Abstract
Leishmania infantum causes visceral leishmaniasis, a severe zoonotic and systemic disease that is fatal if left untreated. Identification of the antigens involved in Leishmania-specific protective immune response is a research priority for the development of effective control measures. For this purpose, we evaluated, in 27 dogs from an enzootic zone, specific humoral and cellular immune response by delayed-type hypersensitivity (DTH) skin test both against total L. infantum antigen and the raw Trichoplusia ni insect-derived kinetoplastid membrane protein-11 (rKMPII), tryparedoxin peroxidase (rTRYP), Leishmania homologue of receptors for activated C kinase (rLACK), and 22-kDa potentially aggravating protein of Leishmania (rpapLe22) antigens from this parasite. rTRYP induced the highest number of positive DTH responses (55% of leishmanin skin test [LST]-positive dogs), showing that TRYP antigen is an important T cell immunogen, and it could be a promising vaccine candidate against this disease. When TRYP-DTH and KMPII-DTH tests were evaluated in parallel, 82% of LST-positive dogs were detected, suggesting that both antigens could be considered as components of a standardized DTH immunodiagnostic tool for dogs.
Introduction
Visceral leishmaniasis (VL), a severe parasitic disease that is usually fatal if left untreated (http://www.who.int/leishmaniasis/en/) has an incidence of more than 500,000 new human cases each year. Zoonotic VL is caused by Leishmania infantum (syn. Leishmania chagasi1) in both the Palearctic and the Neotropical ecozones. Dogs are the natural hosts and the main domestic reservoirs of the parasite.2 In ecoregions around the Mediterranean basin, the prevalence of canine infection reaches 67–80% in highly enzootic areas3,4 with at least 2.5 million dogs infected on the northern shore.2 Control of parasite burden in dogs by vaccination or treatment reduces the infectivity to the vector and the incidence of the disease in humans.5,6 Identification of the mechanisms and components involved in Leishmania-specific immune responses to improve diagnosis, prognosis, and vaccination is a research priority.
L. infantum-infected humans and dogs can develop several immune responses, ranging from predominantly specific cell-mediated immunity (CMI) to a predominantly humoral immune response, which has been associated with active disease.7,8 The cellular mechanisms underlying the protective immune response against L. infantum seem to involve a Th1-like response, including production of interferon (IFN)-γ by sensitized T cells to induce macrophage activation.9,10 Measurement of IFN-γ production using cultured peripheral blood cells in response to Leishmania antigens is expensive, time consuming, and cumbersome. Delayed-type hypersensitivity (DTH) reaction to an intradermal injection of a suspension of inactivated promastigotes (leishmanin skin test, LST) is an easy and semiquantitative test to measure Leishmania-specific CMI in vivo.8,11 This test has been extensively used in large-scale immunoepidemiological studies in Southwestern Europe as a marker of exposure to Leishmania in humans12–14 and much less in dogs.15,16 The LST result is usually negative in advances stages of clinical VL and canine leishmaniasis (CanL),7,8,17 whereas a positive LST result is detected during subclinical infection,15,16 in early stages of VL and CanL,18,19 or after successful treatment.20,21 Several vaccination studies conducted in dogs have shown that animals that converted to a positive LST result were protected against the disease,6,22,23 like in humans.24,25 Unfortunately, the development of this immunodiagnostic method has been hampered by the lack of a standardized product, and a test to evaluate Leishmania-specific CMI in vivo in humans or dogs is currently not available in clinical practice.
Recombinant proteins make it possible to identify specific B cell and T cell immunogens so that more accurate diagnostic and prognostic tools can be developed. Moreover, discrimination between potentially protective and potentially aggravating antigens would allow a more rational development of experimental vaccines. Baculovirus–insect larvae expression system has proven to be a valuable system for the production of large amounts of high-quality, yet inexpensive recombinant proteins.26 Folding and processing of proteins related to eukaryotic systems improve with insect larvae expression strategy compared with prokaryotic systems. However, some processing protein pathways of insect cells, such as the protein glycosylation pathway, are not exactly equivalent to those of other animals, especially vertebrates.27 A wide variety of recombinant proteins have been expressed in insect larvae to be used for serodiagnosis,28,29 vaccination strategies, or production of reagents.
In the present study, we evaluated and compared the serological and DTH response to crude total L. infantum antigen (CTLA) and four recombinant evolutionarily conserved antigens from this parasite: kinetoplastid membrane protein-11 (KMPII; formerly known as KMP-11), tryparedoxin peroxidase (TRYP; previously known as TSA), Leishmania homologue of receptors for activated C kinase (LACK), and 22-kDa potentially aggravating protein of Leishmania (papLe22). These antigens have been used elsewhere as targets for vaccines30–32 and serodiagnosis of CanL33,34; however, their role in L. infantum-specific CMI in naturally infected dogs has not been evaluated. The main objective of this work was to determine whether these antigens produced in recombinant baculovirus-infected Trichoplusia ni larvae are T cell immunogens in dogs naturally infected with L. infantum and to assess their inclusion in a standardized DTH-based immunodiagnostic tool or their usefulness as possible vaccine candidates in the canine model.
Materials and Methods
Production of recombinant KMPII, TRYP, LACK, and papLe22 proteins.
Recombinant proteins were obtained in baculovirus-infected T. ni larvae as previously described.34 Table 1 shows the Genbank accession numbers of the sequences used for primer design and the nucleotide sequences of forward and reverse primers for each L. infantum gene cloned. Recombinant bacmids carrying KMPII, TRYP, LACK, and papLe22 genes and an additional bacmid with non-insert clones produced by the Bac-to-Bac system (Invitrogen, Carlsbad, CA) were used to transfect Spodoptera frugiperda Sf21 cells to obtain the recombinant baculoviruses and the wild-type baculovirus, respectively. Wild-type baculovirus was used to obtain the control raw protein extract (Ni) for both enzyme-linked immunosorbent assay (ELISA) and the DTH skin test. Fifth instar T. ni larvae were intracoelomically injected near the proleg with the recombinant baculovirus preparations. Inoculated larvae were kept at 28°C for 96 hours and then frozen immediately at −20°C. Total protein was solubilized from frozen larvae by homogenizing on ice using extraction buffer: phosphate buffered saline, pH 7.2, 0.01% Triton X-100, 25 mM dithiothreitol, and a protease inhibitor cocktail (Complete; Roche, Basel, Switzerland). After centrifugation, the supernatant was filtered, recentrifuged, and lyophilized overnight.
Table 1.
