovery and stability of PTX using QC samples prepared in drug-free human control plasma at 6.0, 30.0, and 60.0 pmol/μL.
Preparation of patient samples
EDTA-blood was collected (1 week after admission) from patients who had received one dose of PTX (500 mg/m2) and who have written consent for using their blood samples. Plasma was prepared by centrifugation for 10 min (4 °C, 1,400×g). Ten microliters of plasma were transferred into a 10.00 mL volumetric flask and spiked with 10 μL MTX (27.5 μmol/L) and deproteinized/diluted with an acetonitrile/water mixture (60/40% v/v). Precipitated proteins were removed by centrifugation (5 min at 2,000×g) at ambient temperature and 20 μL supernatant were mixed with MALDI matrix (10 μL) and 1.5 μL were spotted (n = 10).
Results
MALDI-QqQ-MS/MS analysis
From all matrices, α-CHCA matrix gave the highest signal intensity for PTX and MTX. Protonated ions of PTX and MTX ((MH)+) were m/z = 428.2 for PTX and m/z = 455.2 for MTX. These protonated ions were used for further assay optimization. Optimized instrument parameters were: collision energy 38 and 25 V and collision cell energy exit potential 13 and 10 V for PTX and MTX, respectively. Collision-induced dissociation resulted in the formation of protonated fragment ions (Fig. 1). Selected MRM transitions were for PTX m/z = 428.2 > 281.2 and for MTX m/z = 455.2 > 308.2.
Assay validation
Analyte recovery
To avoid laborious sample preparations by SPE or extraction, we tried to isolate PTX from plasma by deproteinization with trichloroacetic acid (TCA). We determined recoveries using QC samples (30 pmol/μL) deproteinized with different TCA concentrations; 5%, 10%, 20% and 30% TCA (% w/v). Recoveries ranged between 13% and 30% for PTX and between 20% and 62% for MTX. To obtain probably higher recoveries, we transferred 100 μL aliquots of the supernatant of QC sample (10% TCA) onto a 96-well SPE method development plate (25 mg/well; Sigma Aldrich, Zwijndrecht, the Netherlands). Recovery rates on following SPE adsorbents; PS/PVB (polysterene divinyl benzene), octadecyl (C18), octyl (C8), cyanopropyl, MCAX (mixed-mode strong cation exchange), and WCX (weak cation exchange) were determined. Conditioning of the wells was done by acetone (2 × 1 mL), methanol (2 × 1 mL), and water (2 × 1 mL) except for WCX/SAX the last conditioning step used was 2 × 1 mL H2O/10% TCA (% w/v). Extraction was done by 100 μL of methanol, 20 μL of the extract was mixed with α-CHCA (10 μL) and 1.5 μL were spotted (n = 10). Recoveries were still low (<60%). Because of the low recoveries, we decided to just deproteinize the QC samples by addition of acetonitrile/water mixtures. Ten microliters were deproteinized/diluted (1:1,000) with different acetonitrile/water mixtures; 40/60, 50/50 and 60/40% (v/v). The highest recoveries were obtained for 60:40% (v/v) acetonitrile/water mixture; PTX (91%) and MTX (80%), while recovery rates dropped to 61% and 66% and 58% and 69% for PTX and MTX if we used 50/50% (v/v) and 40/60% (v/v) mixtures, respectively.
Linearity
The selected matrix-based calibrators (0.94–60 pmol/μL) displayed a linear relationship between MRM peak area and PTX concentration (r 2 = 0.9973, y = 101.96 x + 84.245). Linear regression of the results was done using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA, USA).
LLOQ and LOD
The LLOQ was defined as the lowest calibrator of the calibration curve that could be analyzed with an accuracy and precision of CV <20% [12]. The LLOQ of PTX was 0.94 pmol/μL and upper limit of quantification was 60 fmol/μL (CV <15%). The LOD of PTX, defined at a signal-to-noise ratio of (3:1) in drug-free human control plasma, was 0.4 fmol/μL.
Accuracy and precision
The accuracy and precision were within FDA criteria (CV <15%) at all three validation levels (Table 1), precision expressed as% CV was between 11.9% and 31.8% for within-run and between 8.5% and 14.5% for between-run experiments. Accuracy expressed as% RSD ranged between 2.5% and 5.7% for within-run and between 1.7% and 6.0% for between-run experiments.
