Abstract
Mosquito surveillance was carried out in three forested regions of Trinidad during July 2007–March 2009. A total of 185,397 mosquitoes representing at least 46 species was collected, divided into pools of 1–50 mosquitoes according to species and sex, and screened for arboviruses using cytopathic effect assays on Vero cell monolayers. Eighty-five viruses were isolated, including members of the genera Alphavirus (Mucambo virus; MUCV) and Orthobunyavirus (Caraparu, Oriboca, Bimiti, and Wyeomyia viruses). Species of the Culex subgenus Melanoconion accounted for 56% of the total number of mosquitoes collected and 97% of the viruses isolated; Cx. (Mel.) portesi accounted for 92% of virus isolations. Our results also implicate for the first time Aedes (Ochlerotatus) hortator as a potential vector of MUCV. Phylogenetic analyses of 43 MUCV strains suggest population subdivision within Trinidad, consistent with the hypothesis of enzootic maintenance in localized rodent populations.
Arboviruses, such as dengue, chikungunya, yellow fever, and West Nile virus, are important public health problems of increasing concern in both tropical and temperate regions. In Trinidad, dengue and yellow fever virus activity have been well documented and characterized, but there has been little recent research on the presence of other arboviruses since the extensive surveillance studies that were performed nearly 50 years ago.1–7 These studies demonstrated the presence of a wide range of arboviruses, particularly in the Bush Bush Forest (BBF) and Aripo Savannah Scientific Reserve (ASSR), which are ecologically distinct scientific reserves in eastern Trinidad. In the intervening years, the island has undergone considerable urbanization and the regions previously surveyed have been encroached on by squatters, and have been impacted by activities, such as quarrying, hunting, and farming.
To characterize the current arboviral diversity in Trinidad, we performed mosquito surveillance studies in the ASSR and BBF during July 2007–March 2009. Limited surveillance was also performed at the Asa Wright Nature Center at the Heights of Aripo (HOA) in September and October 2008. We recently reported the detection of Mucambo virus (MUCV, a member of the Venezuelan equine encephalitis [VEE] virus complex) and Guama group and group C orthobunyaviruses in mosquitoes from the ASSR, none of which were identified to species.8 We report additional surveillance in the ASSR (total = 21 months), 7 months at the BBF, and 1 month at the HOA, and further identification and characterization of the viruses isolated.
Mosquitoes were collected on average once per week using 6, 12, or 18 dry ice–baited miniature Centers for Disease Control light traps (John Hock, Gainesville, FL) that were deployed 1.5 meters above the ground and operated for 12–16-hour periods. Throughout the study period, traps were set along the same transects (6 traps per transect), which traversed different ecologic zones, including savannah, ecotone, forest, and marshlands. During a period of yellow fever virus (YFV) epizootic activity, limited canopy and human landing collections were also performed at the BBF. Trapped mosquitoes were sorted into pools of 1–50 individuals according to species and sex and assayed for viruses by cytopathic effect assays on Vero cell monolayers as described.8 If cytopathic effect was detected, the virus was re-isolated in Vero cells for confirmation, and the cell culture supernatant was collected and stored at −70°C for further characterization by serologic analysis and viral sequencing.
Isolates were identified by complement fixation tests adapted from Fulton and Dumbell9 by using monoclonal antibodies specific for alphaviruses (family Togaviridae, genus Alphavirus), flaviviruses (family Flaviviridae, genus Flavivirus) and orthobunyaviruses (group C, Guama group and Bunyamwera group) with supernatants from infected Vero cells as the source of antigens. After serologic classification, viral RNA was extracted by using viral RNA extraction kits (Qiagen, Valencia, CA), and reverse transcription–polymerase chain reactions were performed on the alphaviruses using group-specific primers that anneal to the nonstructural protein 1 gene.10 Amplicons were sequenced and used for homology searches in GenBank. All isolated alphaviruses were subsequently identified as MUCV based on nucleotide homology. Amplification primers (26S and 6kE1), sequencing primers (8432F, 8638R, 8811F, 9132R), and polymerase chain reaction conditions were used as described by Auguste and others8 to amplify and sequence a 2,079-nucleotide fragment specifying envelope glycoproteins E3 and E2 for further phylogenetic analysis. All nucleotide sequences derived in this study were submitted to GenBank (accession nos. HM598641–HM598665).
