Abstract
Previous reports have described obtaining mature Plasmodium vivax ookinetes in vitro using blood from infected patients using a simplified, field-based protocol. Here, we report protocols that produce improved P. vivax ookinete yields and morphological development. Optimal conditions included induction of gametogenesis using 10 mM Tris, 170 mM NaCl, 10 mM glucose, 25 mM NaHCO3, and 100 μM xanthurenic acid for 90 minutes at pH 8.0–8.2, followed by culture in RPMI-1640, 50 mg/mL hypoxanthine, 25 mM HEPES, 29 mM NaHCO3, 2 mM L-glutamine, and 20% fetal bovine serum at pH 8.4 for 36 hours. Ookinetes were produced in 86% (18/21) of optimized in vitro cultures; yields ranged from 6.5 × 104 to 2.8 × 106; percent gametocyte conversion ranged from 1.4% to 4.7%. This improved method is suitable for preparation of P. vivax ookinetes in quantities sufficient for biochemical, molecular, and cell biological analysis where basic laboratory facilities are in proximity to patients with vivax malaria.
The study of Plasmodium vivax biology lags behind that of other Plasmodium species.1,2 Significant differences in details of life cycle, epidemiology, and disease mechanisms of P. vivax and other Plasmodium species, such as the early appearance of gametocytes compared with Plasmodium falciparum, highlight the importance of detailed species-specific investigations.3
Relevant to the development of transmission-blocking strategies is an understanding of the sporogonic development of malaria parasites within the mosquito midgut. Once a mosquito ingests gametocytes, parasites transform into diploid zygotes and then into motile ookinetes.4–6 Plasmodium sporogonic development within the mosquito midgut is asynchronous: Plasmodium vivax ookinete density in the mosquito midgut peaks at 36 hours and ookinetes persist up to 48 hours.4,7 The ookinete penetrates the midgut wall and matures as an oocyst, subsequently releasing sporozoites that invade the mosquito salivary glands thereby enabling transmission to the next human on which the mosquito feeds. New strategies to develop and validate transmission-blocking vaccine strategies involve enhancing our detailed knowledge of malaria parasite sporogonic stage biology. Being able to culture the sexual stage forms of human-infecting malaria parasites in quantities sufficient for systems biology analysis (gene expression profiling and proteomic analysis), immunological, cell biological, and biochemical analysis is essential to move the field forward.
Previous reports have described in vitro production of P. vivax ookinetes. An important study produced P. vivax ookinetes in vitro, obtaining blood ex vivo from patients in Thailand with vivax malaria.8 Key findings from this study were that of 98 patients (all with patent gametocytemia), only half produced ookinetes (after 45-minute fertilization and 24-hour incubation), with yields ranging from 10 to 248,500 ookinetes from 5 mL blood. Furthermore, these investigators found that two-thirds of patients infected laboratory-reared Anopheles dirus mosquitoes, but that gametocyte concentrations did not correlate with oocyst production or in vitro ookinete yield. Whether xanthurenic acid was necessary for ookinete formation was not assessed.8,9 Other brief reports have described incidental findings of exflagellating P. vivax microgametes10,11 and P. vivax ookinete formation produced in peripheral blood left for prolonged periods at room temperature during diagnostic testing.12
We present here an improved methodology for the production of P. vivax ookinetes and other sexual stage parasites from field samples in quantities suitable for systems biology, biochemical and cell biological analysis. Photomicrographs of P. vivax sexual stage parasites produced in vitro that confirm previous work9 and provide additional morphological exemplars. Elsewhere we have recently reported gene expression profiling of P. vivax sporogonic forms.13
This study was conducted on 37 P. vivax malaria patients reporting to health clinics in Iquitos, Peru. All patients provided written informed consent and the study was approved by the Institutional Review Board/Ethical Committees of the University of California San Diego and Asociación Benéfica PRISMA.