Table 1.
Precision and accuracy and stability experiments of developed assay at three different plasma concentration levels (n = 3)
| Analyte | Pemetrexed | Storage Conditions/Timea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within-run validationa | Between-run validationb | Refrigerator (4 °C) | Freezer (−20 °C) | Freezer (−20 °C) | ||||||||
| 24 h | 20 days | 3 freeze/thaw cycles | ||||||||||
| Mean (SD) | %Errorc | Mean (SD) | %Errorc | Mean (SD) | %Errorc | |||||||
| Nominal concentration (pmol/μL) | 6.0 | 30.0 | 60.0 | 6.0 | 30.0 | 60.0 | ||||||
| Mean observed concentration (pmol/μL) | 6.2 | 31.7 | 58.5 | 5.9 | 31.8 | 62.2 | ||||||
| Accuracy (% RSDd) | 3.3 | 5.7 | 2.5 | 1.7 | 6.0 | 3.7 | ||||||
| Precision (% CV) | 12.9 | 13.8 | 11.9 | 13.7 | 14.5 | 8.5 | ||||||
| Nominal concentration (pmol/μL) | ||||||||||||
| 60.0 | 62.8 (6.0) | 4.7 | 59.3 (5.8) | −1.2 | 58.7 (4.9) | −2.2 | ||||||
| 30.0 | 29.5 (3.8) | −1.7 | 31.5 (3.9) | 5.0 | 29.2 (2.7) | −2.7 | ||||||
| 6.0 | 6.5 (1.2) | 8.3 | 6.2 (0.8) | 3.3 | 5.9 (0.5) | −1.7 | ||||||
aResults summarize ten spots per QC sample at each concentration level in one experiment
bResults summarize three different experiments from three consecutive days with 10 spots per QC sample at each concentration level
c%Error = (mean observed concentration−nominal concentration)/(nominal concentration) × 100%
dRSD, relative standard deviation of the mean observed concentration
PTX stability
PTX, according to FDA criteria, is defined as stable when the concentration decrease in plasma matrix was <15% (expressed as % error). This was the case at all three QC validation levels (Table 1).
Application of assay to patient samples
Observed PTX plasma concentrations in analyzed patient samples are presented in Table 2. Results show that patient A has an increased plasma concentration of PTX after the second administration compared to first administration, patient C though showed no increased plasma concentration level after the second administration. Other patients (B, D, E, F, and G) had received their first administration of PTX at the moment the samples were collected.
Table 2.
Measured pemetrexed concentrations in plasma from NSCLC patients (n = 3)
| Patient code | Pemetrexed concentration (pmol/μL)a mean (SD) | Genderb | Age |
|---|---|---|---|
| A | 80.0 (9.3) | f | 51 |
| A | 142.7 (20.3)c | f | 51 |
| B | 8.7 (1.4) | f | 56 |
| C | 63.0 (7.0) | m | 66 |
| C | 60.7 (8.3)c | m | 66 |
| D | 33.3 (4.9) | m | 75 |
| E | 42.7 (9.7) | m | 48 |
| F | 49.1 (10.8) | f | 56 |
| G | 121.0 (20.2) | m | 61 |
aResults summarize 10 spots per patient sample
bFemale (f) and male (m)
cSecond administration of 500 mg/m2, 3 weeks after first administration
Discussion
Recently, it was demonstrated that MALDI-QqQ-MS/MS is an ultrafast, high-throughput and sensitive mass spectrometric technique [7 ,8, 13–16] for small molecules. Analysis times for pemetrexed on HPLC- or LC-MS instrumentations are between 7 and 20 min [3, 5, 17] per sample measured by HPLC and up to 30 min per sample for LC-MS [18] while the MALDI-QqQ-MS/MS assay has an analysis time of approx. 10 s per sample (Fig. 1).
Figure 1 illustrates that multiple measurement of a plasma sample (n = 24) can be accomplished within 4 min with acceptable accuracy (CV 13.1%).
