Abstract
Bacterial meningitis is an important cause of morbidity and mortality in children living in low-resource settings. Pediatric bacterial meningitis cases < 5 years of age were identified through a regional hospital surveillance system for 3 years after introduction of routine immunization with Haemophilus influenzae type b (Hib) conjugate vaccine in Senegal in July 2005. Cases from the national pediatric hospital were also tracked from 2002 to 2008. The regional surveillance system recorded 1,711 suspected pediatric bacterial meningitis cases. Of 214 laboratory-confirmed cases, 108 (50%) were caused by Streptococcus pneumoniae, 42 (20%) to Hib, and 13 (6%) to Neisseria meningitidis. There was a 98% reduction in the number of hospitalized Hib meningitis cases from Dakar Region in 2008 compared with 2002. The surveillance system provides important information to the Ministry of Health as they consider self-funding Hib vaccine and introducing pneumococcal vaccine.
Introduction
Pediatric bacterial meningitis (PBM) is an important cause of child morbidity and mortality in low-resource settings.1–3 The Pediatric Bacterial Meningitis Surveillance Network in the World Health Organization (WHO) African Region (AFRO PBM Network) reports that, from 2002–2008, there were over 74,500 cases of suspected PBM identified in children < 5 years of age in 24 countries in the African Region.4 Among the 4,674 children with cerebrospinal fluid (CSF) specimens culture-positive for the three bacteria under surveillance, 47% were positive for Streptococcus pneumoniae, 34% for Haemophilus influenzae type b (Hib), and 19% for Neisseria meningitidis.
Supported by the GAVI Alliance (formerly The Global Alliance for Vaccines and Immunization), the Ministry of Health (MOH) of Senegal introduced the pentavalent diphtheria-tetanus-pertussis (DPT)–hepatitis B–Hib conjugate vaccine into routine immunization in July 2005. Hospital surveillance for PBM started in Senegal in January 2002 at the national pediatric hospital and expanded to seven regions of the country in February 2006. This expanded PBM surveillance system provides important regional data to the MOH as they consider self-funding the relatively expensive Hib conjugate vaccine once the GAVI Alliance donation ends and prepare to introduce additional vaccines against bacterial meningitis.
In this work, we present PBM data reported from the regional surveillance system for the 3 years after Hib conjugate vaccine introduction in Senegal. Data from the national hospital are presented for 3 ½ years both before and after introduction of Hib vaccine. We recommend that the MOH maintain the regional PBM surveillance system and continue to strengthen laboratory capacity in regional sentinel hospitals.
Materials and Methods
Hospital surveillance study area and population.
The MOH began PBM surveillance in children < 5 years of age in Senegal as part of the AFRO PBM Network at the national pediatric hospital, Albert Royer, in Dakar in January 2002. The estimated population of children < 5 years of age in Dakar Region was 269,900 in 2002.5 In February 2006, the Hib Impact Project, a collaboration between the Senegal MOH and PATH, an international, nonprofit organization, expanded PBM surveillance to a second hospital in Dakar, Hôpital Aristide Le Dantec, and six regional hospitals in Diourbel, Kaolack, Saint Louis, Tambacounda, Thiès, and Ziguinchor regions (Figure 1). During the study period, these regions comprised 74% of the population of Senegal, including 1,312,300 children < 5 years of age in 2006.5 (Three new regions were created in Senegal in September 2008 for a total of 14; in this article, we use the former designation of 11 regions and their boundaries.)
Figure 1.
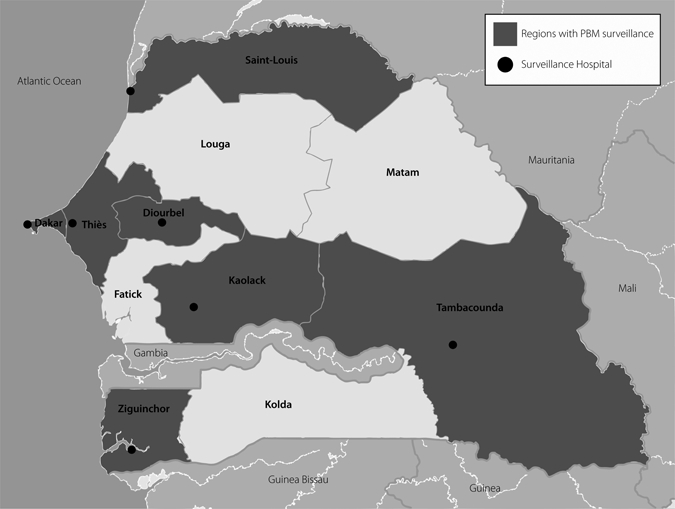
Map of Senegal and regions of pediatric bacterial meningitis (PBM) surveillance.