Sources of parasites were adult patients (≥ 18 years of age) with light microscopy-diagnosed, acutely symptomatic patients with P. vivax malaria. Mixed infections with P. falciparum (as determined by light microscopy) were excluded from the study. For each patient, 10 mL of blood were drawn into heparinized vacutainer tubes and immediately placed in a 37 to 40°C portable incubator for transport from the clinic to the laboratory for processing. Four protocols were attempted before an optimized protocol was selected for ookinete production (Table 1). Within 20 minutes of the initial blood draw samples were pelleted by centrifugation and resuspended in 3 volumes filtration media (FM).8 To remove white blood cells, resuspended blood cells were allowed to pass through sterile methylcellulose packed into a 20 mL syringe (CF-11, Whatman Ltd., Piscataway, NJ) and allowed to filter by gravity while being maintained in an incubator at 37°C. Cells were then centrifuged (500 g for 5 minutes), resuspended in 3 volumes of gametogenesis solution (GS), and allowed to fertilize by incubation for 45 to 90 minutes at ambient temperature lying sideways in a 50 mL conical tube.8 Following incubation, parasites were separated by overlaying the suspension on a 47% percoll/RPMI-1640 density gradient followed by 15-minute centrifugation at 500 g.14 Zygotes were collected from the interface using a 200 μL micropipetter (Gilson, Middletown, WI), twice washed in ookinete medium, pelleted, and resuspended in ookinete medium, and finally incubated for 24 to 48 hours in a 50 mL unvented culture flask, changing the medium every 12 hours. Following slide preparation and ookinete enumeration by hemacytometer, ookinetes were concentrated and purified on a discontinuous gradient of 80%, 65%, 50%, and 35% Percoll/RPMI-1640.15,16 Ookinetes were collected from the 35/50% interface using a 200 μL micropipetter, washed two times in incomplete culture medium (RPMI-1640, 50 mg/mL hypoxanthine, 29 mM NaHCO3) and processed for further study.
Table 1.
Summary of protocols tested to produce Plasmodium vivax ookinetes in vitro*
| Protocol 1 | Protocol 2 | Protocol 3 | Protocol 4 | |
|---|---|---|---|---|
| Filtration media | 10 mM Tris, 170 mM NaCl, 10 mM glucose, pH 7.3 | 10 mM Tris, 170 mM NaCl, 10 mM glucose, pH 7.3 | 10 mM Tris, 170 mM NaCl, 10 mM glucose, pH 7.3 | 10 mM Tris, 170 mM NaCl, 10 mM glucose, pH 7.3 |
| Gametogenesis solution | 100 μM xanthurenic acid in PBS | 100 μM xanthurenic acid in 25 mM NaHCO3/PBS | 10 mM Tris, 170 mM NaCl, 10 mM glucose, 25 mM NaHCO3, 100 μM xanthurenic acid, 10% FBS, pH 8.0–8.2 | 10 mM Tris, 170 mM NaCl, 10 mM glucose, 25 mM NaHCO3, 100 μM xanthurenic acid, pH 8.0–8.2 |
| Gametogenesis time | 45 min | 45 min | 90 min | 90 min |
| Percoll separation | Yes | Yes | Yes | No |
| Ookinete media | RPMI-1640, 50 mg/mL hypoxanthine, 25 mM HEPES, 20% FBS pH 7.8–8.2 | RPMI-1640, 50 mg/mL hypoxanthine, 25 mM HEPES, 20% FBS, pH 7.8–8.2 | RPMI-1640, 50 mg/mL hypoxanthine, 25 mM HEPES, 29 mM NaHCO3, 2 mM L-glutamine, 20% FBS, pH 8.4 | RPMI-1640, 50 mg/mL hypoxanthine, 25 mM HEPES, 29 mM NaHCO3, 2 mM L-glutamine, 20% FBS, pH 8.4 |
| % Of cultures producing ookinetes | 40% | 67% | 60% | 86% |
| Mean ookinete yield | 3 × 103 | 1.3 × 105 | 3.7 × 105 | 7.3 × 105 |
| Mean % gametocyte conversion | 0.05% | 0.6% | 1.6% | 3.0% |
PBS = phosphate buffered saline; FBS = fetal bovine serum.
The χ2 tests and analysis of variance were used to test differences in patient characteristics, parasitemia, and gametocytemia among field samples. Statistical significance was defined at a level of 0.05. Correlation between gametocyte numbers, ookinete yield, and percent gametocyte conversion (the proportion of ookinetes in relation to zygotes + macrogametes [round forms] at the end of the culture incubation period) was tested by Spearman correlation coefficients. Statistical Analysis Software (SAS, version 9.2, Raleigh, NC) was used to perform the analyses.
