Abstract
We describe 11 cases of suspected Dracunculus medinensis infection in which the worm recovered was identified as Onchocerca volvulus. Identification was based on morphology of the examined specimen.
A global campaign to eradicate Guinea worm disease (dracunculiasis) began in 19801 and, in the ensuing 30 years, tremendous progress has been achieved.2,3 Fewer than 4,000 cases of Guinea worm disease were reported in 2009, and only 764 cases were reported during January–June 2010; more than 97% of the cases reported in 2010 have been from only one country, (Sudan).2,3 Part of the rigorous monitoring and surveillance inherent in the eradication programs within countries has been the vigilant reporting and investigation of suspected cases of Guinea worm disease. As control efforts reduce the number of cases, this reporting of suspected cases becomes more and more critical to certify interruption of transmission has occurred. As part of this process, the World Health Organization Collaborating Center for Research, Training, and Eradication of Dracunculiasis at the Centers for Disease Control and Prevention (CDC) receives specimens from national Guinea worm eradication programs to verify them as true cases of human Guinea worm (Dracunculus medinensis). To assist in this identification, a molecular method was developed at CDC to distinguish D. medinensis from other potential zoonotic Dracunculus spp.4
To date, 58 specimens have been submitted for verification, and most have proven to be D. medinensis. However, not all submitted worms are D. medinensis. We first reported three such cases of Onchocerca volvulus from the Central African Republic in 2000 and noted the unusual nature of such cases mimicking and being misidentified as D. medinensis.5 Since 2000, additional cases of O. volvulus in skin lesions and often lesions similar in appearance to those of Guinea worm disease have been received. This report details and further illustrates those cases, provides further insight into the biology of onchocerciasis, and discusses implications for Guinea worm eradication programs.
Eleven additional specimens representing O. volvulus have been received during 2000–2010. These specimens are represented by eight cases from Ghana (one in 2006, two in 2008, three in 2009, and two in 2010), and one each from Uganda (2004), Cameroon (2010), and Côte d'Ivoire (2007). Seven of the cases were reported from males, three from females, and in one the sex was not recorded. Ages ranged from 6 to 36 years, but in six cases the age was not recorded.
The worms emerged from various sites on the body, including the sole of the foot, under the chin, and hip (Figures 1–3), there was no predominant location. In many instances, a small blemish, pimple, or open lesion was present from which the worm was removed. Several of these lesions resembled the emergence of a true Guinea worm, except that the worm was smaller or thinner than normal (Figures 1 and 2). In others lesions (Figure 3), the appearance was not typical of Guinea worm, but because the worm was removed through the skin, it was classified initially by program staff in country as Guinea worm.
Figure 1.
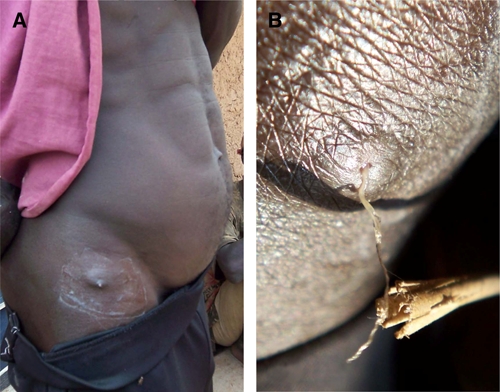
Ghanaian man from which an Onchocerca volvulus female worm was removed. A, Image of small pimple over right hip. B, Closer image showing portion of worm being pulled from small opening in skin. This figure appears in color at www.ajtmh.org.