Genebank accession number and primer sequences of L. infantum recombinant antigens
| Gene (accession number) | Direction | Primer |
|---|---|---|
| KMPII (X95627) | Forward | 5′-ATGGCCACCACGTACGAGGAGTTTTCG-3′ |
| Reverse | 5′-TTACTTGGATGGGTACTGCGCAGC-3′ | |
| TRYP (AF044679) | Forward | 5′-CCAGCCATGTCCTGCGGTAACGCCAAG-3′ |
| Reverse | 5′-AGGTTTACTGCTTGCTGAAGTATCCTTC-3′ | |
| LACK (U49695) | Forward | 5′-ACCATGAACTACGAGGGTCACC-3′ |
| Reverse | 5′-TTACTCGGCGTCGGAGATGGACC-3′ | |
| papLe22 (AF123892) | Forward | 5′-GGCCACTTCTCTCTTCTCCA-3′ |
| Reverse | 5′-CTTGCCACATACACCAATCG-3′ |
Total soluble protein (TSP) extracts were analyzed for production of recombinant proteins by sodium-dodecyl-sulphate polyacrilamide gel electrophoresis (SDS-PAGE), Western blot, and glycoprotein detection assays. Thirty micrograms of each TSP extract were electrophoretically separated by 15% SDS-PAGE and stained with Coomassie brilliant blue (Bio-Rad Laboratories Inc., Hercules, CA), and bands corresponding to L. infantum recombinant proteins were quantified using a Tina 2.0 image analyzer software package (Raytest, Straubenhardt, Germany).
Western blotting was performed to detect recombinant antigens in protein larva extracts without further purification, as previously described.34 Briefly, proteins were electrophoretically transferred onto an Immobilon nitrocelulose membrane (GE Healthcare, Fairfield, CT). The membrane was blocked overnight with phosphate-buffered saline 0.05% Tween 20 (PBST) and 4% dried skimmed milk and subsequently incubated at room temperature with a pool of six sera that were highly positive to CTLA-based ELISA for 1 hour. After washing with PBST (one time for 15 minutes and then two times for 5 minutes), protein A–horse radish peroxidase (HRP) (Pierce, Rockford, IL) was used as secondary antibody and incubated for 1 hour at room temperature, and the membrane was thoroughly washed as described above. Color was developed by enhanced chemiluminescence system (ECL), and chemiluminiscence was detected using Chemidoc (Bio-Rad) and analyzed using the Image laboratory Software, version 2.0.1 (Bio-Rad).
No evidences of N-glycosylation in KMPII, TRYP, LACK, and papLe22 proteins exist, but glycosylation prediction analysis (http://www.oppf.ox.ac.uk/opal/OPAL.php) detected the possible presence of several O-glycosilation sites in both TRYP (N = 19) and papLe22 (N = 5) proteins. For glycosylation detection assays, 200 μg of TSP per lane were resolved in a 15% SDS-PAGE, and glycosylated proteins were stained using the GLYCOPRODetection Kit (Sigma-Aldrich, St. Louis, MO) following the manufacturer's instructions.
Dogs.
The study was conducted in June 2008 in Mallorca, Spain, a highly enzootic area for L. infantum.4 Twenty-seven dogs of different breeds (14 females and 13 males) from the Animal Pound of Palma de Mallorca were included in the study. Their ages ranged from 6 months to 7.5 years, with a mean ± standard deviation (SD) of 3 ± 1.8 years. Before sampling, all dogs were examined to detect clinical signs compatible with CanL. The dogs were then sedated using 10 μg.kg−1 of medetomidine (Domtor; Pfizer, New York, NY) intramuscularly to take blood samples and perform DTH tests. Dogs were considered infected by Leishmania when they had a positive result to serology testing with CTLA and/or LST and/or real-time polymerase chain reaction (qPCR) in blood samples.16,35 All the procedures were performed with the approval of the Animal Pound of Palma de Mallorca Ethics Committee.
Blood samples.
Blood was collected by jugular venipuncture before DTH tests were performed. Sera were obtained after centrifugation of blood samples at 2,000 × g for 20 minutes. Serum and blood clot samples were stored at −20°C until use.
Real-time PCR amplification of L. infantum DNA in blood samples.
L. infantum DNA was specifically detected and quantified by Taqman qPCR (Applied Biosystems, Foster City, CA) performed by the Servei Veterinari de Genètica Molecular, Universitat Autònoma de Barcelona, as described elsewhere.36 Briefly, DNA was extracted from blood clots, and qPCR was performed, targeting conserved DNA regions of the kinetoplast minicircle DNA from L. infantum. The eukaryotic 18S rRNA Pre-Development TaqMan Assay reagents (Applied Biosystems) were used as an internal reference. L. infantum DNA load in each sample was determined by relative quantification using the 2−▵▵Ct method.37 Results were expressed as x-fold more DNA copies than the calibrator sample (dog M7).
Recombinant and crude total L. infantum antigen-based ELISA.
ELISA to detect serum antibodies against rKMPII, rTRYP, rpapLe22, and rLACK antigens and against CTLA was performed as previously described.34,38 Microtiter plates were coated with raw protein larva extracts containing the recombinant proteins (1.3 µg per well for rKMPII, 5.0 μg for rTRYP, 24.4 μg for rLACK, and 10.0 µg for rpapLe22), with the control Ni extract at the same concentrations or with CTLA (2 µg per well). Protein A-HRP was used as secondary antibody. Each serum sample was tested under equal conditions against both protein larva extracts containing each recombinant antigen and control Ni extract in the same plate. A known positive serum used as calibrator (~1 optical densities [OD]) was included in all plates, and plates with interassay variations ≥ 10% were ruled out. The ELISA cut-off values (mean + 3 SD for 76 dogs from non-enzootic areas) were 0.180 OD for CTLA, 0.100 OD for rKMPII, 0.082 OD for rTRYP, 0.060 OD for rpapLe22, and 0.005 OD for rLACK.
Results were expressed as OD. For ELISAs using recombinant antigens, absorbances were corrected by subtracting the absorbance achieved by the serum on the control antigen Ni extract from that achieved on the protein larva extract containing specific recombinant antigen.
DTH tests using leishmanin and recombinant L. infantum proteins.
Leishmanin reagent consisted of a suspension of 3 × 108 inactivated L. infantum (MHOM/FR/78/LEM75) promastigotes per milliliter in 0.4% phenol-saline solution (provided by Instituto de Salud Carlos III). Working concentrations of recombinant protein extracts for use in DTH reactions were set up in a pilot study (data not shown). Recombinant DTH test reagents were set at 4 μg·mL−1 of specific Leishmania antigen diluted in pyrogen-free saline solution. The negative control, Ni reagent, was set at 730 μg·mL−1 of raw larva extract in pyrogen-free saline solution, equivalent to the maximum concentration of raw larva extract used in specific Leishmania antigen preparations (rTRYP reagent). A volume of 0.1 mL of each reagent was intradermally inoculated. The six injections were distributed 3 cm apart on both sides of the groin skin. The DTH response was assessed by measuring the size of the induration and erythematous area (mean of two perpendicular diameters) produced against each reagent at 48 and 72 hours post-injection. A DTH response against leishmanin reagent or against each of the recombinant antigens > 5 mm was considered positive.8 Dogs with a positive DTH response to Ni reagent were not included in the analysis.
