Abstract
Rapid diagnostic tests (RDTs) were developed as an alternative to microscopy for malaria diagnosis. The RDTs detect malaria parasite antigen(s) in whole blood with high sensitivity and specificity. We assessed health worker malaria treatment practices after the introduction of RDTs in peripheral health facilities without microscopy. From December 2007 to October 2008, we introduced histidine-rich protein II (HRP-2)-based ParaHIT RDTs for routine use in 12 health facilities in Rufiji District, Tanzania. Health workers received training on how to perform RDTs for patients 5 years of age or older with fever or suspected malaria. Children < 5 years of age were to be treated empirically per national guidelines. Among the 30,195 patients seen at these 12 health facilities, 10,737 (35.6%) were tested with an RDT for malaria. 88.3% (9,405/10,648) of tested patients reported fever or history of fever and 2.7% (289/10,677) of all tested individuals were children < 5 years of age. The RDT results were recorded for 10,650 patients (99.2%). Among the 5,488 (51.5%) RDT-positive patients, 5,256 (98.6%) were treated with an appropriate first-line antimalarial per national guidelines (artemether-lumefantrine or quinine). Among the 5,162 RDT-negative patients, only 205 (4.0%) were treated with an antimalarial. Other reported treatments included antibiotics and antipyretics. Implementation of RDTs in rural health facilities resulted in high adherence to national treatment guidelines. Patients testing negative by RDT were rarely treated with antimalarials. Unapproved antimalarials were seldom used. Health workers continued to follow guidelines for the empiric treatment of febrile children.
Introduction
Developing countries have, for many years, relied on clinical signs and symptoms to guide the diagnosis of the majority of malaria cases. Microscopic examination of blood smears requires trained personnel and relatively expensive laboratory equipment, typically unavailable in peripheral health facilities in sub-Saharan Africa. As a result patients are often diagnosed and treated based only on clinical symptoms. This results in significant over-diagnosis of malaria and subsequent over-prescription of antimalarial drugs, which may promote antimalarial drug resistance.1,2
Because of increasing parasite resistance to antimalarial drugs the World Health Organization (WHO) has recommended the use of artemisinin-based combination therapy (ACT) for the treatment of uncomplicated malaria. Given the relatively high cost of ACTs, there is renewed interest in treating only laboratory-confirmed malaria cases to reduce expenditure on ACTs, to diminish side effects from unnecessary treatments, and to decrease drug pressure on parasites that leads to resistance. Rapid diagnostic tests (RDTs) are seen as a way of extending parasitological diagnosis of malaria to peripheral health facilities without microscopy. The RDTs detect parasite antigens from a peripheral blood sample with reasonable sensitivity and specificity and can be used at peripheral health facilities with minimal training.3,4 However, persistence of parasite antigen in the blood following successful treatment of malaria infection may complicate clinical decisions in patients with persistent fever.
When laboratory confirmation of malaria is available, studies have shown that clinicians tend to prescribe antimalarial drugs even with a negative test result.2,5–7 This prescription practice may result in higher morbidity and mortality because the true cause of illness can be left untreated.2 The World Health Organization's integrated management of childhood illness (IMCI) strategy and the Tanzanian National Malaria Control Program 2006 guidelines for the treatment of malaria recommend presumptive treatment of children < 5 years of age presenting to health facilities with fever or history of fever in malaria endemic areas. Studies have reported extension of this practice to older children and adults, even when laboratory diagnosis of malaria is available.6,8 Tanzanian malaria treatment guidelines further recommend that patients with suspected malaria receive an RDT when available and that only those patients with positive results receive treatment with artemether-lumefantrine (AL) for uncomplicated malaria or quinine for severe disease. To observe the impact of RDT availability and final result on clinical decision making, we evaluated healthcare worker use of rapid diagnostic tests in the routine management of uncomplicated malaria in rural Tanzania.
Methods
Design.
This was an observational study of the implementation of malaria rapid diagnosis tests (ParaHIT, Span Diagnostics, Ltd., Surat, India) in 12 dispensaries (peripheral health facilities), in Rufiji District, Tanzania from December 2007 to October 2008. The RDTs introduced target the histidine-rich protein II (HRP-2) specific to Plasmodium falciparum parasites. The 12 dispensaries were chosen by convenience for their location within a demographic and health surveillance site.
