Abstract
We report a case of human immunodeficiency virus–associated pericardial tuberculosis complicated by cardiac tamponade. Emergency management and subsequent therapeutic interventions are described and then discussed with particular focus on resource-limited settings. The paucity of evidence to support clinical decisions is emphasized and the need for well designed diagnostic and therapeutic studies is highlighted.
The convergence of human immunodeficiency virus (HIV) and tuberculosis (TB) epidemics in southern Africa has contributed to a significant increase in reported cases of extrapulmonary TB (EPTB).1 One of the most common presentations of disease is pericardial TB, which in HIV co-infection is associated with significant mortality and morbidity.2 Cardiac tamponade is a common and life-threatening complication of TB pericarditis that requires immediate medical intervention.
We report a case of TB pericarditis associated with HIV infection and its management in Hlabisa Hospital, a rural district hospital in northern KwaZulu-Natal, South Africa. Hlabisa is located at the epicenter of the intertwined HIV and TB epidemics: the prevalence of HIV is 21.5% in the adult population (15–49 years of age); the TB notification rate was 1,700 per 100,000 persons in 2008; and the HIV co-infection rate for TB patients is 76%.3 We discuss the identification and management of this condition in HIV-infected persons, with a focus on settings with limited resources for diagnosis and intervention. We highlight some of the key areas where further evidence is required to inform clinical practice.
Case Report
A 34-year old HIV-infected man (CD4 cell count = 310 cells/mm3) came to our hospital with a short history of increasing dyspnea and dizziness. He reported chest pain, weight loss, night sweats, but no cough. On initial assessment, he was unwell, diaphoretic, tachycardic (heart rate = 128/min), hypotensive (blood pressure = 100/60 mm of Hg), and severely tachypnoeic (respiratory rate = 40/min). Jugular venous pressure was increased. Chest radiograph demonstrated enlarged cardiac shadow. Because echocardiography was available on site, ultrasound was performed using a conventional B-Mode ultrasound scanner (Just Vision 400; Toshiba, Tokyo, Japan) with a 3.5-MHz abdominal probe and a sub-xiphoidal approach. A 3–4 cm pericardial effusion was seen, with impaired filling of the right ventricle and right atrium, the characteristic sonographic signs of cardiac tamponade (Figure 1 and Supplementary Video 1).
Figure 1.
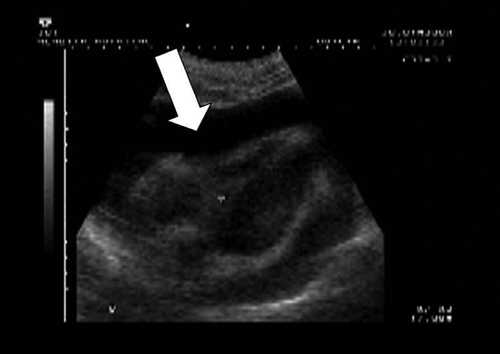
Ultrasound at presentation for the patient, showing large pericardial effusion and right ventricular collapse.
The patient was transferred to the high care unit for pericardiocentesis and continuous electrocardiography (ECG) monitoring. No dedicated pericardiocentesis set or central venous cannula was available at that time. Localization for intercostal puncture approximately in the mid-clavicular line was determined by using ultrasound, and a 16-gauge intravenous cannula was inserted in the lower portion of the intercostal space. The needle was removed and 400 mL of clear yellow fluid was drained before removal. Despite the persistence of a large effusion, cardiac function improved significantly, and the clinical status improved significantly within thirty minutes (Figure 2 and Supplementary Video 2). Anti-tuberculous therapy (Rifafour®; Sanofi-Aventis, Midrand, South Africa, four tablets/day, a fixed-dose combination of rifampicin, isoniazid, ethambutol, and pyrazinamide)) and treatment with prednisolone (80 mg/day) were commenced. After two days, the condition had stabilized and the patient requested discharge; at this time the effusion measured 1–2 cm (Figure 3). Prednisolone was prescribed for one month and continued TB therapy was supervised at the primary health care clinic. After two months, antiretroviral therapy (stavudine/lamivudine/efavirenz) was initiated. The six-month course of antituberculous therapy was completed uneventfully with no evidence of immune reconstitution inflammatory syndrome and ultrasound at the end of treatment demonstrated no residual pericardial effusion.