Case definition and data collection.
Pediatric bacterial meningitis cases were identified using WHO surveillance definitions and protocols.6,7 Suspected bacterial meningitis was defined as clinical illness in children 0 to 59 months of age consistent with meningitis, including sudden onset of fever (≥ 38°C axillary or ≥ 38.5°C rectal) and one or more of the following: stiff neck, bulging fontanelle, poor sucking, altered consciousness, irritability, seizures, other meningeal signs, toxic appearance, and petechial or purpural rash. Probable bacterial meningitis was defined as a suspected case with leukocytosis (> 100 white blood cell/mm3 with > 60% neutrophils) in CSF or a turbid (“cloudy”) CSF if the white blood cell count was not available. Laboratory confirmation of the etiologic agent was made by one of the following: 1) bacteria isolation from CSF by culture, 2) antigen detection from CSF by latex agglutination, or 3) bacteria isolation from blood if the child met criteria for probable bacterial meningitis.
Beginning in January 2002 at Albert Royer Hospital, information was collected on patient demographics, vaccination history, bacterial culture and latex laboratory results, and disease outcome (recovery or death). A child was reported to have died of bacterial meningitis if this was indicated either on the PBM surveillance form or in hospital discharge records. With the advent of regional surveillance in February 2006, the PBM surveillance case investigation form was revised to include additional information on clinical presentation and antibiotic use and sensitivity; it also expanded disease outcome classifications to include referral, complications, and loss to follow-up. Disease complications including hydrocephaly, seizures, hearing loss, paralysis, and cognitive difficulties were also indicated.
From February 2006 to December 2008, a study coordinator visited all eight surveillance hospitals every 2 months to collect surveillance forms, review laboratory records, and gather missing information. During the Hib Impact Project (2006–2008), proficiency testing at regional surveillance laboratories was conducted on-site three times by the head of the Haemophilus Reference Unit from the Respiratory and Systemic Infection Laboratory at London's Health Protection Agency Center for Infections, a WHO Collaborating Center for Haemophilus influenzae.
Laboratory methods.
Lumbar puncture was routine practice for suspected bacterial meningitis cases at all study hospitals during the study period. The CSF samples were cultured on chocolate agar made with horse blood using standard operating procedures adapted from the WHO laboratory manual for diagnosing bacterial meningitis.8 Streptococcus pneumoniae, H. influenzae, and N. meningitidis species were identified by Gram stain, colony morphology, dependency on X and V growth factors for Hib, optochin sensitivity for S. pneumoniae, and serotyping (Difco Laboratories, Detroit, MI). Blood culture was performed using biphasic culture medium (Hemoline performance diphasique, bioMérieux, Marcy-l'Etoile, France). Specific etiologic antigens were also detected by latex agglutination of CSF (Pastorex, Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France, or Slidex, bioMérieux, Marcy-l'Etoile, France).
Data management and statistical analysis.
Surveillance data were entered into a Microsoft Access 2003 database (Microsoft Corporation, Redmond, WA) and were analyzed with Access and Epi Info, version 3.5.1 (Centers for Disease Control and Prevention, Atlanta, GA). Descriptive statistics were calculated using mean scores and proportions. Chi-square (χ2) tests for linear trend were used for testing significance of decline in cases over time. Administrative data from the MOH from 2002 to 2008 were used for estimates of vaccination coverage.
Hib conjugate vaccination.
The target population for routine immunization in Senegal is infants 0–11 months of age. Hib conjugate vaccine was introduced into the routine immunization schedule in all regions simultaneously in July 2005. Two different vaccine presentations were used during the study period. First used was a lyophilized Hib vaccine (Hiberix®, GlaxoSmithKline Biologicals, Rixensart, Belgium), which was reconstituted with a diphtheria, tetanus, whole cell pertussis, and hepatitis B diluent (Tritanrix®, GlaxoSmithKline Biologicals). This was replaced in January 2007 by a fully liquid presentation (Quinvaxem®, Crucell, Leiden, The Netherlands), again composed of diphtheria, tetanus, whole cell pertussis, hepatitis B, and Hib vaccine. In 2008, the cost of one dose of Hib conjugate vaccine was approximately US$3.50.9
The Hib conjugate vaccine schedule includes three doses—given at 6, 10, and 14 weeks of age—administered concurrently with oral polio vaccine. No catch up campaign was held for children previously unvaccinated with Hib, and there is no national policy related to vaccination of children beyond 11 months of age in Senegal.10
The proportion of children < 1 year of age immunized with three doses of diphtheria, pertussis, and tetanus vaccine (DPT3) in Dakar Region in 2002, 2003, and 2004 was 45%, 54%, and 79%, respectively (Senegal MOH, unpublished administrative data, 2009). The DPT3 coverage was tracked until July 2005, when it was replaced by reporting receipt of three doses of Hib conjugate vaccine (Penta3). On the basis of administrative data from the MOH, coverage for DPT3/Penta3 in 2005 and Penta3 from 2005 to 2008 for surveillance regions ranged from 67% in Tambacounda in 2005 to 100% in Kaolack in 2006 (Senegal Ministry of Health, unpublished administrative data, 2009) (Table 1). The country average for Penta3 coverage was 90%, 91%, and 86% in 2006, 2007, and 2008, respectively.