Data analysis.
All statistical tests were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL). Data were analyzed by means of non-parametric tests. A P value < 0.05 was considered significant.
Results
Detection of L. infantum recombinant proteins in T. ni larvae.
The recombinant KMPII, papLe22, TRYP, and LACK proteins were successfully produced in the inoculated T. ni larvae (Figure 1). Specific bands corresponding to rKMPII and rLACK in T. ni extracts were visible using Coomassie brilliant blue staining (Figure 1A) and Western blotting (Figure 1B). Because of bands of Ni antigen having the same size as rTRYP and rpapLe22 in Coomassie blue-stained gels, expression of these antigens was confirmed by Western blotting (Figure 1B). rKMPII, rpapLe22, rTRYP, and rLACK were identified as the expected single band with an electrophoretic mobility of around 11, 20, 22, and 34 kDa, respectively. In the case of rLACK, the band with a higher molecular weight (37 kDa) could be because of alternative post-raductional modifications of the antigen conserving the common epitopes. Because no bands appear in the negative control, it is very unlikely a non-specific immunoreactivity with seemingly unrelated proteins. In the case of rpapLe22, a proteolytic degradation of the sample should be suspected, because a band of lower molecular weight (18 kDa) is detected.
Figure 1.
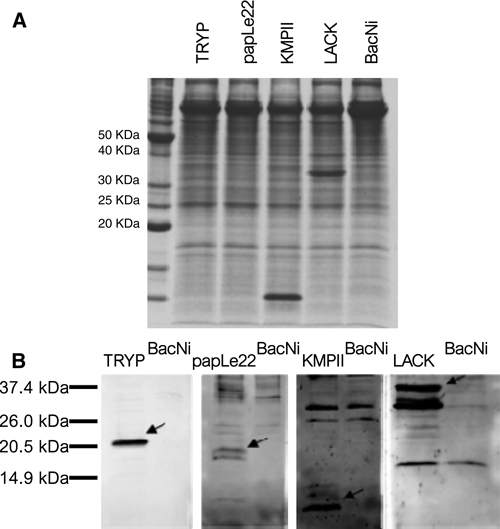
Detection of the recombinant proteins rKMPII, rpapLe22, rTRYP, and rLACK in T. ni larvae. Total protein extracts were analyzed by (A) Coomassie brilliant blue staining of SDS-PAGE gels and (B) Western blotting using a pool of canine sera from total Leishmania antigen-positive samples. Arrows indicate the position of the recombinant proteins.
Glycosylation assay did not show any positive band in the recombinant protein larvae extracts.
Clinical status and L. infantum infection rates.
Clinical signs compatible with CanL, parasite load, specific antibody reactivity, and DTH test results against CTLA and the recombinant proteins in the 27 dogs from Mallorca are listed in Table 2.
Table 2.
Clinical signs, specific antibody reactivity, DTH test results against total L. infantum antigen, Ni reagent, and recombinant Leishmania proteins rKMPII, rTRYP, rLACK, and rpapLe22, and qPCR in the blood of 27 dogs in Mallorca, Spain
| ID | Signs | ELISA* | DTH† | qPCR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTLA | KMP11 | TRYP | LACK | papLe22 | LST | Ni | KMPII + Ni | TRYP + Ni | LACK + Ni | papLe + Ni | Blood‡ | ||
| M1 | − | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 | 5.5§ | 0.0 | 0.0 | 5.5 | 0.0 | 0.0 | 131.2 |
| M2 | − | 0.063 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 8.5 | 7.5 | 9.5 | 4.0 | 0.0 | 0.0 |
| M3 | − | 0.035 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 6.5 | 0.0 | 0.0 | 0.0 | 0.0 | 34.7 |
| M4 | − | 0.045 | 0.000 | 0.000 | 0.000 | 0.000 | 13.0§ | 0.0 | 0.0 | 5.5 | 0.0 | 0.0 | 1.0 |
| M5 | − | 0.036 | 0.000 | 0.000 | 0.000 | 0.000 | 8.0 | 8.5 | 14.0 | 7.5 | 6.0 | 6.5 | 0.0 |
| M6 | − | 0.039 | 0.000 | 0.000 | 0.000 | 0.000 | 7.5 | 5.0 | 10.5 | 15.5 | 11.5 | 12.5 | 1.0 |
| M7 | + | 0.260 | 0.000 | 0.000 | 0.000 | 0.000 | 10.5§ | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| M8 | − | 0.133 | 0.000 | 0.000 | 0.000 | 0.000 | 8.5 | 5.0 | 8.5 | 15.0 | 0.0 | 0.0 | 22.8 |
| M9 | − | 0.08 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.5 |
| M10 | − | 0.015 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 5.5 | 0.0 | 0.0 | 0.0 |
| M11 | − | 0.031 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M12 | + | 0.330 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M13 | − | 0.037 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 6.0 | 0.0 | 6.0 | 14.0 |
| M14 | − | 0.028 | 0.000 | 0.000 | 0.000 | 0.000 | 6.5§ | 0.0 | 12.0 | 0.0 | 0.0 | 7.0 | 1.9 |
| M15 | − | 0.141 | 0.000 | 0.000 | 0.000 | 0.000 | 14.0§ | 0.0 | 0.0 | 12.5 | 6.0 | 0.0 | 2.2 |
| M16 | + | 1.860 | 0.000 | 0.000 | 0.000 | 0.369 | 6.5§ | 0.0 | 6.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M17 | − | 0.043 | 0.000 | 0.000 | 0.000 | 0.000 | 8.0 | 6.5 | 8.5 | 17.5 | 12.0 | 6.0 | 7.0 |
| M18 | − | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 0.0 | 5.5 | 7.5 | 0.0 |
| M19 | + | 3.000 | 1.737 | 0.000 | 0.000 | 0.255 | 5.5§ | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 45.7 |
| M20 | − | 0.047 | 0.000 | 0.000 | 0.000 | 0.000 | 5.5§ | 0.0 | 8.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M21 | − | 0.057 | 0.000 | 0.000 | 0.000 | 0.000 | 8.0 | 5.0 | 0.0 | 0.0 | 0.0 | 13.0 | 0.0 |
| M22 | − | 0.079 | 0.000 | 0.000 | 0.000 | 0.000 | 9.0 | 5.0 | 0.0 | 13.0 | 15.0 | 0.0 | 0.0 |
| M23 | − | 0.061 | 0.000 | 0.000 | 0.000 | 0.000 | 16.5 | 5.0 | 0.0 | 0.0 | 6.5 | 8.0 | 0.0 |
| M24 | + | 0.330 | 0.000 | 0.000 | 0.000 | 0.000 | 8.0§ | 0.0 | 0.0 | 8.5 | 0.0 | 0.0 | 0.0 |
| M25 | − | 0.032 | 0.000 | 0.000 | 0.000 | 0.000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M26 | − | 0.024 | 0.000 | 0.000 | 0.000 | 0.000 | 5.5§ | 0.0 | 0.0 | 7.0 | 0.0 | 0.0 | 0.0 |
| M27 | − | 0.037 | 0.000 | 0.000 | 0.000 | 0.000 | 10.0§ | 0.0 | 15.0 | 6.0 | 0.0 | 0.0 | 13.7 |
| n | 5 | 5 | 1 | 0 | 0 | 2 | 18 | 4 | 8 | 2 | 3 | 13 | |
CTLA = crude total L. infantum antigen; LST = leishmanin skin test.