The 25 MUCV sequences derived in this study were aligned with the 18 MUCV strains available in GenBank and trimmed to 2,079 nucleotides. The data set of 43 sequences was used to construct a maximum likelihood (ML) phylogeny, and bootstrapping was subsequently performed using 1,000 replicate neighbor-joining trees under an ML substitution model. All ML analyses were performed with PAUP*.11
A total of 185,397 mosquitoes representing at least 46 species was collected from the three sites over 752 trap nights during July 2007–March 2009 (Supplemental Table S1). Most sampling was performed at the ASSR (605 trap nights), where 123,384 mosquitoes belonging to 37 species were collected. At the BBF, 58,162 mosquitoes representing 35 species were collected over 104 trap nights, and at the HOA, 3,851 mosquitoes (21 species) were collected over 43 trap nights. Members of the Culex subgenus Melanoconion accounted for 56% of the total number of mosquitoes. The four species that predominated in the collection were Cx. (Mel.) portesi (30%), Coquillettidia (Rhynchotaenia) venezuelensis (14%), Cx. (Mel.) pedroi (13%), and Aedes (Ochlerotatus) serratus (9%), all of which have been implicated as arboviral vectors.6,7 Coquillettidia venezuelensis, a reported vector of Oropouche and Mayaro viruses2,6 was only collected at the BBF, and mosquitoes of the other three species were collected at all three sampled locations. No YFV was isolated from the small numbers of Haemagogus and Sabethes spp. mosquitoes (known YFV vectors) collected during a period of YFV epizootic activity12,13 at the BBF.
The mosquitoes collected were processed in 9,148 pools that were screened for arboviruses. Eighty-five (85) Vero cell cytopathic viruses were isolated resulting in an overall maximum likelihood estimation of pooled mosquito infection rate (MLE) of 0.47 (95% confidence interval [CI] = 0.37–0.57). Although species of the Melanoconion subgenus accounted for only 56% of the total number of mosquitoes collected, 97% of virus isolations (n = 82) were from Culex subgenus Melanoconion mosquitoes [Cx. (Mel.) portesi, Cx. (Mel.) pedroi, Cx. (Mel.) spissipes, and Cx. (Mel.) vomerifer]; Cx. (Mel.) portesi accounted for 92% (n = 78, MLE = 1.43, 95% CI = 1.14–1.78). The remaining three isolations were from Ae. (Och.) hortator, Trichoprosopon digitatum, and Wyeomyia spp. mosquitoes. The high isolation rates for the Melanoconion subgenus and Cx. (Mel.) portesi in particular, highlight the importance of this subgenus in the transmission of alphaviruses and orthobunyaviruses in Trinidad. Similar results were also reported for the Amazon region of Peru by Turrell and others.14
The ASSR was the most productive of the three study sites with 70 isolations (MLE = 0.58 (95% CI = 0.46–0.73), while the BBF yielded 13 (i.e., MLE = 0.22, 95% CI = 0.13–0.37). At the HOA, there were only two isolations but the MLE of 0.52 (95% CI = 0.09–1.7) was similar to that of the ASSR. At the ASSR, there was a statistically significant difference (P < 0.05) between the number of viruses detected in each ecological zone, and the ecotone yielded the largest proportion (n = 38, 54%) and highest diversity of viruses, followed by the forest (n = 30, 43%) and savannah (n = 2, 3%). In contrast, at the BBF, the differences between ecologic zones were not statistically significant. For the BBF, assuming only one mosquito was positive in a pool, the minimum infection rate in the current study was estimated to be 0.2/1,000 specimens, in contrast to the rate of 0.7/1,000 in a five-year BBF mosquito surveillance study during 1959–1964.6 This lower infection rate may be accounted for by differences in mosquito species diversity resulting from the use of a larger range of mosquito collection techniques in the earlier studies, which included human and rodent bait, canopy trapping, and sampling over larger areas. Although the overall mosquito diversity and infection rate were different, the five viruses isolated and their relative proportions were similar, with MUCV isolated in the largest numbers, followed by Bimiti, Caraparu and Oriboca virus, although many other viruses were isolated at lower frequencies during the earlier study.