There was no difference in patient gender (P = 0.8), temperature (P = 0.3), pulse (P = 0.5), number of past clinical cases of vivax malaria (P = 0.3), the number of asexual parasites (P = 0.4), or the number of gametocytes (P = 0.9) observed in the field samples used for the four different culture protocols. The number of P. vivax gametocytes observed in the 37 patients ranged from 1.6 × 106 to 7.3 × 107 gametocytes per 10 mL of blood (mean = 2.3 × 107, median = 1.8 × 107). Protocol 1 produced ookinetes in 40% (2/5) of cultures with ookinete yields of 840 (percent gametocyte conversion = 0.005%) and 5,120 (percent gametocyte conversion = 0.09%). Protocol 2 produced ookinetes in 67% (4/6) of cultures with ookinete yields ranging from 113 to 3 × 105 (mean = 1.3 × 105) and percent gametocyte conversion ranging from 0.0007% to 1.3% (mean = 0.6%). Protocol 3 produced ookinetes in 60% (3/5) of cultures with ookinete yields ranging from 9.5 × 104 to 9.8 × 105 (mean = 3.7 × 105) and percent gametocyte conversion ranging from 1.2% to 2.1% (mean = 1.6%). The best method, Protocol 4, led to ookinete production in 86% (18/21) of cultures with ookinete yields ranging from 6.5 × 104 to 2.8 × 106 (mean = 7.3 × 105) and percent gametocyte conversion ranging from 1.4% to 4.7% (mean = 3.0%). The number of gametocytes present in field samples weakly correlated (Spearman R2 = 0.64) with ookinete yield but did not correlate (Spearman R2 = 0.15) with percent gametocyte conversion (Figure 1). Early retort forms were first observed 6 hours after blood was placed in ookinete medium. Maturing retorts were observed 8–24 hours after and mature ookinetes were observed 24–48 hours after initiation of ookinete culture. Ookinetes with the most characteristic mature morphology were produced after 36 hours of incubation (Figure 2). These in vitro-produced ookinetes were used in an indirect immunofluorescence assay to visualize the P. vivax sexual stage protein Pvs230, which proteomic analysis of Plasmodium gallinaceum ookinetes has shown to be secreted,17 and which gene expression profiling recently has shown to be up-regulated in both P. vivax and P. falciparum ookinetes relative to preceding stages.13,18 We have shown that purified P. vivax zygotes and ookinetes have been successfully used to produce sufficient RNA for large scale gene expression profiling.17
Figure 1.
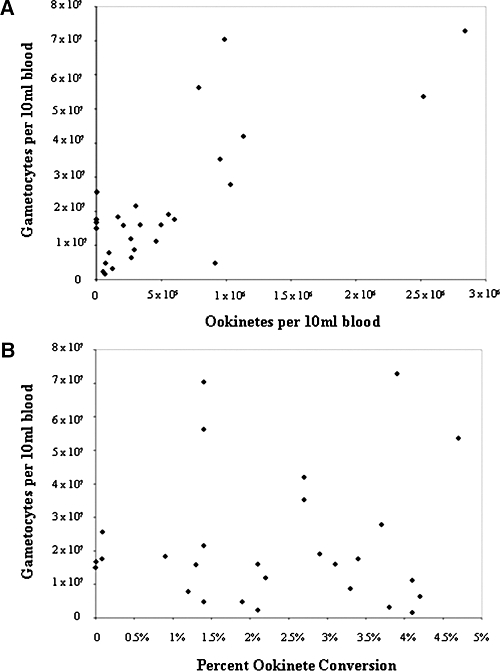
Relationship between (A) the number of gametocytes in patient samples and percent gametocyte conversion and (B) the number of gametocytes in patient samples with ookinete yield.
Figure 2.
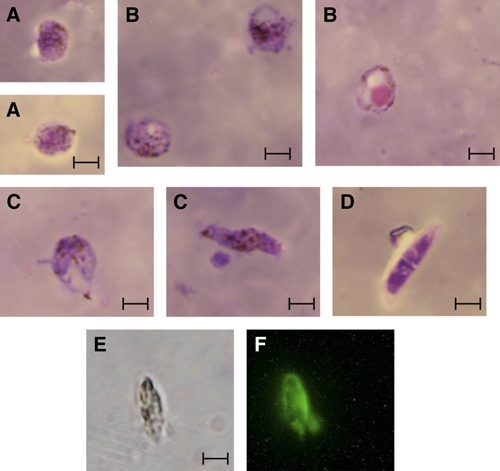
Photomicrographs of in vitro produced Plasmodium vivax sexual stage parasites. Panels: Giemsa-stained thin blood smears: A, Zygotes; B, Early retort forms; C, Maturing retort forms/Early ookinetes; D, Mature ookinete; E, Bright-field microscope of P. vivax ookinete; F, Immunofluorescence microscopy of a fixed P. vivax ookinete stained with polyclonal rabbit anti-Pvs230, with FITC-labeled goat anti-rabbit antibody used as secondary antibody. Bar 5 μm. This figure appears in color at www.ajtmh.org.