Table 1.
Administrative vaccination coverage for third dose of diphtheria, pertussis, tetanus (DPT3) or pentavalent Hib conjugate vaccine (Penta3) in children < 1 year of age, surveillance regions, Senegal, 2005–2008
| Region | Coverage (%) | |||
|---|---|---|---|---|
| DPT3 or Penta3 | Penta3 | |||
| 2005* | 2006 | 2007 | 2008 | |
| Dakar | 78 | 83 | 91 | 89 |
| Diourbel | 88 | 99 | 99 | 97 |
| Kaolack | 90 | 100 | 95 | 92 |
| St. Louis | 90 | 93 | 98 | 93 |
| Tambacounda | 67 | 82 | 91 | 88 |
| Thiès | 93 | 90 | 96 | 94 |
| Ziguinchor | 75 | 82 | 77 | 69 |
Pentavalent Hib conjugate vaccine replaced the trivalent diphtheria, pertussis, tetanus vaccine in July 2005.
Results
Regional PBM surveillance, 2006–2008.
Suspected cases.
There were 1,711 suspected PBM cases in children < 5 years of age identified through the regional surveillance system from 2006 to 2008 (Table 2); 1,695 of 1,711 (99%) of suspected cases had CSF drawn through lumbar puncture. Laboratory tests (blood or CSF culture and/or latex agglutination) were performed on 1,691 specimens. Of the 1,653 children who received a CSF or blood culture, 139 were positive (136 CSF and 3 blood); overall culture positivity was 8% (139/1,653). Latex agglutination was performed on 881 (52%) CSF specimens; among these, 20% (176/881) were positive. Out of 1,656 suspected cases with partial or full information available on use of antibiotics, 631 (38%) received antibiotics before their CSF draw.
Table 2.
Number of pediatric bacterial meningitis (PBM) cases in children < 5 years of age, regional surveillance, Senegal, 2006–2008
| Year | Suspected PBM* | Laboratory results N (%) | Confirmed PBM | Bacterial etiology N (%) | |||
|---|---|---|---|---|---|---|---|
| S. pneumoniae | Hib | N. meningitidis | Other† | ||||
| 2006‡ | 620 | 615 (99) | 81 | 31 (38) | 30 (37) | 3 (4) | 17 (21) |
| 2007 | 527 | 514 (98) | 72 | 39 (54) | 8 (11) | 8 (11) | 17 (24) |
| 2008 | 564 | 562 (99) | 61 | 38 (62) | 4 (7) | 2 (3) | 17 (28) |
| Total | 1711 | 1691 (99) | 214 | 108 (50) | 42 (20) | 13 (6) | 51 (24) |
Includes confirmed cases.
Includes group B Streptococcus (24), Klebsiella pneumoniae (5), Staphylococcus aureus (5), Streptococcus spp. (5), Escherichia coli (3), Pseudomonas aeruginosa (3), group D Streptococcus (2), Acinetobacter sp. (1), Enterobacter cloacae (1), Proteus mirabilis (1), and group F Streptococcus (1).
Regional surveillance began February 2006.
Confirmed cases.