The 85 viruses isolated during our study were serologically classified as VEE complex viruses, Guama group (Bimiti virus), Wyeomyia complex viruses, and group C (Caraparu virus and Oriboca virus), all of which had been isolated in Trinidad.6,7 All of the VEE complex viruses were confirmed as MUCV on the basis of nucleotide sequencing and homology searches, and showed > 98% sequence similarity across an ~400-nucleotide fragment of the nonstructural protein 1 gene of the reference MUCV strain (BeAn8; AF075253). Our isolation of MUCV from Ae. (Och.) hortator is the first to be described for this species. However, given the high MUCV infection rate and the fact that only one isolate was obtained from this species, the possibility of contamination during the preparation of mosquito pools [e.g., inclusion of a leg from another species in the Ae. (Och.) hortator pool] cannot be ruled out. The observed vector–virus associations are shown in Table 1.
Table 1.
Virus and vector associations observed in this study (Trinidad)
| Virus (total no. isolates) | Mosquito species | No. isolates | % Isolates from mosquito species indicated | MLE pooled infection rates (95% CI)* |
|---|---|---|---|---|
| Family Togaviridae, genus Alphavirus | ||||
| Mucambo virus (n = 47) | Culex (Melanoconion) portesi (Senevet & Abonnenc)† | 45 | 95.7 | 0.82 (0.60–1.08) |
| Culex (Mel.) spissipes (Theobald) | 1 | 2.1 | 0.29 (0.02–1.39) | |
| Aedes (Ochlerotatus) hortator (Dyar & Knab) | 1 | 2.1 | 0.4 (0.02–1.95) | |
| Family Bunyaviridae, genus Orthobunyavirus | ||||
| Wyeomyia virus (n = 2) | Trichoprosopon digitatum | 1 | 50.0 | 3.12 (0.18–14.94) |
| Wyeomyia spp. | 1 | 50.0 | 0.19 (0.01–0.93) | |
| Guama group | ||||
| Bimiti virus (n = 17) | Culex (Mel.) portesi (Senevet & Abonnenc) | 17 | 100.0 | 0.31 (0.18–0.48) |
| Group C | ||||
| Caraparu virus (n = 16) | Culex (Mel.) portesi (Senevet & Abonnenc)† | 13 | 81.3 | 0.23 (0.13–0.39) |
| Culex (Mel.) vomerifer (Komp) | 2 | 12.5 | 0.70 (0.12–2.27) | |
| Culex (Mel.) pedroi | 1 | 6.3 | 0.04 (0.00–0.20) | |
| Oriboca virus (n = 3) | Culex (Mel.) portesi (Senevet & Abonnenc) | 3 | 100.0 | 0.05 (0.01–0.14) |
MLE = mosquito infection rate; CI = confidence interval.
Three pools were co-infected with Mucambo virus and Caraparu virus.
The number of mosquitoes collected (all species) and the number of Cx. (Mel.) portesi collected per trap-night and viruses isolated for each month of collection are shown in Figure 1. Most virus isolates were from mosquitoes collected during the rainy season (June–September), with a peak in October (Figure 1). No statistically significant correlations (P < 0.05, by Pearson's correlation) were detected among the parameters investigated, which included numbers of mosquitoes (all species) and number of Cx. (Mel.) portesi collected per trap-night, monthly MLE pooled infection rate, monthly rainfall, wind speed, barometric pressure, or temperature. However, meteorologic data used were from the main weather station of the Trinidad and Tobago Meteorological Services and may not reflect local conditions at the individual study sites.
Figure 1.
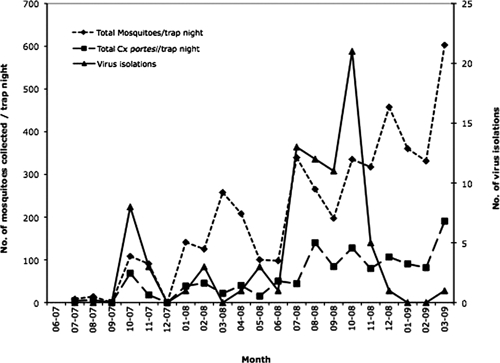
Number of mosquitoes (all species) collected per trap night for each month and number of Culex (Melanoconion) portesi mosquitoes collected per trap night for each month of the study during July 2007–March 2009, Trinidad. Dates are indicated as month-year. Viruses isolated for each month at all three sites of the study are also shown. Virus isolations are plotted on the secondary y-axis.