ELISA results are expressed as optical densities (OD). OD results above the cut-off values were considered positive (numbers in bold). The cut-off values (mean + 3 SD for 76 dogs from non-endemic area) were established at 0.180 OD for CTLA, 0.100 OD for rKMPII ELISA, 0.082 OD for rTRYP ELISA, 0.060 OD for rpapLe22 ELISA, and 0.005 OD for rLACK ELISA.
Mean millimeter of the largest perpendicular diameter of induration and eritamatous area and its perpendicular one at 48 and 72 hours post-injection. DTH responses > 5 mm were considered positive (numbers in bold).
Results expressed as x-fold more DNA copies than the calibrator sample (dog M7).
Dogs with a positive LST result and no reaction to Ni reagent. Ni reagent is raw non-infected larva extract in pyrogen-free saline solution.
Five of twenty-seven dogs (18%) included in the study presented clinical signs compatible with CanL. Two showed generalized lymphadenomegaly, two presented periauricular cutaneous lesions, and one showed classic clinical signs of CanL, namely severe emaciation and generalized exfoliative dermatitis. These five dogs were the only ones that showed specific antibodies against CTLA. In addition, the dog that manifested more severe clinical signs (M19) was the one with the highest L. infantum-specific antibody levels (3.0 OD). The remaining dogs (82%) showed neither clinical signs compatible with CanL nor specific antibodies against CTLA.
Eighteen dogs (67%), including four dogs with clinical signs, developed a positive LST reaction at 48 and/or 72 hours post-injection. The intensity of the reaction recorded in dogs with a positive LST response (median in millimeters; interquartile interval) was 8 mm (6.5–9.8).
L. infantum DNA was detected in the blood samples of 13 dogs (48%). The amount of Leishmania DNA was generally low, with a median of 7.0-fold increase over M7 (range = 1.00–131.2). Although only 2 of these 13 dogs showed antibodies against CTLA, 10 of them developed a positive LST reaction.
Combining the results of the three techniques (CTLA serology, LST, and qPCR), we estimated the rate of L. infantum infection in this group of dogs to be 82% (22/27).
Specific humoral response against recombinant L. infantum antigens.
Two dogs (M16 and M19) showed positive anti-rpapLe22 antibody levels. One of these dogs (M19) also showed antibodies against rKMPII. Anti-rTRYP or anti-rLACK antibodies were not detected in any dog.
DTH response against recombinant L. infantum antigens.
In the 11 LST-positive Ni-negative dogs, the highest percentage of DTH responses was detected against rTRYP (6/11; 55%). Lower percentages of positive DTH reactions were detected against rKMPII (4/11; 36% of the LST-positive dogs). Finally, only one dog showed a DTH-positive response against rLACK, and one showed a DTH-positive result against rpapLe22 (1/11; 9% of LST-positive dogs, respectively). The remaining seven LST-positive dogs showed a DTH-positive reaction against raw antigen Ni at 48 and/or 72 hours post-injection.
The intensity of reaction recorded in dogs with a positive DTH response (median; interquartile interval) was 10.0 mm (7.5–12.8) against rKMPII and 6.0 mm (5.5–7.4) against rTRYP.
We evaluated the potential of the recombinant antigens for detecting dogs with a positive LST reaction. rTRYP produced the highest number of DTH-positive reactions among dogs with positive LST (6/11), but its sensitivity in relation to LST was low: 0.55 (95% confidence interval [CI] = 0.22–0.84). Thus, we combined in parallel the results of rTRYP with the results obtained with the second most recognized antigen (rKMPII) to improve sensitivity. The combination of the rTRYP and rKMPII DTH results revealed a sensitivity of 0.82 (CI = 0.49–0.95), a specificity of 0.71 (CI = 0.31–0.93), and an accuracy of 0.78 (CI = 0.59–0.97) in relation to LST. This combination showed a significant relationship with the LST test (Fisher exact test, P = 0.049).
Discussion
In the present study, we used DTH tests to investigate the role of four evolutionarily conserved L. infantum proteins proposed as vaccine candidates—KMPII, TRYP, LACK and papLe22—as T cell immunogens in dogs living in an enzootic area. To our knowledge, only one recently published study has used a recombinant Leishmania protein as a DTH test reagent (the recombinant cysteine proteinase rLdccys1) to detect CMI in vivo in naturally infected dogs, with very promising results.39 Our study is the first to use recombinant proteins expressed in baculovirus-infected larvae for DTH testing. One limitation of other expression platforms is the high cost associated with large-scale production. As an alternative, the use of lepidoptera larvae has been reported as a suitable method for inexpensive production of large amounts of recombinant proteins. Moreover, previous studies34,40–42 show that crude protein extracts without further purification can be used as immunodiagnostic reagents, because the recombinant proteins preserve their antigenic activities. However, some interference of insect proteins, such as cross-reactions, cannot be ruled out, especially in dogs with a positive DTH response against Ni proteins. Therefore, if Ni-positive dogs are excluded, DTH tests with raw insect-derived recombinant proteins without further purification can be used as a low-cost tool for rapid screening of potential DTH or vaccine antigen candidates. Future studies using purified recombinant L. infantum proteins will be conducted by our group.