Overall, 214 laboratory-confirmed bacterial meningitis cases were reported (Table 2). Fifty percent (108/214) of these were caused by S. pneumoniae, 20% (42/214) to Hib, and 6% (13/214) to N. meningitidis. One hundred one of the 214 children were confirmed positive by both latex and culture (47%), whereas 74 (35%) were latex positive but culture negative, and 32 (15%) were positive by culture but negative by latex (Table 3). Excluding two children with missing data, 71% of confirmed cases (150/212) were < 1 year of age and 87% (185/212) were < 2 years of age (Table 4). Streptococcus pneumoniae and Hib were more likely to be found in children < 1 year of age compared with N. meningitidis (66% and 69%, respectively, versus 42%). Thirty-eight percent (30/80) of all confirmed cases with information on antibiotic use received antibiotics before their CSF draw; antibiotic use before CSF draw was highest for children with N. meningitidis, with 80% (4/5) (Table 5). Average duration of hospital stay was 12 days (range 0–68); children with N. meningitidis were more likely to have a stay of longer than 1 week compared with those with S. pneumoniae and Hib (92% versus 48% and 65%, respectively). For those discharged alive, the average length of stay was 17 days for children with both S. pneumoniae and Hib and 13 days for children with N. meningitidis, compared with 3, 4, and 14 days, respectively, for children who died in the hospital.
Table 3.
Suspected and confirmed pediatric bacterial meningitis cases in children < 5 years of age, regional surveillance, Senegal, 2006–2008
| Cases | N (%) |
|---|---|
| Suspected | 1711 (100) |
| Confirmed | 214 (13) |
| Means of confirmation for confirmed cases* | N (%) |
|---|---|
| Culture and/or latex + | 214 (100) |
| Culture +/latex + | 101 (47) |
| Culture +/latex − | 32 (15) |
| Culture +/latex not done | 6 (3) |
| Culture −/latex + | 74 (35) |
| Culture not done/latex + | 1 (0.4) |
Culture includes blood or cerebrospinal fluid.
Table 4.
Characteristics of confirmed pediatric bacterial meningitis cases in children < 5 years of age, regional surveillance, Senegal, 2006–2008
| Characteristics | Bacterial etiology N (%) | ||||
|---|---|---|---|---|---|
| S. pneumoniae | Hib | N. meningitidis | Other | Total | |
| Age (year) | |||||
| < 1 | 70 (66) | 29 (69) | 5 (42) | 46 (90) | 150 (71) |
| 1 | 26 (24) | 7 (17) | 1 (8) | 1 (2) | 35 (16) |
| ≥ 2 | 11 (10) | 6 (14) | 6 (50) | 4 (8) | 27 (13) |
| Total | 107 | 42 | 12 | 51 | 212 |
| Hospitalization (days) | |||||
| 0–7 | 51 (52) | 15 (36) | 1 (8) | 13 (26) | 80 (39) |
| 8–15 | 24 (24) | 15 (36) | 10 (77) | 15 (30) | 64 (31) |
| > 15 | 24 (24) | 12 (29) | 2 (15) | 22 (44) | 60 (30) |
| Total | 99 | 42 | 13 | 50 | 204 |
| Clinical outcome | |||||
| Death | 49 (49) | 15 (36) | 1 (8) | 14 (28) | 79 (38) |
| Full recovery | 38 (38) | 19 (45) | 9 (69) | 27 (54) | 93 (45) |
| Complications* | 10 (9) | 6 (14) | 2 (15) | 7 (14) | 25 (12) |
| Loss to follow-up | 4 (4) | 2 (5) | 1 (8) | 2 (4) | 9 (5) |
| Total | 101 | 42 | 13 | 50 | 206 |
Includes hydrocephaly, seizures, hearing loss, paralysis, and cognitive difficulties.
Table 5.
Antibiotic use and bacterial species in culture positive meningitis cases in children < 5 years of age, regional surveillance, Senegal, 2006–2008
| Antibiotic use before culture draw | Bacterial etiology N (%) | ||||
|---|---|---|---|---|---|
| S. pneumoniae | Hib | N. meningitidis | Other | Total | |
| Yes | 8 (7) | 6 (14) | 4 (31) | 12 (24) | 30 (14) |
| No | 26 (24) | 9 (22) | 1 (8) | 14 (27) | 50 (23) |
| Unknown | 74 (69) | 27 (64) | 8 (61) | 25 (49) | 134 (63) |
| Total | 108 | 42 | 13 | 51 | 214 |
Information on clinical outcome at hospital discharge was available for 206 of 214 confirmed cases (Table 4). Case fatality was 49% (49/101) for S. pneumoniae compared with 36% (15/42) for Hib and 8% (1/13) for N. meningitidis. Data collected for a case-control study on vaccine effectiveness identified an additional three deaths in Hib cases < 2 years of age who were discharged from the hospital with known complications (Fleming JA, personal communication), bringing the total case fatality of Hib meningitis to 43% (18/42).
A decline in the number of Hib cases was seen in the 3 full years after Hib vaccine introduction; 30 cases were reported in 2006, 8 in 2007, and 4 in 2008 (χ2 test for linear trend: 24.14, P < 0.001) (Table 2). Case numbers for pneumococcal and meningococcal disease were consistent across this time period (χ2 test for linear trend: 0.68, P = 0.41 and 0.12, P = 0.73, respectively).
