Abstract
Cutaneous leishmaniasis caused by Leishmania major infection affected 172 (18.3%) of 938 Dutch military troops deployed in northern Afghanistan in 2005. The high attack rate was a result of initial insufficient availability of means of prevention and insufficient adherence to preventive measures. At presentation, the lymphatic system was involved in 24.8%. Treatment with intralesional injections of antimony with or without cryotherapy was satisfactory, but 19.5% of patients received secondary treatment with miltefosine. Six months after treatment, 128 (77.1%) of 166 treated patients were cured, 16 (9.6%) were lost to follow-up, and 22 (13.3%) already experienced cure at six weeks but were not seen at six months. Natural evolution played a role in this observational study, which showed cure of all patients seen at six months. In general, management of cutaneous leishmaniasis was feasible under field conditions.
Introduction
In Afghanistan, anthroponotic cutaneous leishmaniasis and zoonotic cutaneous leishmaniasis (ZCL) occur. Anthroponotic cutaneous leishmaniasis is caused by Leishmania tropica, may cause urban outbreaks, and is transmitted by Phlebotomus sergenti.1,2 Zoonotic cutaneous leishmaniasis is caused by Leishmania major, which has a reservoir in rodents and is transmitted by Ph. papatasi. In northern Afghanistan, the great gerbil (Rhombomys opimus) is the reservoir.3,4 Dutch military personnel were deployed to the area around Mazar-e-Sharif in northern Afghanistan where ZCL occurs in yearly epidemics and where anthroponotic cutaneous leishmaniasis occurs less frequently.5 Within six weeks of arrival, personnel reported ulcerative skin lesions that were initially treated as bacterial infections. Four cases with lesions not responding to antibacterial treatment were repatriated and L. major infection was confirmed.6 Subsequently, diagnosis and treatment were made available in the field and military personnel who had already returned to The Netherlands were advised to report any skin lesion. We report the epidemiologic features, clinical aspects, diagnosis, and results of treatment in this military cohort with ZCL.
Methods
Sources of data.
Data were retrieved from 1) the Dutch Ministry of Defence (demographic data and dates of arrival and departure), 2) medical records of the Field Dressing Station, Mazar-e-Sharif and of the Academic Medical Center, Amsterdam, the Netherlands (clinical data), and 3) questionnaires on adherence to preventive measures filled out during medical examination at debriefing after redeployment of the main body. Results were anonymously shared with military authorities.
Procedures at the Field Dressing Station.
Diagnosis of leishmaniasis was made by microscopic demonstration of parasites in Giemsa-stained smears of biopsy specimens from lesions. Treatment consisted of injections of sodium stibogluconate (pentavalent antimony [Sbv]) (Pentostam®; GlaxoSmithKline, Bernard Castle, United Kingdom) into the margin of each lesion, 1–2 mL all around, until blanching (intralesional Sbv [ilSbv]). All lesions were treated in one session at intervals of 1–3 days. Treatment was continued until clinical improvement (see below). After redeployment, all patients were examined and those with skin lesions either received immediate treatment or were given appointments at the Academic Medical Center. Already diagnosed and treated patients were followed-up at six weeks and six months after treatment, and those receiving treatment continued treatment within a few days. All patients were advised to report new skin manifestations.
Procedures at the Academic Medical Center.
Parasitologic methods were microscopy (Giemsa-stained smear), culture in Novy-MacNeal-Nicolle medium, polymerase chain reaction with molecular characterization of the parasites by sequence analysis of the polymerase chain reaction amplicon,6 and quantitative nucleic acid sequence–based amplification.7 Treatment consisted of three ilSbv injections with intervals of 1–3 days with cryotherapy preceding the first injection. Cryotherapy was applied with a liquid nitrogen spray apparatus (Cry-Ac; Brymill, CT) within rubber cones matching the diameter of the lesions. A double freeze–thaw cycle was performed with a mean duration of 20 seconds for the freezing cycle and thawing time between cycles of 45–90 seconds. Treatment was repeated until clinical improvement (see below). Patients with unsatisfactory response or recurrence (see below) with a single satellite lesion or slight activity of lesions only were treated with additional ilSbv injections but others were treated with miltefosine, as reported.8 Adverse events were recorded. Follow-up was approximately at six weeks and six months after treatment.
Assessment of treatment response.