Figure 2.
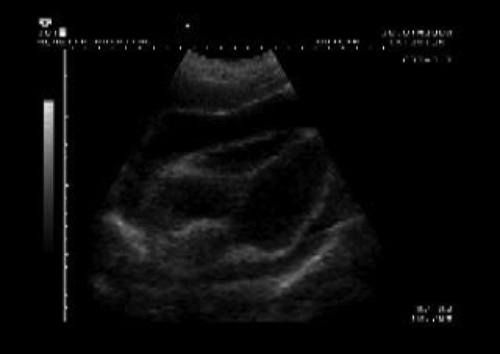
Ultrasound post-pericardiocentesis for the patient, showing persistent large effusion but improved right ventricular filling.
Figure 3.
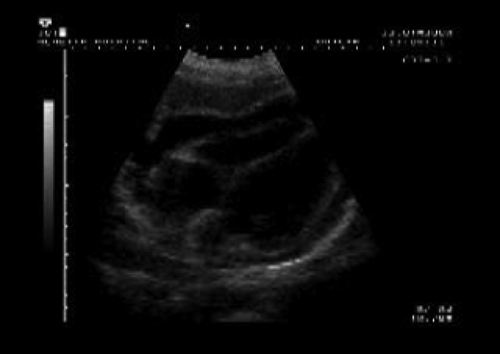
Ultrasound on day 3 for the patient, showing reduction in pericardial effusion.
Discussion
Clinical manifestations of pericarditis (chest pain, cough, dyspnea) overlap with other disease entities in HIV-infected persons: most importantly pulmonary and pleural TB, but also bacterial pneumonia and Pneumocystis jiroveci pneumonia. Systemic signs (weight loss, night sweats, fatigue) may be present but are non-discriminatory. Signs of heart failure may result from progression of pericardial effusion but HIV-infected persons are also more likely to have myocardial involvement and hemodynamic instability.4
A high index of suspicion for pericardial disease is therefore required in symptomatic HIV-infected persons, and it is important that imaging is included in algorithms for smear-negative and extrapulmonary TB.5 Although expansion of radiographic facilities would be welcomed, we would also like to see more extensive use of ultrasound techniques. Although cardiomegaly observed by chest radiograph is suggestive of pericardial effusion, distinction from dilated cardiomyopathy requires ultrasound and formal echocardiography is rarely available in the district hospital setting. Recognition of the anechoic effusion is normally straightforward; thickened pericardium with echogenic, exudative coating and fibrin strands may be seen and increases the likelihood of tuberculous etiology.6 If expertise is available, it is also important to assess the right ventricular cavity because in cases of tamponade the diameter will be reduced and there might be a paradoxical inward motion or even collapse of the free wall.
Preliminary work from our group suggests that non-specialist physicians can be trained to identify key features of EPTB by using ultrasound.7 However, pericardial effusion was a common finding in HIV-infected patients in this study and further research is required to establish clinical decision–making tools based on clinical features and sonographic appearance.
In countries with a high prevalence of TB, this disease has been reported as the cause of more than 90% of large pericardial effusions in HIV-infected persons.8 Other potential causes include bacterial pericarditis (e.g., non-typhoidal Salmonella), human herpesvirus-8–related disease, and lymphoma. Definitive diagnosis of tuberculous etiology is challenging because studies have shown the yield from direct smear examination of pericardial fluid to be as low as 2%9; culture of pericardial fluid is positive in 38–56% of cases.9,10 Other indirect methods used to support TB diagnosis, such as adenosine deaminase levels in pericardial fluid, have the limitation of lower sensitivity in persons with advanced HIV infection.11 All diagnostic studies have the common problem of the lack of a definitive gold standard. Whether novel diagnostic technologies that use the polymerase chain reaction have sufficient accuracy with samples such as pericardial fluid requires well-designed studies.