PBM cases, Dakar Region, 2002–2008.
Overall, 405 cases of confirmed PBM in children < 5 years of age living in Dakar Region were reported from Albert Royer Hospital from 2002 to 2008, 45% (182/405) of them caused by Hib, 35% (142/405) S. pneumoniae, and 3% (13/405) N. meningitidis (Table 6). Compared with 2002, there was a 98% reduction in the number of hospitalized Hib meningitis cases from Dakar Region in 2008, 3 ½ years after Hib vaccine introduction (χ2 test for linear trend: 83.35, P < 0.001). A slight decline was also seen in the number of pneumococcal cases (χ2 test for linear trend: 9.94, P = 0.002), but the number of meningococcal cases remained static (χ2 test for linear trend: 0.35, P = 0.55).
Table 6.
Pediatric bacterial meningitis (PBM) hospitalizations < 5 years of age at Albert Royer Hospital among residents of Dakar Region, Senegal, 2002–2008
| Year | Confirmed | Bacterial etiology | |||
|---|---|---|---|---|---|
| N (%) | |||||
| PBM | S. pneumoniae | Hib | N. meningitidis | Other | |
| 2002 | 77 | 25 (32) | 41 (53) | 2 (3) | 9 (12) |
| 2003 | 66 | 29 (44) | 30 (45) | 1 (2) | 6 (9) |
| 2004 | 91 | 18 (20) | 56 (62) | 2 (2) | 15 (16) |
| 2005* | 78 | 20 (26) | 40 (51) | 4 (5) | 14 (18) |
| 2006 | 35 | 15 (43) | 12 (34) | 1 (3) | 7 (20) |
| 2007 | 32 | 19 (60) | 2 (6) | 2 (6) | 9 (28) |
| 2008 | 26 | 16 (61) | 1 (4) | 1 (4) | 8 (31) |
| Total | 405 | 142 (35) | 182 (45) | 13 (3) | 68 (17) |
Vaccine introduced in July 2005; number of cases January through June vs. July through December: Streptococcus pneumoniae 12 vs. 8; Hib 24 vs. 16; Neisseria meningitidis 4 vs. 0.
Discussion
Performance indicators for countries in the AFRO PBM Network include a target of 80% of clinically suspected bacterial meningitis cases receiving a lumbar puncture and 20% of purulent (> 100 white blood cells or turbid CSF) CSF specimens showing bacterial growth.7 The Senegal regional surveillance system exceeded these targets, as 99% of suspected PBM cases had CSF drawn and 53% of purulent CSF samples were culture positive. This is similar to 2002–2008 data from over 22 African countries in the AFRO PBM Network, reporting 97% and 46%, respectively, for these indicators.4 Thirteen percent of suspected PBM cases reported to the regional surveillance system in Senegal were laboratory-confirmed; the AFRO PBM Network reported 7% (4,674/69,208).
Disease burden cannot be calculated using passive, sentinel surveillance as there is a high probability of incomplete case ascertainment and the catchment population is usually not well defined. The PBM surveillance in Senegal relied on sentinel hospitals in seven of eleven regions; however, referral from outside surveillance areas did occur: seven confirmed cases (3%) came from the regions of Fatick, Kolda, Louga, and Matam, which were not part of the surveillance network. Although the severity of meningeal symptoms often prompts swift medical attention, rural populations have less access to health care, and cases from these areas are less likely to be identified by a sentinel surveillance system.11 From 2006 to 2008, 48% of PBM cases came from Dakar Region, and while it has the highest population, it is also the area with the highest proportion of urban to rural population. Meanwhile, no cases were reported from the predominantly rural region of Ziguinchor. Extrapolating the average number of cases reported in the remaining five surveillance regions (excluding Dakar and Ziguinchor) per the population < 5 years of age,5 we would expect to have seen at least 10 PBM cases in Ziguinchor over the surveillance period. It is therefore likely that cases were missed from this and other predominantly rural areas, and from regions without a surveillance reference hospital.
Even if a child with suspected PBM is seen at a health facility, true cases may be missed through failure to perform lumbar punctures, inappropriate specimen handling, and improper laboratory techniques.11,12 In Senegal, however, 99% of suspected PBM cases reported in the project period received a lumbar puncture. False-negative cultures have been reported elsewhere caused by poor media preparation and suboptimal culture conditions.13 Field reports in Ziguinchor cited insufficient laboratory materials as one potential factor contributing to the lack of PBM cases identified in the region. Our case ascertainment was likely improved by conducting training for clinical and laboratory staff from surveillance regions on proper diagnosis and standard laboratory procedures. Correct identification of organisms at the species level rose from 43% in 2006 to 92% in 2008.14 However, challenges were still reported in bacteria identification; on the second and third proficiency tests, respectively, 45% and 66% of participants correctly identified organisms to the serotype level (results unavailable for the first test).