In a previous report, we showed strong geographic structuring in an MUCV phylogeny based on 18 sequences from Brazil (n = 4) and Trinidad (n = 14) and temporal structuring within Trinidad, which showed at least three major clades.8 The ML phylogeny generated in the present study, which includes 25 additional sequences from Trinidad, also supports the existence of at least three clades, with sequences from Trinidad and Brazil in separate clades and two temporally defined subclades in Trinidad (Figure 2). The phylogeny also suggests that there is geographic structure within Trinidad because the sequence from the 2008 BBF isolate (Tri5991), although closely related, was distinct from all of the 2007 and 2008 sequences from the ASSR. This topology is consistent with enzootic maintenance of the virus within localized rodent populations with limited dispersal and range. However, additional sequence data for MUCV isolates from the BBF would be required to confirm the extent of population subdivision within Trinidad.
Figure 2.
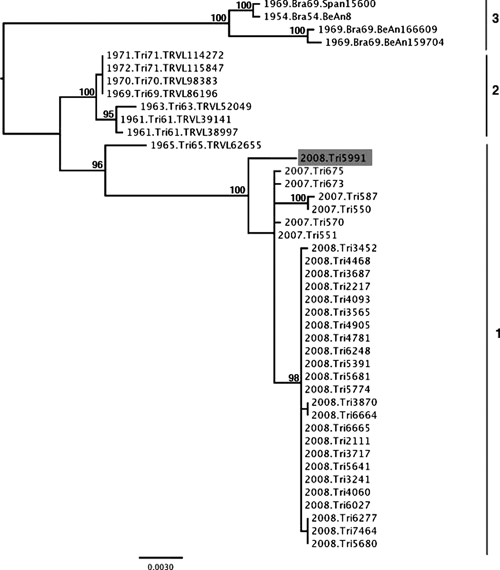
Maximum likelihood tree generated using the general time reversible model and a discrete, gamma-distributed across-site rate variation. The Mucambo virus (MUCV) phylogeny is based on 2,079 nucleotides encompassing the envelope 3 (E3) and E2 gene regions for all available MUCV isolates. The three major clades are indicated in Arabic numerals. Strain Tri5991 (highlighted in gray) is from the Bush Bush Forest site and all of the other 2007 and 2008 isolates were obtained at the Aripo Savannah Scientific Reserve site. Nodes are labeled with bootstrap values ≥ 95%. Tip labels include year of isolation and strain name.
Our study highlights the importance of Cx. (Mel.) portesi as an arboviral vector, and for the first time, implicates Ae. (Och.) hortator as a potential vector of MUCV. No viruses with known epidemic potential were isolated, although there was documented sylvatic YFV activity during the surveillance period. Subsequent studies aimed at virus isolation from different forests and investigations of vector competence, behavior, and biology are needed to develop a more complete understanding of arbovirus ecology and epidemiology in Trinidad, and to assess the risk of emergence of arboviral infections other than dengue and yellow fever.
Supplementary Material
Acknowledgments
We thank Vernie Ramkissoon, Jason Gobin, and the staff of the Forestry division of the Ministry of Agriculture, Land and Marine Resources for assistance in the field, and Shamjeet Singh for assistance with statistical analyses.
Note: Supplemental table is available at www.ajtmh.org.
Footnotes
Financial support: This study was supported by grants from the Trinidad and Tobago Government Research Fund, the Caribbean Health Research Council, The University of the West Indies St. Augustine Campus Research and Publications Fund, National Institutes of Health grants AI25489 and AI071192, and the John S. Dunn Foundation.
Authors' addresses: Albert J. Auguste, Raymond Martinez, and Christine V. F. Carrington, Department of Preclinical Sciences, Faculty of Medical Sciences, The University of the West Indies, St. Augustine, Republic of Trinidad and Tobago, E-mails: albert.auguste@sta.uwi.edu, raytrini6@gmail.com, and christine.carrington@sta.uwi.edu. A. Paige Adams, Amelia P. A. Travassos da Rosa, Robert B. Tesh, and Scott C. Weaver, Institute for Human Infections and World Health Organization Collaborating Center for Tropical Diseases, University of Texas Medical Branch, Galveston, TX, E-mails: apadams@utmb.edu, aptravas@utmb.edu, rtesh@utmb.edu, and sweaver@utmb.edu. Nicole C. Arrigo, Institute for Human Infections and World Health Organization Collaborating Center for Tropical Diseases, University of Texas Medical Branch, Galveston, TX, and Center for Infection and Immunity, Columbia University, New York, NY, E-mail: ncarrigo@gmail.com. Abiodun A. Adesiyun, School of Veterinary Medicine, Faculty of Medical Sciences, The University of the West Indies, St. Augustine, Republic of Trinidad and Tobago, E-mail: abiodun.adesiyun@sta.uwi.edu. Dave D. Chadee, Department of Life Sciences, Faculty of Science and Agriculture, The University of the West Indies, St. Augustine, Republic of Trinidad and Tobago, E-mail: dave.chadee@sta.uwi.edu.