Clinical improvement was defined as a reduction in size, infiltration, induration, perilesional erythema, crusting of the lesion, or any combination thereof without new manifestations. An unsatisfactory response was defined as extension of the lesion, development of satellite lesions, newly developed lymphatic involvement (sporotrichoid spread, lymphangitis, lymphadenitis, or lymph node enlargement), or any combination thereof during treatment up to the first follow-up visit. Recurrence was defined as the appearance of new manifestations at the original site or elsewhere after initial improvement. An unsatisfactory response and recurrence were based on clinical examination and presence of Leishmania parasites. Definite cure was defined as a complete re-epithelialization of all lesions at six months. Probable cure was defined as clinical cure (re-epithelialization without new manifestations of leishmaniasis) six weeks after treatment but not assessed at six months.
Statistical analysis.
The contingent attack rate was calculated as the total number of patients with CL divided by total number of deployed troops. Attack rates were calculated per arrival cohort (all military personnel arriving on the same day). The incidence rate of the self-reported first CL lesion was calculated as total number of cases divided by total person-months at risk. The at-risk period was defined as the time between arrival and the day CL was confirmed in the four index patients because from that day onwards preventive measures were more strictly emphasized and implemented. Total person-months at-risk was calculated as the period between date of arrival and 1) date of departure, 2) end of the at-risk period, or 3) date of noticing a skin lesion, whichever came first. For 32 patients who did not recall when they had noticed the skin lesion, person-months at-risk was imputed as 35 days, which was the median interval between arrival and observation of first lesion in all cases.
Multivariate Poisson regression (GENLIN procedure in SPSS version 15.0 for Windows; SPSS Inc., Chicago, IL) was used to assess differences in incidence rates comparing poor or good adherence to preventive measures. Models were adjusted for arrival date, as a proxy for changes in infection pressure (because of entomologic factors and changes in size of the susceptible population). Multivariate exponential risk models (GENLIN in SPSS) were used to assess differences in response to first line treatment. Robust standard errors were used for calculation of confidence intervals (CIs) for the incidence rate ratios (IRRs) and risk ratios (RRs). The incubation period was estimated for a cohort of 25 persons with CL who were present in the area during the same at-risk period (the camp build-up phase) for a relatively short duration (30–35 days) by using a non-parametric method described by Tam and Wong,9,10 which estimates the distribution of incubation periods on the basis of a sample of persons with multiple exposure times.
Results
During June–November 2005, 938 Dutch military personnel were deployed to the rural area near Mazar-e-Sharif for a median of 86 days (interquartile range [IQR] = 80–91 days, range = 5–134 days). After the construction of the camp, the force gradually increased to full strength (Figure 1). Before departure, personnel were informed about vector-borne diseases prevalent in the region, in particular malaria and CL. Preventive measures discussed included use of anti-malarial drugs, a dress code of wearing long sleeves and long trousers in the late afternoon and evening and use of insect repellents (N,N-diethyl-m-toluamide) and permethrin-impregnated bed nets and uniforms. Unfortunately, factory-impregnated uniforms were not available before deployment; instead, insecticide and instructions for impregnation of uniforms were supplied. The greater part of the force was in the field before the arrival of impregnated-bed nets.
Figure 1.
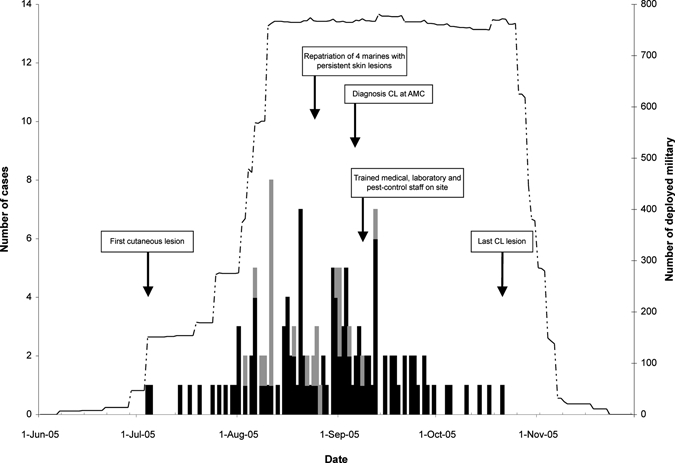
Epidemic curve of self reported first occurrence of lesion(s) later diagnosed as cutaneous leishmaniasis (CL) in Dutch military personnel deployed in northern Afghanistan during July–November 2005. Black and gray bars represent number of first occurrences in military personnel deployed in northern Afghanistan or redeployed to the Netherlands, respectively (left y-axis). The dotted line represents total number of Dutch military personnel deployed in northern Afghanistan over time (right y-axis). The time line presents important events related to the cutaneous leishmaniasis epidemic.