Antibiotic use before CSF collection may lead to an underestimate of true case numbers through inhibiting bacterial growth in culture.11 Thirty-eight percent of both suspected and confirmed cases reported use of antibiotics before their CSF draw, which may have contributed to the low percentage of laboratory-confirmed cases (13%). Additional use of latex agglutination for detecting antigen likely improved our laboratory sensitivity over culture results alone. Eighty-two percent of confirmed cases were latex positive, whereas only 65% tested positive by culture.
A decrease in confirmed Hib meningitis cases was seen in children identified through the regional PBM surveillance system over the 3 years after Hib conjugate vaccine was introduced in Senegal. Although the number of Hib cases in children < 5 years of age decreased from 30 in 2006 to just four in 2008, the number of S. pneumoniae or N. meningitidis cases reported through the regional system remained relatively unchanged. Similar static trends in non-H. influenzae bacterial meningitis have been reported after Hib conjugate vaccine introduction in other African countries, including The Gambia, Kenya, Rwanda, and Mali.15–18 Although a 169% increase in S. pneumoniae meningitis was found in South Africa after Hib vaccine introduction,19 the authors suggested that the increase may have been caused by an increase in human immunodeficiency virus infection and improvements in case ascertainment (increases were also reported for non-b typable [213%] and non-typable [217%] H. influenzae serotypes during this time). Regional surveillance found no cases of non-b H. influenzae in Senegal, and evidence for serotype replacement in the literature remains uncertain.12 Ribiero and others20 reported an 8-fold increase in the incidence of meningitis from H. influenzae serotype a in the year after Hib vaccine introduction in Brazil (coincident with a 69% decrease in incidence of Hib meningitis), whereas Cowgill and others16 reported no increase in Hib disease caused by non-b typable or noncapsular H. influenzae serotypes in Kenya.
Although it is likely that the use of Hib conjugate vaccine contributed to the decrease in the number of Hib cases recorded in the 3 years after vaccine introduction, with high immunization coverage in Senegal (69–97% in surveillance regions in 2008), it is also possible that indirect effects of vaccination may have contributed to the decline. Herd immunity, the protection of unvaccinated individuals through reduced transmission from those vaccinated, has been well documented with Hib conjugate vaccines specifically through a reduction in nasopharyngeal colonization by the bacteria.21,22 Adegbola and others23 reported a 60% reduction in oropharyngeal carriage in the second year of life in children fully vaccinated during infancy in The Gambia.
Albert Royer Hospital is the only health facility in Senegal to systematically track PBM cases both before and after Hib conjugate vaccine introduction. From 2002 to 2004, Hib was the leading cause of laboratory-confirmed PBM in children < 5 years of age presenting at the hospital, representing 54% of all confirmed cases resident in Dakar Region. Streptococcus pneumoniae and N. meningitidis represented 31% and 2% of confirmed cases from the region, respectively. In the three years after Hib conjugate vaccine introduction, S. pneumoniae replaced Hib as the leading cause of PBM in Dakar Region (54% versus 16%, respectively).
Although introduction and wide coverage of the Hib conjugate vaccine likely contributed to the decline in the number of Hib cases in Dakar Region, reasons for the slight decrease in the number of reported pneumococcal cases from Albert Royer Hospital in the years after Hib conjugate vaccine introduction remain unclear. The improvements seen in laboratory capacity would likely have increased case ascertainment over the life of the project for all bacterial species, leading to an increase in case numbers over time, which was not seen. Information was not collected on antibiotic use before CSF draw before regional surveillance began in 2006, or on health care utilization differences over time, both of which may have impacted disease trends. One possible contribution to the decline in both Hib and S. pneumoniae meningitis cases from Dakar Region may be the introduction from 2003 to 2006 of several community-based health programs, including education for parents in nutrition and sanitation and the introduction of Community-based Integrated Management of Childhood Illness, which included the recognition of illness danger signs and enabled parents to provide antibiotics for signs of pneumonia to their children at home. However, as discussed previously, similar reductions in S. pneumoniae meningitis cases were not reported throughout the country by the regional system from 2006 to 2008.