The dogs included in the study were from Mallorca, a highly L. infantum-enzootic area.16,43 By combining the detection of anti–Leishmania-specific humoral (CTLA ELISA) and cellular (LST reaction) immune responses with the detection of parasite DNA in blood (qPCR), we estimated the rate of L. infantum infection in the study dogs to be 82% (22/27). This high rate of infection is similar to that of previous reports in this area (77%).16 Although 18% of dogs presented clinical leishmaniasis, 67% developed a positive reaction to LST. A positive LST result indicates previous Leishmania exposure44 and is generally thought to reflect durable and protective CMI.6,8,15,22 In agreement with previous studies, our results show that most dogs living in a Leishmania-enzootic area display a resistant immunological profile against L. infantum infection15,16 and that clinical disease represents only a small part of the real infection by the parasite.45
To avoid false-positive DTH reactions against recombinant antigens because of possible cross-reactions with insect proteins, dogs with a positive DTH response against Ni reagent were not included in the analysis of results. rTRYP induced the highest percentage of positive DTH responses in the 11 LST-positive dogs (6/11; 55%). TRYP has shown immunogenicity in dogs46 and protection against cutaneous or mucosal leishmaniasis in mice and non-human primates,47 and it is a component of the trifusion Leish-111f vaccine, now being assayed in humans (http://clinicaltrials.gov/show/NCT00121862). Supporting its value as a vaccine candidate against VL, TRYP has proven highly immunogenic in a DNA/modified vaccinia virus ankara (MVA) vaccine strategy against CanL.32 Furthermore, the DTH response against this antigen in vaccinated dogs correlated with high production of Leishmania-specific IFN-γ. These results indicate that TRYP could be a potent T cell immunogen in dogs and that it might be involved in protective L. infantum-specific CMI, at least in one-half of naturally infected dogs. However, it was not possible to verify the potential glycosilation sites detected by prediction analysis of neither rTRYP nor rpapLe22 antigens with the detection method used in this work. If these predicted glycosylated sites are real and located in immunodominant regions of native TRYP antigen, then the results obtained in the present study might be underestimating the true percentage of dogs that recognized this protein.
The present results show that only 4 of 11 LST-positive dogs (36%) developed a positive DTH skin reaction against rKMPII, indicating that, compared with rTRYP, this antigen is a weak inducer of CMI in naturally L. infantum-infected dogs. Accordingly, the antigenic and T cell immunogenic potential of KMPII studied during experimental canine L. infantum infection showed that, although this antigen induced a Th1 cytokine expression pattern, the production of IFN-γ was only moderate.48 In humans, KMPII has been shown to stimulate T cells from patients who have recovered from L. donovani infection49 and to induce production of both IFN-γ and interleukin (IL)-4,50 whereas both IFN-γ and IL-10 were found during subclinical L. infantum infection.51 Thus, KMPII is not playing a Th1 role but a mixed Th1/Th2 role in these individuals.
Only one LST-positive dog showed a DTH-positive response against rLACK and another one against rpapLe22 in the present study. These results indicate that a specific CMI against LACK or papLe22 is not common in clinically healthy, naturally L. infantum-infected dogs. In this sense, LACK does not induce lymphoproliferation in patients cured of VL,52 and papLe22 elicits production of IL-10 but not IFN-γ.53 Both antigens play a prominent role in the pathogenesis of Leishmania infection in patients with different clinical presentations.53,54 However, vaccine strategies, such as DNA or prime-boost vaccinations, which are able to induce a strong Th1 immune response against these antigens, have proven able to obtain protection against L. infantum, probably by promoting a redirection of their Th2 immune response to a Th1 profile.30,55
The lack of a standardized antigen is the main drawback of the LST. Leishmanin reagent is an amalgam of antigens, some of which could be activating a strong Th1 immune response, such as TRYP, and others that could be inducing a Th2 or weak Th1 immune response, such as KMPII, LACK, or papLe22. Screening Leishmania antigens using DTH tests makes it possible to identify the antigens that may be related to host protection against the parasite. Recombinant Leishmania antigens could be used in the future as a standardized tool for diagnosis of subclinically L. infantum-infected dogs, in clinical trials with vaccines, and even to predict the outcome of disease and treatment. This approach has been applied in the diagnosis of bovine tuberculosis56 and to evaluate clinical trials with vaccine against malaria57 or hepatitis B58 in humans. In the present study, a high percentage of dogs reacting to leishmanin reagent also showed a positive DTH response against rTRYP and/or rKMPII (82% of LST-positive dogs). Because of the different MHC-II repertory in dogs,59 a cocktail of different antigens would provide better coverage for diagnosis of L. infantum. The estimated sensitivity, specificity, and accuracy of the combination of these two antigens used in parallel compared with LST were 82%, 71%, and 78%, respectively. Our results suggest that a multiantigenic DTH test including rTRYP and rKMPII could constitute a useful diagnostic tool for the detection of dogs subclinically infected with L. infantum.
Only dogs showing clinical signs (18%) showed specific antibodies against CTLA. In two of them, seroreactivity was found against rKMPII and/or rpapLe22. KMPII, TRYP, LACK, and papLe22 antigens have proven to be B cell immunogens in humans and dogs with clinical leishmaniasis,34,60–62 but their seroprevalence in the study group was very low. It is important to highlight that most of the dogs in the present study did not manifest clinical signs and that overall CTLA seroprevalence was low. These findings are consistent with the association between negative to medium levels of specific antibodies and subclinical L. infantum infection in dogs.63
Interestingly, four of five dogs with clinical leishmaniasis developed a positive LST reaction. This result raises the question of the existence of a defined polarized dichotomy between LST(+) protection and LST(−) susceptibility in CanL. A broad spectrum of immunological profiles coexists in clinically healthy canine populations living in Leishmania-enzootic areas.15,16,64 Our results support the fact that such a wide range also exists in dogs with clinical leishmaniasis63: from dogs with self-limiting disease65 or even dogs at the early stages of disease that develop specific CMI19 to dogs with severe disease, a suppressed T cell response, and an exaggerated humoral response.8,66 A positive LST result in a Leishmania-infected dog probably reflects control of parasites, even in the presence of clinical signs, although several factors can break the balance and severe disease can develop.45 Longitudinal studies will be required to confirm the long-term protective capability of the observed LST responses.
In conclusion, our results indicate that the raw insect-derived recombinant antigens KMPII, TRYP, LACK, and papLe22 present T cell epitopes responsible for the induction of a DTH response in dogs living in a L. infantum-enzootic area. Whereas TRYP showed the highest DTH-positive percentage in clinically healthy dogs, KMPII, LACK, and papLe22 proved to be weak T cell immunogens. Thus, our results suggest that TRYP could be a promising vaccine candidate or a component of a standardized DTH test, particularly when combined with KMPII. Further studies are needed to confirm the diagnostic, prognostic, and vaccination potential of this approach.