References
- 1.Aitken TH, Anderson CR, Downs WG. The isolation of Ilheus virus from wild caught forest mosquitoes in Trinidad. Am J Trop Med Hyg. 1956;5:621–625. doi: 10.4269/ajtmh.1956.5.621. [DOI] [PubMed] [Google Scholar]
- 2.Aitken TH, Downs WG, Anderson CR, Spence L, Casals J. Mayaro virus isolated from a Trinidadian mosquito, Mansonia venezuelensis. Science. 1960;131:986. doi: 10.1126/science.131.3405.986. [DOI] [PubMed] [Google Scholar]
- 3.Aitken TH, Downs WG, Spence L, Jonkers AH. St. Louis encephalitis virus isolations in Trinidad, West Indies, 1953–1962. Am J Trop Med Hyg. 1964;13:450–451. doi: 10.4269/ajtmh.1964.13.450. [DOI] [PubMed] [Google Scholar]
- 4.Aitken TH, Spence L, Jonkers AH, Downs WG. A 10-year survey of Trinidadian arthropods for natural virus infections (1953–1963) J Med Entomol. 1969;6:207–215. doi: 10.1093/jmedent/6.2.207. [DOI] [PubMed] [Google Scholar]
- 5.Downs WG, Aitken TH, Spence L. Eastern equine encephalitis virus isolated from Culex nigripalpus in Trinidad. Science. 1959;130:1471. doi: 10.1126/science.130.3387.1471. [DOI] [PubMed] [Google Scholar]
- 6.Jonkers AH, Spence L, Downs WG, Aitken TH, Tikasingh ES. Arbovirus studies in Bush Bush forest, Trinidad, W. I., September 1959–December 1964. V. Virus isolations. Am J Trop Med Hyg. 1968;17:276–284. doi: 10.4269/ajtmh.1968.17.276. [DOI] [PubMed] [Google Scholar]
- 7.Jonkers AH, Spence L, Downs WG, Aitken TH, Worth CB. Arbovirus studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. VI. Rodent-associated viruses (VEE and agents of groups C and Guama): isolations and further studies. Am J Trop Med Hyg. 1968;17:285–298. [PubMed] [Google Scholar]
- 8.Auguste AJ, Volk SM, Arrigo NC, Martinez R, Ramkissoon V, Adams AP, Thompson NN, Adesiyun AA, Chadee DD, Foster JE, Travassos da Rosa AP, Tesh RB, Weaver SC, Carrington CV. Isolation and phylogenetic analysis of Mucambo virus (Venezuelan equine encephalitis complex subtype IIIA) in Trinidad. Virology. 2009;392:123–130. doi: 10.1016/j.virol.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulton F, Dumbell KR. The serological comparison of strains of influenza virus. J Gen Microbiol. 1949;3:97–111. doi: 10.1099/00221287-3-1-97. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer M, Proebster B, Kinney RM, Kaaden OR. Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am J Trop Med Hyg. 1997;57:709–718. doi: 10.4269/ajtmh.1997.57.709. [DOI] [PubMed] [Google Scholar]
- 11.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (and Other Methods) Sunderland, MA: Sinauer Associates; 2003. Version 4. [Google Scholar]
- 12.Pan American Health Organization . Epidemiological Alert: Increase in Circulation of Jungle Yellow Fever Virus in the Americas. Washington, DC: Pan American Health Organization; 2010. [Google Scholar]
- 13.Auguste AJ, Lemey P, Pybus OG, Suchard MA, Salas RA, Adesiyun AA, Barrett AD, Tesh RB, Weaver SC, Carrington CV. Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas. J Virol. 2010;84:9967–9977. doi: 10.1128/JVI.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turell MJ, O'Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi CA, Ludwig V, Mangiafico JA, Kondig J, Wasieloski LP, Jr, Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA. Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru. J Med Entomol. 2005;42:891–898. doi: 10.1603/0022-2585(2005)042[0891:IOVFMD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