Location.
Rufiji District is an area of holoendemic malaria transmission with > 200,000 inhabitants served by ~59 health facilities. Acute febrile illnesses, including malaria, are the leading cause of death in the district.9
Training.
A single day of training was provided to 99 health workers and volunteers at the implementation health facilities or at nearby schools. Contents of the training included local malaria epidemiology, importance of rapid diagnostic tests, patient testing criteria, what to do for patients with positive and negative test results, demonstration of how to perform a rapid test, practice in reading and recording test results, proper waste disposal, and practical sessions where participants performed RDTs on febrile patients or each other. Job aids, IMCI guidelines, and national malaria treatment algorithms were reviewed and copies were provided to each facility.
Implementation.
Health workers were instructed to use RDTs in patients 5 years of age and older presenting with fever or history of fever in the past 48 hours or in whom they suspected malaria. Presence of fever, RDT result, diagnosis, and treatment provided were recorded for each patient tested. For the purpose of RDT quality control, thick blood smears were collected from all patients tested with RDTs for 2–3 days every week and sent to a central facility for staining and reading by a reference microscopist. Results of the blood smear and RDTs were compared to assess test performance in terms of test sensitivity and specificity.10 Once a month, a member of the district health management team or study team member conducted a supervisory visit to assess health workers' performance of RDTs and progress of the implementation. Health facility patient registers were reviewed to determine the total number of patients seen during the study period and the number diagnosed with malaria. Data was also abstracted from every fifth patient recorded in registers to estimate the percentage of patients presenting to the health facilities with fever, and the percent of febrile patients tested with RDTs.
Data analysis.
Patient level data on age, sex, presence of fever, and RDT results were recorded on pre-printed study forms. After review by the study team data were entered in EPI Info version 3.3.2 (CDC, Atlanta, GA) and analyzed with STATA version 10 (StataCorp, College Station, TX).
Ethical review.
The evaluation was granted a non-research determination by the Ifakara Health Institute and U.S. Centers for Disease Control and Prevention (CDC) institutional review boards.
Results
Description of study patients.
A total of 30,195 clinical visits for all-causes were recorded at the 12 facilities during the study period. In facility registers 15,043 (49.8%) patients had a diagnosis of malaria of which 9,292 (61.7%) were children < 5 years of age. Of the 9,292 children < 5 years of age diagnosed with malaria, 9,109 (98.0%) were treated presumptively for malaria without performing an RDT. 10,737 (35.6%) patients were sent for malaria RDTs and RDT results were recorded for 10,650 (99.2%) of them and 5,488 (51.5%) had a positive test result (Figure 1). Only 226 adults and children 5 years of age or older were treated presumptively for malaria without a malaria RDT being performed and the frequency of presumptive treatment did not vary seasonally. 205 (4.0%) of the 5,162 patients who tested negative were treated for malaria, and 15 people whose malaria test results were not recorded were also treated for malaria.
Figure 1.
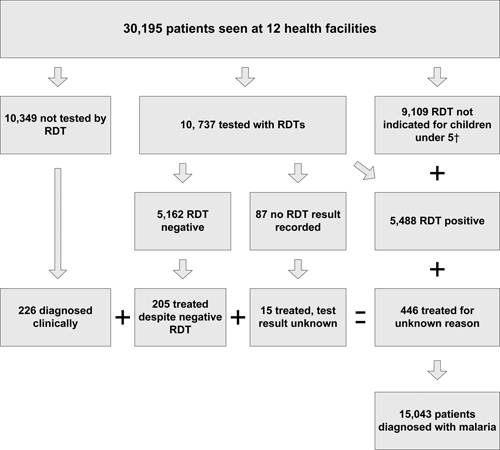
Description of patients seen, tested, and diagnosed with malaria at 12 health facilities where rapid diagnostic tests (RDTs) were introduced, Rufiji District, Tanzania, December 2007–October 2008. †Per Tanzanian National Guidelines (2006) febrile children < 5 years of age should be treated presumptively for malaria.