Acknowledgments
The authors thank the staff of the Animal Pound of Palma de Mallorca for their interest and collaboration.
Footnotes
Financial support: F.T. was supported by Generalitat de Catalunya Grant 2005 FI 01116. This work was financed by Projects BIO2004-03893 and AGL2008-00748 (both for J.A.) from the Spanish Government.
Authors' addresses: Felicitat Todolí, Jordi Alberola, and Alhelí Rodríguez-Cortés, Unitat de Farmacologia Veterinària and LeishLAB–Servei d'Anàlisi de Fàrmacs, Departament de Farmacologia, de Terapèutica i de Toxicologia, Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain, E-mails: Felicitat.Todoli@uab.cat, Jordi.Alberola@uab.cat, and Alheli.Rodriguez@uab.cat. Laia Solano-Gallego, Department of Pathology and Infectious Diseases, Royal Veterinary College, University of London, Hertfordshire, United Kingdom, E-mail: lsolano@rvc.ac.uk. Rafael de Juan and Pere Morell, Centre Sanitari Municipal de Protecció Animal, Palma, Mallorca, Spain, E-mails: rdjuan@sf.a-palma.es and centreproteccioanimal@sf.a-palma.es. Maria del Carmen Núñez, Rodrigo Lasa, and Silvia Gómez-Sebastián, Alternative Gene Expression S.L. (ALGENEX), Centro Empresarial, Parque Científico y Tecnológico de la Universidad Politécnica de Madrid, Campus de Montegancedo, Madrid, Spain, E-mails: mcn@algenex.es, rlc@algenex.es, and sgs@algenex.es. José M. Escribano, Departamento de Biotecnología, Instituto Nacional de Investigación y Tecnologia Agraria y Alimentaria, Madrid, Spain, E-mail: escriban@inia.es.
References
- 1.Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 2.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 3.Berrahal F, Mary C, Roze M, Berenger A, Escoffier K, Lamouroux D, Dunan S. Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am J Trop Med Hyg. 1996;55:273–277. doi: 10.4269/ajtmh.1996.55.273. [DOI] [PubMed] [Google Scholar]
- 4.Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol. 2001;39:560–563. doi: 10.1128/JCM.39.2.560-563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gradoni L, Maroli M, Gramiccia M, Mancianti F. Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Med Vet Entomol. 1987;1:339–342. doi: 10.1111/j.1365-2915.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 6.Borja-Cabrera GP, Correia Pontes NN, da Silva VO, Paraguai de Souza E, Santos WR, Gomes EM, Luz KG, Palatnik M, Palatnik de Sousa CB. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (Sao Goncalo do Amarante, RN) Vaccine. 2002;20:3277–3284. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho EM, Barral A, Pedral-Sampaio D, Barral-Netto M, Badaro R, Rocha H, Johnson WD., Jr Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992;165:535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- 8.Pinelli E, Killick-Kendrick R, Wagenaar J, Bernadina W, del Real G, Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect Immun. 1994;62:229–235. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meller-Melloul C, Farnarier C, Dunan S, Faugere B, Franck J, Mary C, Bongrand P, Quilici M, Kaplanski S. Evidence of subjects sensitized to Leishmania infantum on the French Mediterranean coast: differences in gamma interferon production between this population and visceral leishmaniasis patients. Parasite Immunol. 1991;13:531–536. doi: 10.1111/j.1365-3024.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 10.Holzmuller P, Bras-Goncalves R, Lemesre JL. Phenotypical characteristics, biochemical pathways, molecular targets and putative role of nitric oxide-mediated programmed cell death in Leishmania. Parasitology. 2006;132((Suppl)):S19–S32. doi: 10.1017/S0031182006000837. [DOI] [PubMed] [Google Scholar]
- 11.Manson-Bahr PE, Heisch RB, Garnham PC. Studies in leishmanifasis in East Africa. IV. The Montenegro test in kala-azar in Kenya. Trans R Soc Trop Med Hyg. 1959;53:380–383. doi: 10.1016/0035-9203(59)90038-0. [DOI] [PubMed] [Google Scholar]
- 12.Gramiccia M, Bettini S, Gradoni L, Ciarmoli P, Verrilli ML, Loddo S, Cicalo C. Leishmaniasis in Sardinia. 5. Leishmanin reaction in the human population of a focus of low endemicity of canine leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84:371–374. doi: 10.1016/0035-9203(90)90322-6. [DOI] [PubMed] [Google Scholar]
- 13.Marty P, Le Fichoux Y, Giordana D, Brugnetti A. Leishmanin reaction in the human population of a highly endemic focus of canine leishmaniasis in Alpes-Maritimes, France. Trans R Soc Trop Med Hyg. 1992;86:249–250. doi: 10.1016/0035-9203(92)90295-n. [DOI] [PubMed] [Google Scholar]
- 14.Acedo Sanchez C, Martin Sanchez J, Velez Bernal ID, Sanchis Marin MC, Louassini M, Maldonado JA, Morillas Marquez F. Leishmaniasis eco-epidemiology in the Alpujarra region (Granada Province, southern Spain) Int J Parasitol. 1996;26:303–310. doi: 10.1016/0020-7519(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso L, Neto F, Sousa JC, Rodrigues M, Cabral M. Use of a leishmanin skin test in the detection of canine Leishmania-specific cellular immunity. Vet Parasitol. 1998;79:213–220. doi: 10.1016/s0304-4017(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 16.Solano-Gallego L, Llull J, Ramos G, Riera C, Arboix M, Alberola J, Ferrer L. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet Parasitol. 2000;90:37–45. doi: 10.1016/s0304-4017(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 17.Dos-Santos WL, Jesus EE, Paranhos-Silva M, Pereira AM, Santos JC, Baleeiro CO, Nascimento EG, Moreira ED, Oliveira GG, Pontes-de-Carvalho LC. Associations among immunological, parasitological and clinical parameters in canine visceral leishmaniasis: emaciation, spleen parasitism, specific antibodies and leishmanin skin test reaction. Vet Immunol Immunopathol. 2008;123:251–259. doi: 10.1016/j.vetimm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Kurkjian KM, Mahmutovic AJ, Kellar KL, Haque R, Bern C, Secor WE. Multiplex analysis of circulating cytokines in the sera of patients with different clinical forms of visceral leishmaniasis. Cytometry A. 2006;69:353–358. doi: 10.1002/cyto.a.20256. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Cortes A, Ojeda A, Lopez-Fuertes L, Timon M, Altet L, Solano-Gallego L, Sanchez-Robert E, Francino O, Alberola J. A long term experimental study of canine visceral leishmaniasis. Int J Parasitol. 2007;37:683–693. doi: 10.1016/j.ijpara.