In the absence of sufficiently rapid, accurate diagnostic techniques, it is appropriate in high prevalence areas to initiate empiric antituberculous therapy in the presence of large pericardial effusion. Diagnostic pericardial aspiration should be reserved for cases where there is evidence for an alternative diagnosis (e.g., skin lesions of Kaposi's sarcoma) or suspicion of drug-resistant TB.
Pericardiocentesis is often a life-saving intervention and is essential in patients with cardiac tamponade. In clinical studies, the proportion of patients with tamponade has ranged between 20% and 90% depending on the selection of patients and definition of tamponade.4,12 Cardiac tamponade is classically a clinical diagnosis characterized by increased jugular venous pressure, hypotension, and diminished heart sounds but cardiac ultrasound may identify typical features such as collapse of the right ventricle or right atrium.
According to standard protocols, a needle attached to the V1 lead of an electrocardiograph machine is inserted into the pericardial space, commonly by a sub-xiphoidal approach. Echocardiographic control is recommended but the expertise is unlikely to be available in many settings. In settings where dedicated pericardiocentesis equipment is not available, the use of alternative approaches, such as intercostal approach with a simple cannula as described in our case, might be necessary but should be guided by ultrasound (Figure 4). It is necessary to drain only enough fluid to relieve the signs of hemodynamic instability. Many patients experience rapid clinical improvement despite the continuing presence of pericardial effusion. It is worth remembering that the aim is reduction of pressure in the pericardial space not volume per se and the drainage of 50–100 mL of fluid might suffice for resolution of tamponade.
Figure 4.
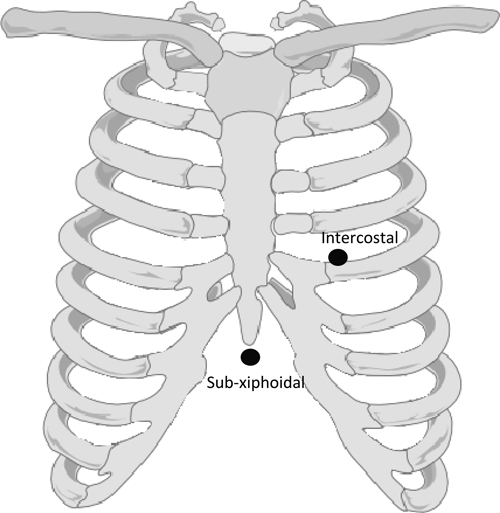
Sites for pericardiocentesis. A, Standard sub-xiphoidal approach. B, Intercostal approach used with ultrasound guidance in this case.
Antituberculous therapy should be commenced as soon as a diagnosis of pericarditis is made in a setting with high prevalence of TB and HIV infection. Treatment regimens recommended for pericardial TB are the same as for pulmonary TB, consisting of rifampicin, isoniazid, ethambutol, and pyrazinamide for two months, followed by rifampicin and isoniazid for four months.13 The paucity of data informing the treatment of TB/HIV co-infection has recently been highlighted, with particular uncertainty for duration of therapy.14 This is particularly striking for EPTB and it is crucial that common forms of EPTB are adequately represented in future trials of antituberculous therapy.