Index cases.
Within a few weeks of deployment, ulcerative skin lesions were noticed and treated as a streptococcal ulcer. Four patients with ulcers not responding to antibiotics were repatriated and CL was diagnosed (Figure 1). A physician and laboratory technician trained in diagnosis of CL and treatment with ilSbv were deployed to Afghanistan where they trained colleagues. Those persons who had already returned to The Netherlands were informed and advised to report any skin lesion.
Epidemic.
Cutaneous leishmaniasis was diagnosed in 172 military personnel (attack rate = 18.3%). Parasitologic investigations were performed for 132 patients; in 129 (97.7%), the diagnosis was confirmed (75% of 172 cases). Parasitologic investigations were not available for 38 patients and not performed for two patients (by mistake). These patients and the three with negative parasitologic results (43 patients [25%]) were clinically diagnosed as being cases of CL. The total number deployed and the epidemic curve of onset of lesions (n = 140) is shown in Figure 1. The overall incidence rate was 17.6 (95% CI = 15.4–20.2) per 100 person-months. Median incubation period was 27 days (IQR = 17–37 days). The crude attack rates per arrival cohort are shown in Figure 2. Cutaneous leishmaniasis was not seen in military personnel who arrived after mid-August.
Figure 2.
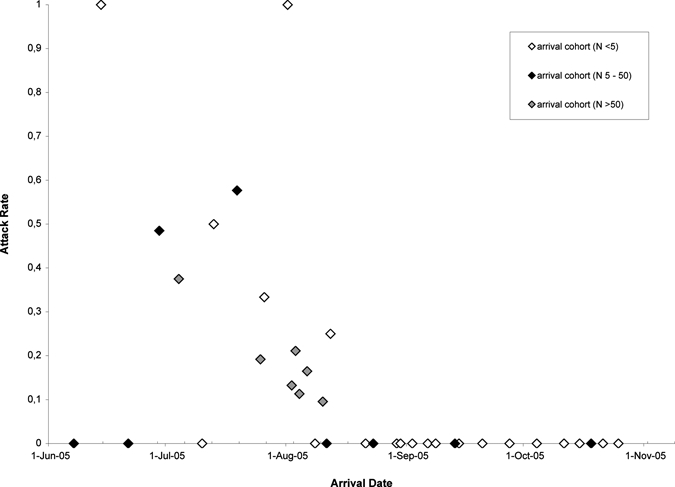
Attack rates per arrival cohort of Dutch military personnel deployed in northern Afghanistan during July–November 2005 by date of arrival in northern Afghanistan. White, black, and gray circles represent arrival cohorts consisting of < 5 military personnel, 5–50 military personnel, and > 50 military personnel, respectively.
Risk factors and personal preventive measures.
At debriefing, 710 questionnaires were completed. Non-adherence to the prescribed dress code and inconsistent use of an impregnated bed net were associated with increased risk of CL (adjusted IRR = 1.40, 95% CI = 0.97–2.03 and 1.47, 95% CI = 0.99–2.17) but inconsistent use of N,N-diethyl-m-toluamide was not associated with these two factors (adjusted IRR = 1.12, 95% CI = 0.79–1.58). The effect of dress code was independent of whether uniforms were insecticide-impregnated. Effect measures were adjusted for arrival date as a proxy for changing infection pressure and concurrent adherence to each of the other preventive measures.
Clinical characteristics.
Clinical characteristics of 169 patients are presented; three patients were treated elsewhere. The median period between noticing a CL lesion and first visit to a physician was 28 days (IQR = 14–35 days). Descriptive statistics are shown in Table 1. Lesions occurred on parts of the body covered by standard uniforms (trunk, legs, or feet) in 81.0%, and the lymphatic system was involved in 24.8%.
Table 1.
Description of cutaneous leishmaniasis lesions at initial clinical presentation for 169 Dutch military personnel deployed in northern Afghanistan during July–November 2005*
| Characteristic | Value |
|---|---|
| No. (%) lesions (n = 161) | |
| 1 | 67 (41.6) |
| 2 | 28 (17.4) |
| 3 | 34 (21.1) |
| ≥ 4 | 32 (19.9) |
| Median (interquartile range) size of lesions, mm (n = 149) | |
| Minimum diameter | 10 (5–13) |
| Maximum diameter | 20 (13–30) |
| Location of lesions, no (%) (n = 163) | |
| Hands, neck, or head | 20 (12.3) |
| Arms | 61 (37.4) |
| Trunk, legs, or feet | 132 (81.0) |
| Aspect of lesions, no. (%) (n = 149) | |
| Ulcerated | 64 (43.0) |
| Nodular | 57 (38.3) |
| Mixed | 28 (18.8) |
| Lymphatic system involvement, no. (%) (n = 153)† | |
| Lymphangitis | 7 (4.6) |
| Lymph node involvement | 32 (20.9) |
| Satelite lesion, no. (%) (n = 121) | 18 (14.9) |
Three other patients were treated elsewhere.