Abstracting information on every fifth patient recorded in health facility registers revealed that ~55.8% of patients presenting to the health facility reported fever in the last 48 hours, and 46.4% of febrile patients were tested with RDTs. According to data recorded in RDT log books, fever or history of fever in the last 48 hours was reported by 88.3% (9,405/10,648) of tested patients. Among the 10,366 adults with RDT results recorded, 5,300 (51.1%) tested positive and 2.7% (289/10,677) of all tested individuals with age recorded were children < 5 years of age. Among the 284 children < 5 tested with RDT results recorded, 188 (66.2%) tested positive. Children tested were more likely to have a positive result than tested adults and children aged 5 and older (odds ratio [OR] = 1.87, 95% confidence interval [CI] = 1.46, 2.40); however, few children < 5 were tested and those tested may not be representative of all children < 5. Female patients accounted for 60.9% (6,514/10,696) of all patients tested with RDTs whose sex was reported, and 352 (5.5%) were pregnant. Among the 352 pregnant women tested, 82.1% (288) reported fever and 45.2% (159) tested positive by RDT. The 585 (5.4%) of tested patients reported use of antimalarial drugs within the past 3 weeks, and 454 (77.6%) tested positive by RDT.
Management of patients tested with RDTs.
98.6% (5,256/5,488) of patients with positive RDT results were treated with an appropriate first-line antimalarial as per national guidelines, i.e., AL or quinine (Table 1). Among the 5,162 RDT negative patients, 205 (4.0%) were treated with an antimalarial. Among patients seen from May to October 2008, other commonly reported treatments included antipyretics and non-steroidal anti-inflammatory drugs such as paracetamol (41.0%), acetyl salicylic acid (11.5%) and/or antibiotics (35.6%). Patients with positive RDT results frequently received both an antimalarial and an antipyretic. Among the 352 pregnant women tested by RDT, 155 (97.5%) of the 159 RDT-positive women received treatment with a first-line antimalarial and 10 (5.2%) of the 193 RDT negative women received antimalarial therapy.
Table 1.
Type of antimalarial drug given to patients treated for malaria in 12 health facilities where RDTs were introduced, Rufiji District, Tanzania, December 2007–October 2008*
| Antimalarial drug | RDT result | Total (%) | |
|---|---|---|---|
| Positive (%) | Negative (%) | ||
| Artemether-lumefantrine | 4,221 (79.2) | 178 (86.8) | 4,399 (79.5) |
| Quinine | 1,035 (19.4) | 17 (8.3) | 1,052 (19.0) |
| Amodiaquine | 43 (0.8) | 3 (1.5) | 46 (0.8) |
| Sulphadoxine-pyrimethamine | 30 (0.6) | 5 (2.4) | 35 (0.6) |
| Chloroquine | 2 (0.1) | 2 (1.0) | 4 (0.1) |
| Total | 5,331 (96.3) | 205 (3.7) | 5,536 (100) |
RDTs = rapid diagnostic tests.
Discussion
This implementation has shown a high level of clinicians' acceptance of malaria rapid diagnostic tests and appropriate use of test results to guide clinical management of febrile patients. The overwhelming majority of patients tested with RDTs had a history of fever or presented with fever to the facility, indicating appropriate case selection for RDT testing. Over half of all patients tested with RDTs had a positive result. The percentage of positive RDT results increased as would be expected in the months following the rainy season and then gradually declined.10 Over half of the RDTs were read as positive, and clinician confidence in RDT results remained high. It is unclear if similar confidence in RDT results would be found in an area of lower transmission where a smaller proportion of RDTs would be positive. However, we did not see an increase in the number of RDT-negative patients treated with antimalarials during months where the percentage of positive RDT results was relatively lower.
The study also showed good adherence to the country's malaria treatment policy. In light of increased antimalarial drug resistance, the Tanzanian NMCP changed national malaria treatment policy twice within less than a decade. In late 2001, chloroquine (CQ) was replaced with sulphadoxine-pyrimethamine (SP) as the first-line treatment of uncomplicated malaria, and in 2006, SP was replaced by artemether-lumefantrine. Quinine remains the drug of choice for severe malaria. A very high proportion of confirmed malaria cases in this study received AL and quinine, as did most of the relatively few treated with antimalarials despite a negative test.