Some papers [2, 3, 5, 18] report the measurement of PTX in plasma and many papers use SPE. Hamilton et al. [3] reported recoveries for PTX depending on the PTX concentration, at a concentration of 15 ng/mL (35 fmol/μL), recoveries were 40% and at 1,200 ng/mL (2,760 fmol/μL) the recovery was 83%. Higher recoveries were reported by [2] (76.9%), while [5] reported recoveries of 99% and 124%. We studied also SPE but obtained only low recoveries for PTX and MTX. Instead, we tested different acetonitrile/water mixtures for PTX and MTX isolation from proteins in plasma. Due to expected PTX patient plasma concentrations in the micro molar range, simultaneous deproteinization and dilution (1:1,000) of patient plasma samples were studied. The highest analyte recoveries were obtained with an acetonitrile/water (60:40% v/v) mixture; recoveries were 91% for PTX and 80% for MTX, respectively.
The LOD of the assay is 0.4 fmol/μL, significantly lower than previously reported LODs of 23 fmol/μL and 6 fmol/μL [3–5], respectively.
The assay had been validated by the determination of linearity, recovery rates, within- and between-run accuracy and precision and stability of PTX. The within- and between-run accuracy and precision as well as stability of PTX were all <15% CV and are in good compliance with the FDA regulation [12] (Table 1).
The total plasma amount spotted is 0.001 μL while others [5] injected in total 3.5 μL of plasma into the LC-MS system, demonstrating the very high sensitivity of the assay.
After the validation, the assay was applied to NSCLC patient samples. PTX concentrations ranged between 8.7 (1.4) and 142.7 (20.3) pmol/μL (SD). Two patients had already received a second dose (Table 2). One of the two patients showed a significant increase (142.7 (20.3) pmol/μL) compared to the first dose (80.0 (9.3) pmol/μL). This significant increase of the PTX plasma concentration cannot be explained by an increasing steady-state plasma concentration level between two administrations, since the half-life time of PTX is 3.5 h [19]. More experiments/studies will be necessary to determine pharmacokinetic properties for extra- and intracellular concentrations of PTX to be able to understand significant increased plasma concentration levels in patients.
Conclusion
We have developed an ultrafast, sensitive, and high-throughput assay for the determination of PTX concentrations in plasma from NSCLC patients. The assay can be used for therapeutic drug monitoring of PTX plasma concentration levels and the assay is so sensitive that it can support pharmacokinetic studies even with plasma amounts of few microliters.
Acknowledgment
This research was financial supported by ZonMw via the granted project (project #152001017): “Biomarkers for improving the cost-effectiveness and safety of pemetrexed”.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jones RJ, Twelves CJ. Pemetrexed: a multitargeted antifolate (ALIMTA, LY-231514) Expert Rev Anticancer Ther. 2002;2:13–22. doi: 10.1586/14737140.2.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Rivory LP, Clarke SJ, Boyer M, Bishop JF. Highly sensitive analysis of the antifolate pemetrexed sodium, a new cancer agent, in human plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;765:135–140. doi: 10.1016/S0378-4347(01)00406-6. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CL, Kirkwood JA. Column-switching high-performance liquid chromatographic method for the determination of a thymidylate synthase inhibitor, LY231514, an investigational agent for the treatment of solid tumors, in human plasma. J Chromatogr B Biomed Appl. 1994;654:297–303. doi: 10.1016/0378-4347(93)E0447-X. [DOI] [PubMed] [Google Scholar]
- 4.Rinaldi DA, Burris HA, Dorr FA, Woodworth JR, Kuhn JG, Eckardt JR, Rodriguez G, Corso SW, Fields SM, Langley C, et al. Initial phase I evaluation of the novel thymidylate synthase inhibitor, LY231514, using the modified continual reassessment method for dose escalation. J Clin Oncol. 1995;13:2842–2850. doi: 10.1200/JCO.1995.13.11.2842. [DOI] [PubMed] [Google Scholar]