One patient had lymphangitis and lymph node involvement.
Diagnosis and treatment.
Diagnosis was made in the field by microscopy and by isolation and characterization of the parasite at the Academic Medical Center. All 68 characterized6 parasite isolates were identical. Isoenzyme analysis (Laboratory of Parasitology, University of Montpellier, Montpellier, France) classified the parasite as MON-26. Data on diagnosis and treatment are shown in Table 2. One patient refused treatment, one experienced spontaneous cure, and one patient had the nodular lesion excised; all three patients were cured when seen approximately six weeks later. The 45 patients given a diagnosis at the Academic Medical Center were patients redeployed after the build-up period, the four index patients, and 11 other patients who were repatriated because of facial or extensive lesions. Overall median treatment duration was 17 days (IQR = 10–34 days, range = 1–163 days) with a median of 5 sessions (IQR = 4–8 sessions) and a cumulative dose of ilSbv of 5.1 mL (IQR = 2.9–9.9 mL). Forty-two (25.8%) patients were treated for more than four weeks because of unsatisfactory response or problems with compliance caused by military duties. Of these patients, 22 received additional ilSbv injections and 20 received miltefosine.
Table 2.
Treatment and outcome of cutaneous leishmaniasis in Dutch military personnel deployed in northern Afghanistan during July–November 2005*
| Characteristic | Treatment location† | |
|---|---|---|
| MeS‡ | AMC | |
| No. patients given first-line treatment§ | 118 | 45 |
| Parasitologic confirmation, no. (%)¶ | 79/80 (98.8) | 42/44 (95.5) |
| Clinical presentation | ||
| No. lesions, median (interquartile range) (range) | 2 (1–3) (1–11) | 2 (1–3) (1–9) |
| Maximal diameter of lesions (mm), median (IQR) (range) | 20 (13–30) (5–80) | 15 (10–20) (4–50) |
| Lymphatic involvement, no. (%) | 22/103 (21.4) | 16/44 (36.4) |
| First-line treatment | ||
| Total treatment duration (days),# median (IQR) (range) | 18 (11–38) (1–163) | 14.5 (7–21) (3–96) |
| MeS | ||
| il SbV (no. sessions),#** median (IQR) (range) | 5 (4–8) (1–13) | NA |
| Duration (days),# median (IQR) (range) | 16 (10–32) (1–56) | NA |
| AMC | ||
| il SbV (no. sessions),#** median (IQR) (range) | 1 (1–3) (1–7) | 4 (3–6) (2–11) |
| Cryotherapy (no. sessions),#†† median (IQR) (range) | 1 (0–1) (0–3) | 2 (1–2) (1–4) |
| Duration (days),# median (IQR) (range) | 1 (1–8) (1–64) | 14.5 (7–21) (3–96) |
| Outcomes | ||
| Recurrence; no./N (%) | 18/118 (15.3) | 5/45 (11.1) |
| Treatment duration > 28 days (prolonged because of unsatisfactory treatment response or poor compliance), no./N (%) | 34/118 (28.8) | 8/45 (17.8) |
| Second-line treatment with oral miltefosine,‡‡ no./N (%) | 24/118 (20.3)9 because of recurrence and 15 because of unsatisfactory treatment response | 6/45 (13.3)1 because of recurrence and 5 because of unsatisfactory treatment response |
| Present at final follow-up (6 months),§§¶¶ no. /N (%) | 65/94 (69.1) | 30/39 (76.9) |
| Definite cure 6-months post-treatment,§§## no. (%) | 65 (100) | 30 (100) |
IQR = interquartile range; ilSbv = with intralesional pentavalent antimony; NA = not available.
MeS = Mazar-e-Sharif, Afghanistan; AMC = Academic Medical Center, Amsterdam, The Netherlands.
Twenty-one persons who started treatment at M-e-S continued treatment at AMC after redeployment, 9 because of recurrence.