Senegal replaced Hiberix® vaccine with Quinvaxen® in 2007. Although the price of Quinvaxen® is comparable to Hiberix®, it is presented as a single dose (versus the two-dose vial of Hiberix®). Although it requires more space in the cold chain, a single-dose vaccine presentation likely decreases wastage and improves coverage through reducing health worker reticence of opening a multi-dose vial for a single child in need of vaccination. Furthermore, being fully liquid, Quinvaxem® vaccine does not require a dilution syringe, ensuring the correct formulation and dosage while being cost saving.
The Hib Impact Project came to an end in December 2008. Continued maintenance of the regional PBM surveillance system and laboratory capacity built by the project is important for several compelling reasons. At US$3.50 for each dose, Hib conjugate vaccine is relatively expensive. If the MOH had been responsible for funding Hib conjugate vaccine, based on the 2008 birth cohort, their annual vaccine expenditures would have doubled compared with the vaccine budget without the Hib antigen included. With high vaccine costs and the end of the GAVI Alliance donation on the horizon, reliable data on which to base vaccine funding decisions is imperative. A regional PBM surveillance system will continue to be necessary to monitor the long-term impact of Hib vaccination in the country and has the capacity of alerting the MOH to outbreaks or epidemics of meningococcal disease. Looking toward the future, strong PBM surveillance will allow an informed decision by the MOH regarding the appropriate choice of serotypes for the forthcoming pneumococcal vaccine and provide critical baseline information for evaluating the impact of this and other vaccines against bacterial meningitis introduced in the future.
Acknowledgments
We thank Deborah Atherly for providing Hib vaccine cost and expenditure estimations and Scott Brown for assistance with map graphics. We also thank Jeffrey Partridge for reviewing the manuscript prior to submission and Erin Kester and Kimberly Burgess for their expert editorial assistance.
Footnotes
Financial support: Funding for this work was provided by a grant from the Children's Vaccine Program at PATH.
Authors' addresses: Osseynou Ba, Yakou Dieye, and Ndiouga Diallo, PATH Senegal Project Office, Dakar, Senegal, E-mails: ousseynouba1@gmail.com, ydieye@yahoo.fr, and ndiougad@yahoo.fr. Jessica A. Fleming, PATH Vaccine Access and Delivery, Seattle, WA, E-mail: jfleming@path.org. Boniface Mutombo wa Mutombo, National Malaria Control Centre, Chainama Hospital, Lusaka, Zambia, E-mail: boniface.mutombo@gmail.com. Mamadou Ba and Moussa Fafa Cisse, Bacterial Meningitis Surveillance, Albert Royer Hospital,Dakar, Senegal, E-mails: madouba@gmail.com and moussafafa@hotmail.com. Aissatou Gaye Diallo, LeDantec Hospital Laboratory, Dakar, Senegal, E-mail: ayougd@orange.sn. Iyane Sow, Fann Hospital Laboratory, Dakar, Senegal, E-mail: profisow@orange.sn. Mary P. E. Slack, Haemophilus Reference Unit, WHO Collaborating Centre for Haemophilus influenzae Respiratory and Systemic Infection Laboratory, Health Protection Agency Centre for Infections, London, UK, E-mail: Mary.Slack@HPA.org.uk. Pape Coumba Faye, Director of Medical Prevention, Senegal Ministry of Health, Dakar, Senegal, E-mail: Papfaye@orange.sn. Mady Ba, Epidemiologic Surveillance, Senegal Ministry of Health, Dakar, Senegal, E-mail: dyma67@gmail.com. Noel S. Weiss, Department of Epidemiology, University of Washington, Seattle, WA, E-mail: nweiss@u.washington.edu.
References
- 1.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 2.Mulholland EK, Adegbola RA. Bacterial infections–a major cause of death among children in Africa. N Engl J Med. 2005;352:75–77. doi: 10.1056/NEJMe048306. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. The WHO Young Infants Study Group. Pediatr Infect Dis J. 1999;18((Suppl)):S17–S22. doi: 10.1097/00006454-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization The pediatric bacterial meningitis surveillance network in WHO's African region, 2001–2008. WER. 2009;84:173–184. [PubMed] [Google Scholar]
- 5.Ministere de L'Economie et Des Finances Senegal . Estimations de la Population du Senegal: 0–5 Ans, 15–49 Ans et 60 Ans et Plus de 2002 a 2009. Dakar, Senegal: Bureau Etat Civil et Projections Démographiques; 2008. [Google Scholar]
- 6.World Health Organization . Generic Protocol for Population-Based Surveillance of Haemophilus influenzae type b. WHO/VRD/GEN/95.05. Geneva: WHO Global Programme for Vaccines and Immunization; 1996. [Google Scholar]
- 7.Otten MW, Soriano-Gabarró M, Shaba K, Kay B, Mhlanga B, Wenger J. Hib-Paediatric Bacterial Meningitis (Hib-PBM) Surveillance Network: Surveillance Manual, Field Test Version, July 2001. Harare, Zimbabwe: World Health Organization Regional Office for Africa; 2001. [Google Scholar]
- 8.Popovic T, Ajello G, Facklam R. Laboratory Manual for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. WHO/CDS/CSR/EDC/99.7. Geneva: World Health Organization, Communicable Disease Surveillance and Response; 1999. [Google Scholar]
- 9.United Nations Children's Fund Product menu for vaccines supplied by UNICEF for the Global Alliance for Vaccines and Immunizations (GAVI) 2008. http://www.unicef.org/supply/files/Product_Menu_23_Sept_2008.pdf Available at. Accessed May 21, 2010.