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Caldas A, Favali C, Aquino D, Vinhas V, van Weyenbergh J, Brodskyn C, Costa J, Barral-Netto M, Barral A. Balance of IL-10 and interferon-gamma plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infect Dis. 2005;5:113. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borja-Cabrera GP, Santos FN, Santos FB, Trivellato FA, Kawasaki JK, Costa AC, Castro T, Nogueira FS, Moreira MA, Luvizotto MC, Palatnik M, Palatnik-de-Sousa CB. Immunotherapy with the saponin enriched-Leishmune((R)) vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine. 2010;28:597–603. doi: 10.1016/j.vaccine.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 22.Molano I, Alonso MG, Miron C, Redondo E, Requena JM, Soto M, Nieto CG, Alonso C. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet Immunol Immunopathol. 2003;92:1–13. doi: 10.1016/s0165-2427(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 23.Rafati S, Nakhaee A, Taheri T, Taslimi Y, Darabi H, Eravani D, Sanos S, Kaye P, Taghikhani M, Jamshidi S, Rad MA. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23:3716–3725. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Khalil EA, Elhassan AM, Zijlstra EE, Osman OF, Eljack IA, Ibrahim ME, Mukhtar MM, Ghalib HW, Modabbers F. Safety and immunogenicity of an autoclaved Leishmania major vaccine. East Afr Med J. 2000;77:468–470. [PubMed] [Google Scholar]
- 25.Khalil EA, Musa AM, Modabber F, El-Hassan AM. Safety and immunogenicity of a candidate vaccine for visceral leishmaniasis (Alum-precipitated autoclaved Leishmania major + BCG) in children: an extended phase II study. Ann Trop Paediatr. 2006;26:357–361. doi: 10.1179/146532806X152890. [DOI] [PubMed] [Google Scholar]
- 26.Medin JA, Gathy K, Coleman MS. Expression of foreign proteins in Trichoplusia ni larvae. Methods Mol Biol. 1995;39:265–275. doi: 10.1385/0-89603-272-8:265. [DOI] [PubMed] [Google Scholar]
- 27.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medin JA, Hunt L, Gathy K, Evans RK, Coleman MS. Efficient, low-cost protein factories: expression of human adenosine deaminase in baculovirus-infected insect larvae. Proc Natl Acad Sci USA. 1990;87:2760–2764. doi: 10.1073/pnas.87.7.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Filgueira DM, Resino-Talavan P, Cubillos C, Angulo I, Barderas MG, Barcena J, Escribano JM. Development of a low-cost, insect larvae-derived recombinant subunit vaccine against RHDV. Virology. 2007;364:422–430. doi: 10.1016/j.virol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Fragaki K, Suffia I, Ferrua B, Rousseau D, Le Fichoux Y, Kubar J. Immunisation with DNA encoding Leishmania infantum protein papLe22 decreases the frequency of parasitemic episodes in infected hamsters. Vaccine. 2001;19:1701–1709. doi: 10.1016/s0264-410x(00)00398-4. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Cortes A, Ojeda A, Lopez-Fuertes L, Timon M, Altet L, Solano-Gallego L, Sanchez-Robert E, Francino O, Alberola J. Vaccination with plasmid DNA encoding KMPII, TRYP, LACK and GP63 does not protect dogs against Leishmania infantum experimental challenge. Vaccine. 2007;25:7962–7971. doi: 10.1016/j.vaccine.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Carson C, Antoniou M, Ruiz-Arguello MB, Alcami A, Christodoulou V, Messaritakis I, Blackwell JM, Courtenay O. A prime/boost DNA/Modified vaccinia virus Ankara vaccine expressing recombinant Leishmania DNA encoding TRYP is safe and immunogenic in outbred dogs, the reservoir of zoonotic visceral leishmaniasis. Vaccine. 2009;27:1080–1086. doi: 10.1016/j.vaccine.2008.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berberich C, Machado G, Morales G, Carrillo G, Jimenez-Ruiz A, Alonso C. The expression of the Leishmania infantum KMP-11 protein is developmentally regulated and stage specific. Biochim Biophys Acta. 1998;1442:230–237. doi: 10.1016/s0167-4781(98)00176-6. [DOI] [PubMed] [Google Scholar]
- 34.Todoli F, Perez-Filgueira M, Galindo I, Gomez-Sebastian S, Escribano JM, Rodriguez-Cortes A, Alberola J. Seroreactivity against raw insect-derived recombinant KMPII, TRYP, and LACK Leishmania infantum proteins in infected dogs. Vet Parasitol. 2009;164:154–161. doi: 10.1016/j.vetpar.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Silveira FT, Lainson R, Pereira EA, de Souza AA, Campos MB, Chagas EJ, Gomes CM, Laurenti MD, Corbett CE. A longitudinal study on the transmission dynamics of human Leishmania (Leishmania) infantum chagasi infection in Amazonian Brazil, with special reference to its prevalence and incidence. Parasitol Res. 2009;104:559–567. doi: 10.1007/s00436-008-1230-y. [DOI] [PubMed] [Google Scholar]
- 36.Francino O, Altet L, Sanchez-Robert E, Rodriguez A, Solano-Gallego L, Alberola J, Ferrer L, Sanchez A, Roura X. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006;137:214–221. doi: 10.1016/j.vetpar.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Riera C, Valladares JE, Gallego M, Aisa MJ, Castillejo S, Fisa R, Ribas N, Carrio J, Alberola J, Arboix M. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet Parasitol. 1999;84:33–47. doi: 10.1016/s0304-4017(99)00084-9. [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro PH, Pinheiro AN, Ferreira JH, Costa FA, Katz S, Barbieri CL. A recombinant cysteine proteinase from Leishmania (Leishmania) chagasi as an antigen for delayed-type hypersensitivity assays and serodiagnosis of canine visceral leishmaniasis. Vet Parasitol. 2009;162:32–39. doi: 10.1016/j.vetpar.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Barderas MG, Wigdorovitz A, Merelo F, Beitia F, Alonso C, Borca MV, Escribano JM. Serodiagnosis of African swine fever using the recombinant protein p30 expressed in insect larvae. J Virol Methods. 2000;89:129–136. doi: 10.1016/s0166-0934(00)00209-3. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Martin E, Grau-Roma L, Argilaguet JM, Nofrarias M, Escribano JM, Gomez-Sebastian S, Segales J, Rodriguez F. Development of two Trichoplusia ni larvae-derived ELISAs for the detection of antibodies against replicase and capsid proteins of porcine circovirus type 2 in domestic pigs. J Virol Methods. 2008;154:167–174. doi: 10.1016/j.jviromet.