Six patients are not included: four received alternative treatment (three received primary miltefosine because of extensive or numerous lesions; in one patient, the lesion was excised), two persons were not treated (one refused one showed spontaneous resolution of the lesion).
Sixty-eight of 68 samples were subtyped as Leishmania major by polymerase chain reaction.
Excluding patients with a recurrence.
One session includes all injections without or with cryotherapy given on one occasion.
Four of 21 redeployed soldiers continuing treatment did not receive cryotherapy.
Details reported elsewhere.6
Excluding patients receiving secondary miltefosine treatment.
Of those absent at final follow-up (6 months) but present at the 6-week follow-up, 16 (84.2%) of 19 at MeS and 6 (100%) of 6 at AMC showed good clinical response.
Of those present at final follow-up (6 months) and present at the 6-week follow-up, 30 (54.5%) of 55 at MeS and 13 (46.4%) of 28 at AMC showed good clinical response.
Of the 23 patients (14.1%) with recurrence, 13 were treated with additional ilSbv injections and 10 were treated with miltefosine. Three patients received miltefosine as primary treatment because of multiple or extensive lesions.8 Details on treatments and treatment results applied at the different sites are shown in Tables 2 and 3. Results of treatment with ilSbv alone or with the combination treatment were not different as assessed six weeks after treatment: 39 (68.4%) of 57 versus 19 (55.9%) of 34, RR = 1.22, 95% CI = 0.87–1.73. Treatment response for nodular lesions (27 [64.3%] of 42) versus ulcerative and mixed lesions (32 [56.1%] of 57) as assessed at six weeks was not different (RR = 1.15, 95% CI = 0.83–1.58).
Table 3.
Results for 163 patients with cutaneous leishmaniasis (Leishmania major infection) acquired in northern Afghanistan treated with intralesional pentavalent antimony with or without cryotherapy in Mazar-e-Sharif, Afghanistan, the Academic Medical Center, Amsterdam, The Netherlands, or in both locations*
| Result | M-e-S,† ilSbv, no. (%) (n = 118) | AMC, ilSbv plus cryotherapy, no. (%) (n = 45) |
|---|---|---|
| Secondary miltefosine | 24 (20.3) | 6 (13.3) |
| Definite cure | 65 (55.1) | 30 (66.7) |
| Probable cure | 16 (13.6) | 6 (13.3) |
| Unknown | 13 (11.0) | 3 (6.7) |
Three of 172 patients were treated elsewhere, 4 received other treatment, 1 refused treatment, and 1 experienced spontaneous cure. MeS = Mazar-e-Sharif, Afghanistan; ilSBv = with intralesional pentavalent antimony; AMC = Academic Medical Center, Amsterdam, The Netherlands
Twenty-one persons who started treatment at M-e-S continued treatment in AMC after redeployment; 17 with the addition of cryotherapy and 9 because of recurrence.
Adverse events.
Pain at the injection site of Sbv was commonly reported. Unfortunately, how much fitness for duties was influenced by the lesions and the treatment could not be obtained from medical records. Secondary bacterial infection was clinically diagnosed and treated in 21 (23.9%) of 88 patients treated in Mazar-e-Sharif (ilSbv) and in 14 (31.1%) of 45 patients treated at the Academic Medical Center (combination treatment) (RR = 0.77, 95% CI = 0.43–1.36). The few gram stains and cultures that were performed showed streptococci and staphylococci. Lymphatic involvement developed during treatment in 54 (60%) of 90 patients treated with ilSbv alone and in 22 (48.9%) of 45 patients treated with combination treatment (no significant difference: RR = 1.23, 95% CI = 0.87–1.73). No patient discontinued treatment and no severe events occurred.
Discussion
The high attack rate of CL (18.3%) in this military contingent may be explained by the influx of a large group of non-immune persons in an area endemic for L. major3,4 in the peak of the transmission season,5,11 initially insufficient means of prevention, and inadequate adherence to preventive measures. Off-duty activities in sports outfit in the late afternoon/early evening likely contributed to the attack rate, a conclusion supported by the association between non-adherence and higher incidence of CL and the presence of lesions on parts of the body normally covered by clothing in > 80%. The attack rate was highest in the build-up period of the camp (Figure 2). In cohorts deployed after mid-August, no cases occurred probably because of better adherence to preventive measures, availability of means of prevention, and possible decrease in transmission. These data are consistent with the findings that flight activity of sand flies already started at 4:00 pm and that the transmission season rapidly decreased in early September as reported by Faulde and others.5,11 Investigations regarding transmission were not performed but reduction of sand flies was attempted by spraying of insecticide within the compound. In 2003–2004, several persons in this cohort had been deployed in southern Iraq where no case of CL has been reported in Dutch military personnel. Therefore, immunity did not likely play a role in the evaluation of this outbreak.