The best in vitro ookinete culture protocol presented here allowed for the reliable production of large quantities of high quality P. vivax ookinetes suitable for further studies. By properly solubilizing xanthurenic acid in NaHCO3 during gametogenesis a 43-fold increase in ookinete yield over a previously published protocol was obtained.8 An additional 3-fold increase was obtained using a gametogenesis solution modified from Peiris and others.19 The final 2-fold increase in ookinete yield was achieved by further modifying the gametogenesis solution by removing fetal bovine serum and by not carrying out Percoll gradient purification before ookinete culture; such handling of the parasites seems to impair the efficiency of ookinete development.20 The final protocol achieved a 243-fold increase in ookinete yield, a 2-fold increase in the percentage of cultures that produced ookinetes, and a 60-fold increase in the percent gametocyte conversion over previously published protocols.
There were three important limitations of this study. First, in vitro ookinete formation was not compared with natural ookinete formation within the mosquito gut of experimentally infected mosquitoes. In the previous study from Thailand, there was only a loose, non-statistically significant association between mosquito midgut and in vitro ookinete formation.9 Second, parasitized blood was only obtained from patients with symptomatic acute vivax malaria accompanied by low parasitemia (~0.1%) as convenience samples. Obtaining blood from patients with higher parasitemia would likely improve ookinete yields. Third, mature P. vivax ookinetes were not assessed for motility or mosquito infectivity. Future work will address these limitations.
Although this improved protocol will allow for advances in the study of P. vivax sexual stages, the production of sexual stage parasites will remain difficult until reproducible methods to continuously propagate P. vivax in vitro are developed, including the production of functional gametocytes.21,22 Obtaining parasites ex vivo from humans introduces unavoidable variability into laboratory studies of P. vivax, which may be caused by host factors or differences in the strains of parasites used in a single study. The yield of P. vivax ookinete remains far less than from the rodent parasites Plasmodium berghei and Plasmodium yoelii, but nonetheless enables investigators to obtain sufficient material to directly study the ookinetes of this human malaria parasite without necessitating the need to analogize with animal models. The development of an in vitro culture method allowing for the enrichment of P. vivax gametocytes would greatly assist in the high volume production of sexual stage parasites.
Acknowledgments
We thank the field staff of the Iquitos Satellite Laboratory for their assistance in sample collection.
Footnotes
Financial support: This work was supported by U.S. Public Health Service grants 5RO1AI45999, 1D43TW007120, K24AI068903, and R01AI067727.
Authors' addresses: Colleen M. McClean, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA; current address: PCV, Peace Corps/TOGO, Lomé, Togo, E-mail: cmmcclean@gmail.com. Haydee Guerra Alvarado, Asociación Benéfica PRISMA, Lima, Peru, E-mail: haydeeprisma@gmail.com. Victor Neyra and Alejandro Llanos-Cuentas, Universidad Peruana Cayetano Heredia, Instituto de Medicina Tropical Alexander von Humboldt, Lima, Perú, E-mails: victor.neyra@upch.pe and elmer.llanos@upch.pe. Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA, E-mail: jvinetz@ucsd.edu.