Only 2.0% of children < 5 years of age that were seen at health facilities were tested with RDTs and the remainder received presumptive treatment of malaria according to the 2006 national malaria guidelines. An even smaller percentage of adults and children 5 years of age and older were treated for malaria without being tested. This shows that different treatment policies can be successfully implemented for children under 5 (presumptive treatment of febrile illness per IMCI guidelines) and adults and older children based on RDT results. Although previous studies have documented concerns that IMCI presumptive treatment policies might be extended to older age groups,6,8 this study did not document such extension. However, no RDT stockouts were observed during the study period. It is likely that in the absence of a diagnostic test such extension might occur.
The WHO has recently recommended that all patients with suspected malaria should receive confirmatory testing.11 Given the limitations in guaranteeing adequate supplies of other malaria commodities (ACTs, long lasting insecticidal nets [LLINs], etc.) and the frequent stockouts of RDTs observed in areas where RDTs have already been introduced, an implementation strategy that limits diagnostic confirmation of malaria to adults and children 5 years of age and older may be a logical way of rationing available rapid diagnostic tests. Given the variability in RDT performance in the WHO/Foundation for Innovative New Diagnostics (FIND) product testing,12 it may take several years to have sufficient numbers of quality manufacturers of RDTs to meet the global demand necessary to follow universal testing guidelines. Insufficient funding of diagnostics, poor forecasting of needs and distribution of supplies, and inadequate human resource capacity may further limit universal testing initiatives. In the interim, continuation of the IMCI policy of presumptive treatment of children < 5 years of age may be a reasonable alternative until RDT stocks are sufficient to meet demand. However, universal testing of suspected malaria cases before treatment should remain the objective.
Training for this implementation was conducted at each health facility or a nearby school. On-site training made it possible for all staff members and volunteers present on the training day to participate. Because nearly every staff member had received RDT training, all personnel performed RDTs and supervisory visits provided an opportunity to inform and train individuals not present on the original training date. Supervisory visits increased confidence in the diagnostic test and provided an opportunity for feedback on how the tests were performing and being used for clinical decision making. Job aides and training materials were also used extensively by health facility staff.
In this study about 5% of patients tested with RDTs had received antimalarial treatment in the 3 weeks before presentation. Because HRP2 antigens can continue to circulate for up to 56 days (median 32 days) after appropriate antimalarial treatment,13 it is possible that some tested patients were not parasitemic and did not require antimalarial treatment. The message that febrile patients who received antimalarial treatment in the 3 weeks before presentation should be referred to a higher level facility with microscopy must be reinforced during supervisory visits to reduce unnecessary retreatment, detect treatment failures, or identify other causes of fever. Appropriate referral of patients previously treated with antimalarials remains a challenge in rural health facilities.
There are several limitations to this evaluation of RDT use in routine case management. First, there was limited data collected from patients who were not tested for malaria. Therefore, we do not know if the patients who were presumptively treated for malaria had signs of severe disease or other reasons for presumptive malaria treatment (jaundice, hepatosplenomegally, etc.). Furthermore, we did not collect additional data on clinical signs and symptoms other than the presence or absence of fever among patients tested with RDTs. As such, we are unable to evaluate the potential reasons that patients may have received antimalarial treatment with a negative RDT result. We are also unable to assess the correct prescription of antibiotics to patients that tested RDT negative. However, we are encouraged that the majority of RDT-negative patients with alternative treatments recorded were treated with antipyretics or anti-inflammatory drugs. This may indicate that as malaria prevalence decreases in Tanzania clinicians are becoming increasingly comfortable with the differential diagnosis of febrile illness, which includes a high proportion of viral illnesses in all epidemiologic settings.
Implementation of RDTs in rural health facilities resulted in high adherence to national treatment guidelines. Emphasis should be placed on providing clear treatment algorithms, quality control, and supervision. Patients testing negative by RDT were rarely treated with antimalarials. Unapproved antimalarials were seldom used. Quality assurance increased health worker's confidence in RDT performance and remains an essential part of RDT implementation. Health workers continued to follow national guidelines for the empiric treatment of febrile children. Clinicians frequently used antipyretics, anti-inflammatory drugs, and antibiotics to treat RDT-negative patients, which may indicate increasing awareness of the differential diagnosis of febrile illness.