The clinical picture of L. major infection is variable and includes nodular, ulcerative, and satellite lesions, lymph node enlargement, and lymphadenitis and lymphangitis with sporotrichoid spread.12–15 The picture differs in various parts of the world in relation to the locally circulating L. major strain.16,17 In the present cohort, 41.6% had only 1 lesion and 19.9% had ≥ 4 lesions; lymphatic involvement was relatively frequent (24.8%). Assessment of the treatment response in the field was based on clinical grounds. At the Academic Medical Center, parasitologic results could be taken into account but decisions to continue treatment were made on the basis of clinical grounds because parasitologic results were available several days later. Duties and redeployment explain the rather long range of treatment duration (Table 2).
Miltefosine was advised for secondary treatment in cases of recurrence or unsatisfactory clinical response in the presence of multiple or extensive lesions. This drug was known to be an effective treatment for visceral leishmaniasis and had been used to limited extend in the treatment of patients with CL. An important advantage is its oral administration. The alternative treatment would have been systemic (intramuscular or intravenous) with antimony that required 20 daily hospital visits. Of the total cohort of 166 treated patients, 128 (77%) were definitely cured, 22 (13.3%) probably experienced cure (seen at six weeks and not at six months), and 16 patients (9.7%) were lost to follow-up. In the last group, lesions most likely resolved because natural cure without treatment eventually is the rule.15 All patients seen six months after treatment were cured as a result of treatment or natural evolution (or a combination of both); assessment at six weeks and three months may provide better information on treatment efficacy. Our results do not confirm those of previous reports on this same cohort;5,11,18 the incubation time was not a minimum of 7 weeks5,11 but a mean of 25 days, and treatment results were better than the reported delayed or poor response to sodium stibogluconate or miltefosine.18 These reports were based on unverified information.
In this study, the combination of ilSbv with cryotherapy was not better than ilSbv alone as reported.19 Because our study was not a controlled study, caution is required in the interpretation of treatment results and differences in results of various treatments. Exacerbation of symptoms of CL caused by bacterial infection, as reported,20 was not encountered in this cohort. Suppurative subcutaneous nodules were aspirated or incised on two occasions, but results of microbiologic and parasitologic investigations were negative for both samples, which indicated a host response rather than pathogen dissemination.
Gaafar and others reported parasites in suppurative nodes in a few patients with CL before treatment.21 Involvement of the lymphatic system was prominent in the study cohort; at presentation, this involvement was observed in 24.8% of the patients and developed during treatment in 54 (60%) of 90 patients treated with ilSbv alone and in 22 (48.9%) of 45 patients treated with ilSbv plus cryotherapy. Reports from Saudi Arabia on L. major infections suggested that development of satellite lesions and lymphatic involvement may be related to treatment.12,13,22 Treatments reported in these studies are local therapies (cryotherapy, ilSbv) and systemic treatments, both oral (ketoconazole, rifampicin) and parenteral (antimony), but numbers of patients treated and specific relationships and associations of lymphatic involvement with respective treatments are not given. In a Cochrane analysis on treatment of Old World CL19 and in a large series of ilSbv treatments of patients infected with L. tropica in Turkey,23,24 lymphatic involvement during treatment is not mentioned. These data suggest that lymphatic involvement during treatment may occur irrespective of the nature of the treatment and that it may be a particular feature of the local parasite strain or the host response to the pathogen or a combination of factors.
In conclusion, the high attack rate of CL caused by L. major infection in Dutch troops deployed in northern Afghanistan in 2005 during the transmission season was caused by initial insufficient availability of means of prevention and insufficient adherence to preventive measures. Microscopic demonstration of parasites in skin biopsy specimens and treatment with ilSbv were feasible under basic field conditions, even in the presence of multiple lesions and dissemination. Treatment with ilSbv with or without cryotherapy was satisfactory, but 19.5% of patients received second-line treatment with miltefosine. We concur with the advice of a Cochrane analysis19 that the quality and standardization of trials and case series on Old World CL must be improved to obtain evidence-based clinical and treatment guidelines.
Acknowledgments
We thank the medical, para-medical, and administrative personnel in the field and in the departments of parasitology, dermatology and tropical medicine during the management of this large outbreak for expert assistance, the patients and staff of the Netherlands Armed Forces for their cooperation, the Laboratory of Parasitology of Montpellier for analyzing the parasite isolate, and Mariska Bot and Lex Schouten (VU Medical Center, Amsterdam, The Netherlands) for their assistance.