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Todoli F, Galindo I, Gomez-Sebastian S, Perez-Filgueira M, Escribano JM, Alberola J, Rodriguez-Cortes A. Dynamics and predictive potential of antibodies against insect-derived recombinant Leishmania infantum proteins during chemotherapy of naturally infected dogs. Am J Trop Med Hyg. 2010;82:795–800. doi: 10.4269/ajtmh.2010.09-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riera C, Fisa R, Lopez-Chejade P, Serra T, Girona E, Jimenez M, Muncunill J, Sedeno M, Mascaro M, Udina M, Gallego M, Carrio J, Forteza A, Portus M. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain) Transfusion. 2008;48:1383–1389. doi: 10.1111/j.1537-2995.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 44.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, Valenzuela JG, Barral-Netto M, Barral A. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 45.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Fujiwara RT, Vale AM, Franca da Silva JC, da Costa RT, Quetz Jda S, Martins Filho OA, Reis AB, Correa Oliveira R, Machado-Coelho GL, Bueno LL, Bethony JM, Frank G, Nascimento E, Genaro O, Mayrink W, Reed S, Campos-Neto A. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential vaccine candidates for canine visceral leishmaniasis. Vet Res. 2005;36:827–838. doi: 10.1051/vetres:2005033. [DOI] [PubMed] [Google Scholar]
- 47.Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, Reed SG, Grimaldi G., Jr Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrillo E, Crusat M, Nieto J, Chicharro C, Thomas Mdel C, Martinez E, Valladares B, Canavate C, Requena JM, Lopez MC, Alvar J, Moreno J. Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine. 2008;26:1902–1911. doi: 10.1016/j.vaccine.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 49.Jensen AT, Gasim S, Ismail A, Gaafar A, Kurtzhals JA, Kemp M, El Hassan AM, Kharazmi A, Theander TG. Humoral and cellular immune responses to synthetic peptides of the Leishmania donovani kinetoplastid membrane protein-11. Scand J Immunol. 1998;48:103–109. doi: 10.1046/j.1365-3083.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 50.Kemp M, Kurtzhals JA, Bendtzen K, Poulsen LK, Hansen MB, Koech DK, Kharazmi A, Theander TG. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993;61:1069–1073. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Carvalho LP, Soto M, Jeronimo S, Dondji B, Bacellar O, Luz V, Orge Orge G, Alonso C, Jesus AR, Carvalho EM. Characterization of the immune response to Leishmania infantum recombinant antigens. Microbes Infect. 2003;5:7–12. doi: 10.1016/s1286-4579(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 52.Maasho K, Wolday D, Edjigu M, Soderstrom K, Britton S, Akuffo H. Induction and abrogation of LACK reactive cells in the evolution of human leishmaniasis. Clin Exp Immunol. 2001;124:255–261. doi: 10.1046/j.1365-2249.2001.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suffia I, Ferrua B, Stien X, Mograbi B, Marty P, Rousseau D, Fragaki K, Kubar J. A novel Leishmania infantum recombinant antigen which elicits interleukin 10 production by peripheral blood mononuclear cells of patients with visceral leishmaniasis. Infect Immun. 2000;68:630–636. doi: 10.1128/iai.68.2.630-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho LP, Passos S, Dutra WO, Soto M, Alonso C, Gollob KJ, Carvalho EM, Ribeiro de Jesus A. Effect of LACK and KMP11 on IFN-gamma production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patients. Scand J Immunol. 2005;61:337–342. doi: 10.1111/j.1365-3083.2005.01581.x. [DOI] [PubMed] [Google Scholar]
- 55.Ramos I, Alonso A, Marcen JM, Peris A, Castillo JA, Colmenares M, Larraga V. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine. 2008;26:333–344. doi: 10.1016/j.vaccine.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 56.Pollock JM, McNair J, Bassett H, Cassidy JP, Costello E, Aggerbeck H, Rosenkrands I, Andersen P. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J Clin Microbiol. 2003;41:1856–1860. doi: 10.1128/JCM.41.5.1856-1860.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kublin JG, Lowitt MH, Hamilton RG, Oliveira GA, Nardin EH, Nussenzweig RS, Schmeckpeper BJ, Diggs CL, Bodison SA, Edelman R. Delayed-type hypersensitivity in volunteers immunized with a synthetic multi-antigen peptide vaccine (PfCS-MAP1NYU) against Plasmodium falciparum sporozoites. Vaccine. 2002;20:1853–1861. doi: 10.1016/s0264-410x(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 58.Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, Van Belle P, Clement F, Hanon E, Wettendorff M, Garcon N, Leroux-Roels G. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 59.Kennedy LJ, Altet L, Angles JM, Barnes A, Carter SD, Francino O, Gerlach JA, Happ GM, Ollier WE, Polvi A, Thomson W, Wagner JL. Nomenclature for factors of the dog major histocompatibility system (DLA), 1998: first report of the ISAG DLA Nomenclature Committee. Anim Genet. 2000;31:52–61. doi: 10.1046/j.1365-2052.2000.00492.x. [DOI] [PubMed] [Google Scholar]
- 60.Berberich C, Requena JM, Alonso C. Cloning of genes and expression and antigenicity analysis of the Leishmania infantum KMP-11 protein. Exp Parasitol. 1997;85:105–108. doi: 10.1006/expr.1996.4120. [DOI] [PubMed] [Google Scholar]
- 61.Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, Reed SG. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maalej IA, Chenik M, Louzir H, Ben Salah A, Bahloul C, Amri F, Dellagi K. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am J Trop Med Hyg. 2003;68:312–320. [PubMed] [Google Scholar]
- 63.Solano-Gallego L, Koutinas A, Miro G, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Cortes A, Fernandez-Bellon H, Ramis A, Ferrer L, Alberola J, Solano-Gallego L. Leishmania-specific isotype levels and their relationship with specific cell-mediated immunity parameters in canine leishmaniasis. Vet Immunol Immunopathol. 2007;116:190–198. doi: 10.1016/j.vetimm.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Ordeix L, Solano-Gallego L, Fondevila D, Ferrer L, Fondati A. Papular dermatitis due to Leishmania spp. infection in dogs with parasite-specific cellular immune responses. Vet Dermatol. 2005;16:187–191. doi: 10.1111/j.1365-3164.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Moreno A, Moreno T, Martinez-Moreno FJ, Acosta I, Hernandez S. Humoral and cell-mediated immunity in natural and experimental canine leishmaniasis. Vet Immunol Immunopathol. 1995;48:209–220. doi: 10.1016/0165-2427(95)05434-8. [DOI] [PubMed] [Google Scholar]