Corticosteroids are frequently prescribed for TB pericarditis but the evidence to support their use in HIV-infected patients is limited. Studies conducted in the pre-HIV era suggested significant reduction in mortality with steroids in pericardial effusion and constrictive pericarditis.15,16 However, HIV infection influences the pathologic process and evidence suggests that constrictive pericarditis is much less common. The only randomized trial in HIV-infected patients included only 58 patients and showed a non-significant trend to lower mortality with adjunctive steroids.10 A Cochrane review on this topic concluded that prednisolone has no proven beneficial effect in patients with TB pericarditis.17 An ongoing multicentre randomized trial aims to address this key question.18
It is important to remember that because rifampicin increases corticosteroid metabolism through hepatic enzyme induction, higher doses might be required.19 Prednisolone doses as high as 120 mg have been used successfully in treating patients with TB pericarditis.20
Pericardial TB is a World Health Organization stage IV condition and thus antiretroviral therapy is indicated regardless of CD4+ lymphocyte count.21 There is recent evidence to support the early initiation of combination antiretroviral therapy (ART) during antituberculous therapy for pulmonary TB, although the optimal time to start has yet to be determined.22 Patients with EPTB were not included in this trial and only observational data including patients with EPTB support the benefit of early antiretroviral therapy in this context.23
The potential risks of early ART initiation include drug toxicity, drug interactions, and immune reconstitution inflammatory syndrome. The occurrence of pericardial TB–immune reconstitution inflammatory syndrome has been reported but the morbidity or mortality associated with this is unknown.24 Theoretically, immune reconstitution could lead to re-accumulation of fluid (with the possibility of tamponade) and/or a higher risk of constrictive pericarditis. Our practice is to initiate ART after 2–8 weeks of TB treatment and, as far as possible, to monitor the clinical status closely.
Conclusions
Pericardial TB is a frequent manifestation of HIV-associated TB and is associated with considerable mortality and morbidity. Many questions remain in terms of optimal diagnosis and therapy for this condition. As we progress into a new era of TB diagnostics and therapeutics to address this public health emergency, it is imperative that clinical trials include patients with extrapulmonary TB to answer some of the questions highlighted here.
Supplementary Material
Supplementary Material
Supplementary Material
Note: Supplementary videos are available at www.ajtmh.org.
Footnotes
Financial support: The Africa Centre for Health and Population Studies is supported by the Wellcome Trust.
Authors' addresses: Tom Heller, Hlabisa Hospital, Hlabisa, KwaZulu-Natal, South Africa, E-mail: echnatom@web.de. Richard J. Lessells and Claudia Wallrauch, Africa Centre for Health and Population Studies, University of KwaZulu-Natal, Mtubatuba, KwaZulu-Natal, South Africa, E-mails: rlessells@africacentre.ac.za and claudiawallrauch@web.de. Enrico Brunetti, Division of Infectious and Tropical Diseases, University of Pavia, S. Matteo Hospital Foundation, Pavia, Italy, E-mail: enricobrunetti@unipv.it.
References
- 1.World Health Organization . Global Tuberculosis Control: Epidemiology, Planning, Financing: WHO report 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Mayosi BM, Wiysonge CS, Ntsekhe M, Gumedze F, Volmink JA, Maartens G, Aje A, Thomas BM, Thomas KM, Awotedu AA, Thembela B, Mntla P, Maritz F, Blackett KN, Nkouonlack DC, Burch VC, Rebe K, Parrish A, Sliwa K, Vezi BZ, Alam N, Brown BG, Gould T, Visser T, Magula NP, Commerford PJ. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S Afr Med J. 2008;98:36–40. [PubMed] [Google Scholar]
- 3.Wallrauch C, Heller T, Lessells R, Kekana M, Barnighausen T, Newell ML. High uptake of HIV testing for tuberculosis patients in an integrated primary health care HIV/TB programme in rural KwaZulu-Natal. S Afr Med J. 2010;100:146–147. doi: 10.7196/samj.3898. [DOI] [PubMed] [Google Scholar]