Footnotes
Authors' addresses: Pieter-Paul van Thiel, Tjalling Leenstra, and Alex C. Krull, Department of Internal Medicine, Division of Infectious Diseases, Tropical Medicine and AIDS and Center for Infection and Immunity, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, and Netherlands Ministry of Defence, The Hague, The Netherlands, E-mails: p.p.vanthiel@amc.uva.nl, t.leenstra@mindef.nl, and a.c.krull@amc.uva.nl. Henry J. de Vries and William R. Faber, Department of Dermatology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mails: h.j.devries@amc.uva.nl and w.r.faber@amc.uva.nl. Allard van der Sluis, Netherlands Ministry of Defence, The Hague, The Netherlands, E-mail: aj.vd.sluis.01@mindef.nl. Tom van Gool and Aldert Bart, Department of Medical Microbiology, Section of Parasitology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mails: t.vangool@amc.uva.nl and a.bart@amc.uva.nl. Michèle van Vugt, Department of Internal Medicine, Division of Infectious Diseases, Tropical Medicine and AIDS and Center for Infection and Immunity, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, and PharmAccess Foundation, Amsterdam, The Netherlands, E-mail: m.vanvugt@amc.uva.nl. Peter J. de Vries and Piet A. Kager, Department of Internal Medicine, Division of Infectious Diseases, Tropical Medicine and AIDS and Center for Infection and Immunity, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mails: p.j.devries@amc.uva.nl and p.a.kager@amc.uva.nl. Jimmy E. Zeegelaar, Department of Dermatology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, and Department of Dermatology, Flevoziekenhuis, Almere, The Netherlands, E-mail: jezeegelaar@flevoziekenhuis.nl. Wendy F. van der Meide, U-Cytech Biosciences, Utrecht, The Netherlands, E-mail: wmeide@ucytech.com. Henk D. F. H. Schallig, KIT Biomedical Research, Parasitology Unit, Royal Tropical Institute/Koninklijk Instituut voor de Tropen, Amsterdam, The Netherlands, E-mail: h.schallig@kit.nl.
References
- 1.Reyburn H, Rowland M, Mohsen M, Khan B, Davies C. The prolonged epidemic of anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: “bringing down the neighbourhood.”. Trans R Soc Trop Med Hyg. 2003;97:170–176. doi: 10.1016/s0035-9203(03)90111-8. [DOI] [PubMed] [Google Scholar]
- 2.Reithinger R, Mohsen M, Aadil K, Sidiqi M, Erasmus P, Coleman PG. Anthroponotic cutaneous leishmaniasis, Kabul, Afghanistan. Emerg Infect Dis. 2003;9:727–729. doi: 10.3201/eid0906.030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadim A, Javadian E, Noushin MK, Nayil AK. Epidemiology of cutaneous leishmaniasis in Afghanistan. Part I: zoonotic cutaneous leishmaniasis. Bull Soc Pathol Exot. 1979;72:31–35. [PubMed] [Google Scholar]
- 4.Ahmad K. War and gerbils compound Afghan leishmaniasis epidemic. Lancet Infect Dis. 2002;2:268. doi: 10.1016/s1473-3099(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 5.Faulde M, Schrader J, Heyl G, Amirih M. Differences in transmission seasons as an epidemiological tool for characterization of anthroponotic and zoonotic cutaneous leishmaniasis in northern Afghanistan. Acta Trop. 2008;105:131–138. doi: 10.1016/j.actatropica.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Marfurt J, Nassereddin A, Niederweiser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J Clin Microbiol. 2003;41:3147–3153. doi: 10.1128/JCM.41.7.3147-3153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Meide WF, Schoone GJ, Faber WR, Zeegelaar JE, de Vries HJ, Özbel Y, Lai A Fat RF, Coelho LI, Kassi M, Schallig HD. Quantitative nucleic acid sequence–based assay as a new molecular tool for detection and quantification of Leishmania parasites in skin biopsy samples. J Clin Microbiol. 2005;43:5560–5566. doi: 10.1128/JCM.43.11.5560-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Thiel PP, Leenstra T, Kager PA, de Vries HJ, van Vugt M, van der Meide WF, Bart A, Zeegelaar JE, van der Sluis AJ, Schallig HD, van Gool T, Faber WR, de Vries PJ. Miltefosine treatment of Leishmania major infection: an observational study involving Dutch military personnel returning from northern Afghanistan. Clin Infect Dis. 2010;50:80–83. doi: 10.1086/648726. [DOI] [PubMed] [Google Scholar]