Acknowledgments
We thank the other members of our research team: Buzingwa Bofu and Abdallah Bakari (data collectors), Bakari Kissa (microscopist), Shaban Masoud (CHMT lab supervisor), Said Mkikima (District Medical Officer), Christopher Membi (Laboratory technologist at Bagamoyo) for their contributions to this evaluation. We also thank Salim Abdulla (IHI Director) and Alex Mwita (National Malaria Programme Manager) for their guidance.
Footnotes
Financial support: This work was financially supported by a cooperative agreement with the U.S. Centers for Disease Control and Prevention and the United States Agency for International Development (USAID) under the U.S. President's Malaria Initiative (PMI).
Authors' addresses: M. Irene Masanja, Ifakara Health Institute, Dar-es-Salaam, Tanzania, E-mail: imasanja@ihi.or.tz. Meredith McMorrow, Malaria Branch, Center for Global Health, U.S. Centers for Disease Control and Prevention, Atlanta, GA, and U.S. Public Health Service, Rockville, MD, E-mail: MMcMorrow@cdc.gov. Elizeus Kahigwa, Ifakara Health Institute, Dar-es-Salaam, Tanzania, E-mail: ekahigwa@yahoo.com. S. Patrick Kachur, Malaria Branch, Center for Global Health, U.S. Centers for Disease Control and Prevention, Atlanta, GA, and U.S. Public Health Service, Rockville, MD, E-mail: spk0@cdc.gov. Peter D. McElroy, CDC–Tanzania office, Dar-es-Salaam, Tanzania, and President's Malaria Initiative, Malaria Branch, Center for Global Health, U.S. Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mcelroyp@tz.cdc.gov.
References
- 1.Singer B, Teklahaimanot A. Background Paper of the Millenium Project Task Force on Major Diseases and Access to Medicine, Subgroup on Malaria. New York: United Nations Development Program (UNDP); 2003. [Google Scholar]
- 2.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood MB, Whitty CJM. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proux S, Hkirijareon L, Ngamngonkiri C, McConnel S, Nosten F. Paracheck-Pf: a new, inexpensive and reliable rapid test for P. falciparum malaria. Trop Med Int Health. 2001;6:99–101. doi: 10.1046/j.1365-3156.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 5.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in low transmission area of Tanzania. Malar J. 2006;5:4. doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJ. Rapid diagnostic tests compare with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomized trial. BMJ. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makani J, Matuja W, Liyombo E, Snow RW, Marsh K, Warell DA. Admission diagnosis of cerebral malaria in adults in an endemic area of Tanzania: implications and clinical description. Q J Med. 2003;96:355–362. doi: 10.1093/qjmed/hcg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zurovac D, Njogu J, Akhwale W, Hamer DH, Larson BA, Snow RW. Effects of revised diagnostic recommendations on malaria treatment across age groups in Kenya. Trop Med Int Health. 2008;13:784–787. doi: 10.1111/j.1365-3156.2008.02072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rufiji DSS. Tanzania. Tanzania Ministry of Health and Tanzania Essential Health Interventions Project. INDEPTH Monograph. 1999;Volume 1 http://www.indepth-network.org/dss_site_profile/rufiji.pdf Part C. Available at. Accessed March 15, 2010. [Google Scholar]
- 10.McMorrow ML, Masanja MI, Kahigwa E, Abdulla SM, Kachur SP. Quality assurance of rapid diagnostic tests for malaria in routine patient care in rural Tanzania. Am J Trop Med Hyg. 2010;82:151–155. doi: 10.4269/ajtmh.2010.09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Guidelines for the Treatment of Malaria. Second edition. 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html Available at. Accessed March 15.
- 12.World Health Organization Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 1. 2009. 2010. http://www.finddiagnostics.org/export/sites/default/media/press/pdf/Full-report-malaria-RDTs.pdf Available at. Accessed March 8.
- 13.Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]