- 4.Mayosi BM, Wiysonge CS, Ntsekhe M, Volmink JA, Gumedze F, Maartens G, Aje A, Thomas BM, Thomas KM, Awotedu AA, Thembela B, Mntla P, Maritz F, Blackett KN, Nkouonlack DC, Burch VC, Rebe K, Parish A, Sliwa K, Vezi BZ, Alam N, Brown BG, Gould T, Visser T, Shey MS, Magula NP, Commerford PJ. Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: the Investigation of the Management of Pericarditis in Africa (IMPI Africa) Registry. BMC Infect Dis. 2006;6:2. doi: 10.1186/1471-2334-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extrapulmonary Tuberculosis among Adults and Adolescents. Recommendations for HIV-Prevalent and Resource-Constrained Settings. Geneva: World Health Organization; 2007. [Google Scholar]
- 6.George S, Salama AL, Uthaman B, Cherian G. Echocardiography in differentiating tuberculous from chronic idiopathic pericardial effusion. Heart. 2004;90:1338–1339. doi: 10.1136/hrt.2003.020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller T, Wallrauch C, Lessells RJ, Goblirsch S, Brunetti E. Short course for focused assessment with sonography for human immunodeficiency virus/tuberculosis: preliminary results in a rural setting in South Africa with high prevalence of human immunodeficiency virus and tuberculosis. Am J Trop Med Hyg. 2010;82:512–515. doi: 10.4269/ajtmh.2010.09-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuter H, Burgess LJ, Doubell AF. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol Infect. 2005;133:393–399. doi: 10.1017/s0950268804003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuter H, Burgess L, van Vuuren W, Doubell A. Diagnosing tuberculous pericarditis. QJM. 2006;99:827–839. doi: 10.1093/qjmed/hcl123. [DOI] [PubMed] [Google Scholar]
- 10.Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart. 2000;84:183–188. doi: 10.1136/heart.84.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuon FF, Litvoc MN, Lopes MI. Adenosine deaminase and tuberculous pericarditis–a systematic review with meta-analysis. Acta Trop. 2006;99:67–74. doi: 10.1016/j.actatropica.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Reuter H, Burgess LJ, Louw VJ, Doubell AF. The management of tuberculous pericardial effusion: experience in 233 consecutive patients. Cardiovasc J S Afr. 2007;18:20–25. [PubMed] [Google Scholar]
- 13.World Health Organization, Stop TB Department . Treatment of Tuberculosis: Guidelines. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, Menzies D. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 15.Strang JI, Kakaza HH, Gibson DG, Allen BW, Mitchison DA, Evans DJ, Girling DJ, Nunn AJ, Fox W. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet. 1988;2:759–764. doi: 10.1016/s0140-6736(88)92415-4. [DOI] [PubMed] [Google Scholar]
- 16.Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, Fox W. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transkei. Lancet. 1987;2:1418–1422. doi: 10.1016/s0140-6736(87)91127-5. [DOI] [PubMed] [Google Scholar]
- 17.Mayosi BM, Ntsekhe M, Volmink JA, Commerford PJ. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev. 2002:CD000526. doi: 10.1002/14651858.CD000526. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health A Pilot Trial of Adjunctive Prednisolone and Mycobacterium w Immunotherapy in Tuberculous Pericarditis (IMPI) 2010. http://clinicaltrials.gov/ct2/show/NCT00810849 Available at. Accessed April 28, 2010.
- 19.McAllister WA, Thompson PJ, Al-Habet SM, Rogers HJ. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed) 1983;286:923–925. doi: 10.1136/bmj.286.6369.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strang JI. Rapid resolution of tuberculous pericardial effusions with high dose prednisone and anti-tuberculous drugs. J Infect. 1994;28:251–254. doi: 10.1016/s0163-4453(94)91593-8. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: World Health Organization; 2007. [Google Scholar]
- 22.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velasco M, Castilla V, Sanz J, Gaspar G, Condes E, Barros C, Cervero M, Torres R, Guijarro C. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–152. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 24.Meintjes G, Rangaka MX, Maartens G, Rebe K, Morroni C, Pepper DJ, Wilkinson KA, Wilkinson RJ. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