- 9.Wong T, Tam W. Estimating SARS incubation period. Emerg Infect Dis. 2004;10:1503–1504. doi: 10.3201/eid1008.040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam WW, Wong T. Estimating incubation period with multiple contact days. Scand J Infect Dis. 2007;39:607–611. doi: 10.1080/00365540601113719. [DOI] [PubMed] [Google Scholar]
- 11.Faulde M, Schrader J, Heyl G, Amirih M, Hoerauf A. Zoonotic cutaneous leishmaniasis outbreak in Mazar-e-Sharif, northern Afghanistan: an epidemiological evaluation. Int J Med Microbiol. 2008;298:543–550. doi: 10.1016/j.ijmm.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Kubba R, El-Hassan AM, Al-Gindan Y, Omer AH, Kutty MK, Saeed MB. Dissemination in cutaneous leishmaniasis. I. Subcutaneous nodules. Int J Dermatol. 1987;26:300–304. doi: 10.1111/j.1365-4362.1987.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 13.Kubba R, Al-Gindan Y, El-Hassan AM, Omer AH, Kutty MK, Saeed MB. Dissemination in cutaneous leishmaniasis. II. Sattelite papules and subcutaneous induration. Int J Dermatol. 1988;27:702–706. doi: 10.1111/j.1365-4362.1988.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 14.Al-Gindan Y, Kubba R, El-Hassan AM, Omer AH, Kutty MK, Saeed MB. Dissemination in cutaneous leishmaniasis. 3. Lymph node involvement. Int J Dermatol. 1989;28:248–254. doi: 10.1111/j.1365-4362.1989.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 15.Dowlati Y. Cutaneous leishmaniasis: clinical aspects. Clin Dermatol. 1996;14:425–431. doi: 10.1016/0738-081x(96)00058-2. [DOI] [PubMed] [Google Scholar]
- 16.Elfari M, Schnur LF, Strelkova MV, Eisenberger CL, Jacobson RL, Greenblatt CL, Presber W, Schönian G. Generic and biological diversity among populations of Leishmania major from Central Asia, the Middle East and Africa. Microbes Infect. 2005;7:93–103. doi: 10.1016/j.micinf.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Gaafar A, Fadl A, El Kadaro AY, El Hassan MM, Kemp M, Ismael AI, Morgos SA, El Hassan AM. Sporothrichoid cutaneous leishmaniasis due to Leishmania major of different zymodemes in the Sudan and Saudi Arabia: a comparative study. Trans R Soc Trop Med Hyg. 1994;88:552–554. doi: 10.1016/0035-9203(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 18.Faulde MK, Heyl G, Amirih ML. Zoonotic cutaneous leishmaniasis, Afghanistan. Emerg Infect Dis. 2006;10:1623–1624. doi: 10.3201/eid1210.060076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008. CD005067. doi:10.1002/14651858.CD005067.pub3. [DOI] [PubMed]
- 20.Kubba R, Al-Gindan Y. Leishmaniasis. Dermatol Clin. 1989;89:149–159. [PubMed] [Google Scholar]
- 21.Gaafar A, Ismail A, El Kadaro AY, Hashim E, Khalil EA, El Hassan AM. Necrotizing and suppurative lymphadenitis in Leishmania major infections. Trop Med Int Health. 1996;1:243–249. doi: 10.1111/j.1365-3156.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Gindan Y, Kubba R, Omer AH, El-Hassan AM. Cryosurgery in old world cutaneous leishmaniasis. Br J Dermatol. 1988;118:851–854. doi: 10.1111/j.1365-2133.1988.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 23.Uzun S, Uslular A, Yücel A, Acar MA, Özpoyraz M, Memişsoğglu HR. Cutaneous leishmaniasis: evaluation of 3074 cases in the Cukurova region of Turkey. Br J Dermatol. 1999;140:347–350. doi: 10.1046/j.1365-2133.1999.02673.x. [DOI] [PubMed] [Google Scholar]
- 24.Uzun S, Durdu M, Culha G, Allahverdiyec AM, Memisoglu HR. Clinical features, epidemiology and efficacy and safety of intralesional antimony treatment of cutaneous leishmaniasis: recent experience in Turkey. J Parasitol. 2004;90:853–859. doi: 10.1645/GE-185R. [DOI] [PubMed] [Google Scholar]