- 5.Bobin-Dubigeon C, Amiand MB, Herrenknecht C, Bard JM. Development and validation of an improved liquid chromatography-mass spectrometry method for the determination of pemetrexed in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2451–2456. doi: 10.1016/j.jchromb.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 6.van Kampen JJ, Verschuren EJ, Burgers PC, Luider TM, de Groot R, Osterhaus AD, Gruters RA. Validation of an HIV-1 inactivation protocol that is compatible with intracellular drug analysis by mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:38–44. doi: 10.1016/j.jchromb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Meesters RJ, van Kampen JJ, Reedijk ML, Scheuer RD, Dekker LJ, Burger DM, Hartwig NG, Osterhaus AD, Luider TM, Gruters RA. Ultrafast and high-throughput mass spectrometric assay for therapeutic drug monitoring of antiretroviral drugs in pediatric HIV-1 infection applying dried blood spots. Anal Bioanal Chem. 2010;398:319–328. doi: 10.1007/s00216-010-3952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatsis P, Brombacher S, Corr J, Kovarik P, Volmer DA. Quantitative analysis of small pharmaceutical drugs using a high repetition rate laser matrix-assisted laser/desorption ionization source. Rapid Commun Mass Spectrom. 2003;17:2303–2309. doi: 10.1002/rcm.1192. [DOI] [PubMed] [Google Scholar]
- 9.Sleno L, Volmer DA. Some fundamental and technical aspects of the quantitative analysis of pharmaceutical drugs by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1928–1936. doi: 10.1002/rcm.2006. [DOI] [PubMed] [Google Scholar]
- 10.Rathore R, Corr J, Scott G, Vollmerhaus P, Greis KD. Development of an inhibitor screening platform via mass spectrometry. J Biomol Screen. 2008;13:1007–1013. doi: 10.1177/1087057108326143. [DOI] [PubMed] [Google Scholar]
- 11.van Kampen JJ, Burgers PC, Gruters RA, Osterhaus AD, de Groot R, Luider TM, Volmer DA. Quantitative analysis of antiretroviral drugs in lysates of peripheral blood mononuclear cells using MALDI-triple quadrupole mass spectrometry. Anal Chem. 2008;80:4969–4975. doi: 10.1021/ac800218a. [DOI] [PubMed] [Google Scholar]
- 12.CDER (2001) Center for Drug Evaluation and Research (CDER) Guidance for Industry: bioanalytical method validation
- 13.Corr JJ, Kovarik P, Schneider BB, Hendrikse J, Loboda A, Covey TR. Design considerations for high speed quantitative mass spectrometry with MALDI ionization. J Am Soc Mass Spectrom. 2006;17:1129–1141. doi: 10.1016/j.jasms.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Sleno L, Volmer DA. Toxin screening in phytoplankton: detection and quantitation using MALDI triple quadrupole mass spectrometry. Anal Chem. 2005;77:1509–1517. doi: 10.1021/ac0486600. [DOI] [PubMed] [Google Scholar]
- 15.Gobey J, Cole M, Janiszewski J, Covey T, Chau T, Kovarik P, Corr J. Characterization and performance of MALDI on a triple quadrupole mass spectrometer for analysis and quantification of small molecules. Anal Chem. 2005;77:5643–5654. doi: 10.1021/ac0506130. [DOI] [PubMed] [Google Scholar]
- 16.Van Kampen JJ, Reedijk ML, Burgers PC, Dekker LJM, Hartwig NG, van der Ende I, de Groot R, Osterhaus AD, Burger DM, Gruters RA, Luider TM. Ultra-fast analysis of plasma and intracellular levels of HIV protease inhibitors in children: a clinical application of MALDI mass spectrometry. PLoS ONE. 2010 doi: 10.1371/journal.pone.0011409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouellet D, Periclou AP, Johnson RD, Woodworth JR, Lalonde RL. Population pharmacokinetics of pemetrexed disodium (ALIMTA) in patients with cancer. Cancer Chemother Pharmacol. 2000;46:227–234. doi: 10.1007/s002800000144. [DOI] [PubMed] [Google Scholar]
- 18.Woodland JM, Barnett CJ, Dorman DE, Gruber JM, Shih C, Spangle LA, Wilson TM, Ehlhardt WJ. Metabolism and disposition of the antifolate LY231514 in mice and dogs. Drug Metab Dispos. 1997;25:693–700. [PubMed] [Google Scholar]
- 19.Latz JE, Chaudhary A, Ghosh A, Johnson RD. Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol. 2006;57:401–411. doi: 10.1007/s00280-005-0036-1. [DOI] [PubMed] [Google Scholar]