References
- 1.Mendis KN. The neglected burden of P. vivax malaria. Am J Trop Med Hyg. 2001;164:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 2.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 4.Sinden RE. Sexual development of malarial parasites in their mosquito vectors. Trans R Soc Trop Med Hyg. 1981;75:171–172. doi: 10.1016/0035-9203(81)90058-4. [DOI] [PubMed] [Google Scholar]
- 5.Kaslow DC. Transmission-blocking vaccines: uses and current status of development. Int J Parasitol. 1997;27:183–189. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 6.Poudel SS, Newman RA, Vaughan JA. Rodent Plasmodium: population dynamics of early sporogony within Anopheles stephensi mosquitoes. J Parasitol. 2008;94:999–1008. doi: 10.1645/GE-1407.1. [DOI] [PubMed] [Google Scholar]
- 7.Zollner GE, Ponsa N, Garman GW, Poudel GW, Bell JA, Sattabongkot J, Coleman RE, Vaughan JA. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar J. 2006;5:68. doi: 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwanabun N, Sattabongkot J, Tsuboi T, Torii M, Maneechai N, Rachapaew N, Yim-amnuaychok N, Punkitchar V, Coleman RE. Development of a method for the in vitro production of Plasmodium vivax ookinetes. J Parasitol. 2001;87:928–930. doi: 10.1645/0022-3395(2001)087[0928:DOAMFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattagongkot JS, Suwanabun N, Punkitchar V, Coleman RE. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun. 2000;68:6618–6623. doi: 10.1128/iai.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein MP, Alcid DV. Occurrence of exflagellation and microgametes in peripheral blood of a patient with malaria. J Infect Dis. 1982;146:448. doi: 10.1093/infdis/146.3.448. [DOI] [PubMed] [Google Scholar]
- 11.Ford JC, Wadsworth LD. Exflagellating Plasmodium vivax in peripheral blood. Arch Pathol Lab Med. 2003;127:117–118. doi: 10.5858/2003-127-117-EPVIP. [DOI] [PubMed] [Google Scholar]
- 12.Hummert BA. Plasmodium vivax ookinetes in human peripheral blood. J Clin Microbiol. 1994;32:2578–2580. doi: 10.1128/jcm.32.10.2578-2580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westenberger SJ, McClean CM, Chattopadhyay R, Dharia NV, Carlton JM, Barnwell JW, Collins WE, Hoffman SL, Zhou Y, Vinetz JM, Winzeler EA. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis. 2010;4:e653. doi: 10.1371/journal.pntd.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihalamulla RL, Mendis KN. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1987;81:25–28. doi: 10.1016/0035-9203(87)90271-9. [DOI] [PubMed] [Google Scholar]
- 15.Knight A, Sinden RE. The purification of gametocytes of Plasmodium falciparum and P. yoelii nigeriensis by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1982;76:503–509. doi: 10.1016/0035-9203(82)90150-x. [DOI] [PubMed] [Google Scholar]
- 16.Kariuki MM, Kiaira JK, Mulaa FK, Mwangi JK, Wasunna MK, Martin SK. Plasmodium falciparum: purification of the various gametocyte developmental stages from in vitro-cultivated parasites. Am J Trop Med Hyg. 1998;59:505–508. doi: 10.4269/ajtmh.1998.59.505. [DOI] [PubMed] [Google Scholar]
- 17.Patra KP, Johnson JR, Cantin GT, Yates JR, 3rd, Vinetz JM. Proteomic analysis of zygote and ookinete stages of the avian malaria parasite Plasmodium gallinaceum delineates the homologous proteomes of the lethal human malaria parasite Plasmodium falciparum. Proteomics. 2008;8:2492–2499. doi: 10.1002/pmic.200700727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, Henson K, Nussenzweig V, Carlton J, Vinetz JM, Duraisingh MT, Winzeler EA. Evidence-based annotation of the malaria parasite's genome using comparative expression profiling. PLoS One. 2008;3:e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiris JS, Premawansa S, Ranawaka MB, Udagama PV, Munasinghe YD, Nanayakkara MV, Gamage CP, Carter R, David PH, Mendis KN. Monoclonal and polyclonal antibodies both block and enhance transmission of human Plasmodium vivax malaria. Am J Trop Med Hyg. 1988;39:26–32. doi: 10.4269/ajtmh.1988.39.26. [DOI] [PubMed] [Google Scholar]
- 20.Carter EH, Suhrbier A, Beckers PJ, Sinden RE. The in vitro cultivation of P. falciparum ookinetes, and their enrichment on Nycodenz density gradients. Parasitology. 1987;95:25–30. doi: 10.1017/s0031182000057516. [DOI] [PubMed] [Google Scholar]
- 21.Hurd H, Al-Olayan E, Butcher GA. In vitro methods for culturing vertebrate and mosquito stages of Plasmodium. Microbes Infect. 2003;5:321–327. doi: 10.1016/s1286-4579(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 22.Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J. Cultivation of Plasmodium vivax. Trends Parasitol. 2008;24:85–88. doi: 10.1016/j.pt.2007.09.010. [DOI] [PubMed] [Google Scholar]