- 10.Breugelmans G. Senegal Hib Vaccine Impact Assessment Mission Report. Paris: Agence de Médecine Préventive; 2008. [Google Scholar]
- 11.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gessner BD. Haemophilus influenzae type b vaccine impact in resource-poor settings in Asia and Africa. Expert Rev Vaccines. 2009;8:91–102. doi: 10.1586/14760584.8.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Munson RS, Kabeer MH, Lenoir AA, Granoff DM. Epidemiology and prospects for prevention of disease due to Haemophilus influenzae in developing countries. Rev Infect Dis. 1989;11:S588–S597. doi: 10.1093/clinids/11.supplement_3.s588. [DOI] [PubMed] [Google Scholar]
- 14.Slack MPE. Senegal Mission Report. London: Haemophilus Reference Unit, Respiratory and Systemic Infection laboratory, Health Protection Agency Centre for Infections; 2009. [Google Scholar]
- 15.Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Oluwalana C, Obaro S, Weber M, Corrah T, Mulholland K, McAdam K, Greenwood B, Milligan PJM. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–150. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 16.Cowgill KD, Ndiritu M, Nyiro J, Slack MP, Chiphatsi S, Ismail A, Kamau T, Mwangi I, English M, Newton CR, Feikin DR, Scott JA. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–678. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muganga N, Uwimana J, Fidele N, Gahimbare L, Gessner BD, Mueller JE, Mhlanga BR, Katsande R, Herbinger KH, Rugambwa C. Haemophilus influenzae type b conjugate vaccine impact against purulent meningitis in Rwanda. Vaccine. 2007;25:7001–7005. doi: 10.1016/j.vaccine.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Sow SO, Tapia MD, Diallo S, Keita MM, Sylla M, Onwuchekwa U, Pasetti MF, Kotloff KL, Levine MM. Haemophilus influenzae Type b conjugate vaccine introduction in Mali: impact on disease burden and serologic correlate of protection. Am J Trop Med Hyg. 2009;80:1033–1038. [PubMed] [Google Scholar]
- 19.von Gottberg A, de Gouveia L, Madhi SA, du Plessis M, Quan V, Soma K, Huebner R, Flannery B, Schuchat A, Klugman KP. Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa. Bull World Health Organ. 2006;84:811–818. doi: 10.2471/blt.06.030361. and the Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro GS, Reis JN, Cordeiro SM, Lima JB, Gouveia EL, Petersen M, Salgado K, Silva HR, Zanella RC, Almeida SC, Brandileone MC, Reis MG, Ko AI. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J Infect Dis. 2003;187:109–116. doi: 10.1086/345863. [DOI] [PubMed] [Google Scholar]
- 21.Takala AK, Santosham M, Almeido-Hill J, Wolff M, Newcomer W, Reid R, Kayhty H, Esko E, Makela PH. Vaccination with Haemophilus influenzae type b meningococcal protein conjugate vaccine reduces oropharyngeal carriage of Haemophilus influenzae type b among American Indian children. Pediatr Infect Dis J. 1993;12:593–599. doi: 10.1097/00006454-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Takala AK, Eskola J, Leinonen M, Kayhty H, Nissinen A, Pekkanen E, Makela PH. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with a Hib conjugate vaccine. J Infect Dis. 1991;164:982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 23.Adegbola RA, Mulholland EK, Secka O, Jaffar S, Geenwood BM. Vaccination with a Haemophilus influenzae type b conjugate vaccine reduces oropharyngeal carriage of H. influenzae type b among Gambian children. J Infect Dis. 1998;177:1758–1761. doi: 10.1086/517440. [DOI] [PubMed] [Google Scholar]