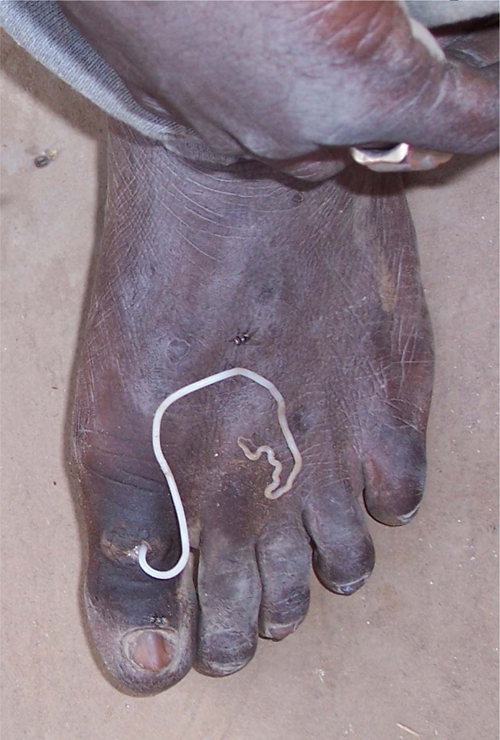
Figure 3.
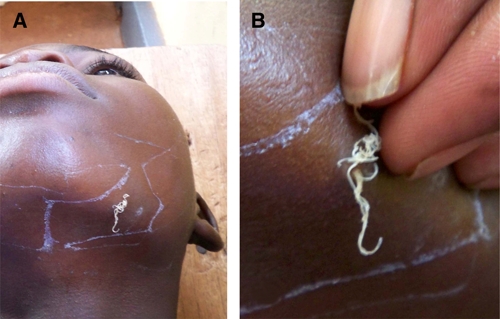
Young Ghanaian man from which a coiled Onchocerca volvulus female worm was removed. A, Image of coiled mass of worm on margin of left jaw. B, Closer image showing coiled mass of worm as it was being extracted. This figure appears in color at www.ajtmh.org.
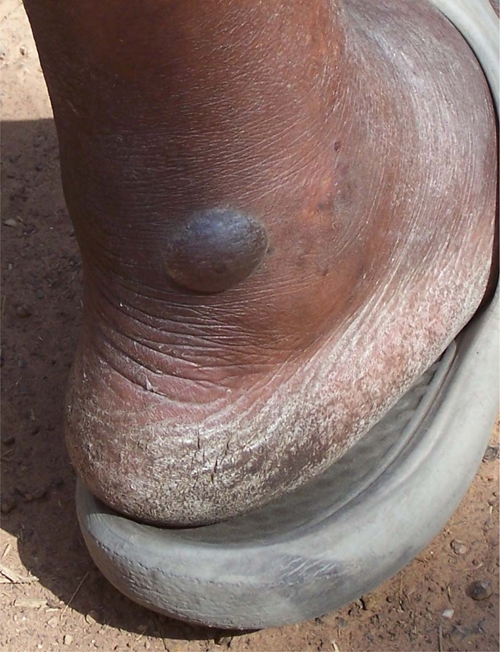
Figure 2.
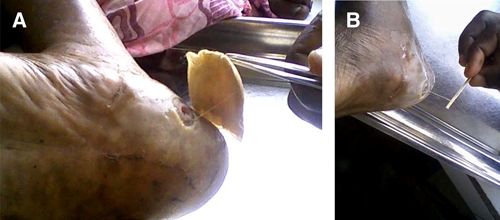
Cameroonian woman from which an Onchocerca volvulus female worm was removed from the heel of the foot. A, Image as dressing was being removed. B, Image of worm being extracted by winding on a stick. This figure appears in color at www.ajtmh.org.
When examined under a microscope, it was immediately apparent that these 11 specimens were not Guinea worm, and they were easily identified as female O. volvulus because of the distinctive nature of the cuticle (Figure 4). The cuticle of female O. volvulus has characteristic features, including external ridges that run around the body, and internal bars or bands called striae; different species have distinctive size and spacing of ridges and number of internal stria per ridge. In O. volvulus, there are two striae per ridge, one under adjacent ridges and one between adjacent ridges (Figure 4). In most cases, the worm was not gravid and did not contain microfilaria; in two cases, microfilariae were seen in utero.
Figure 4.
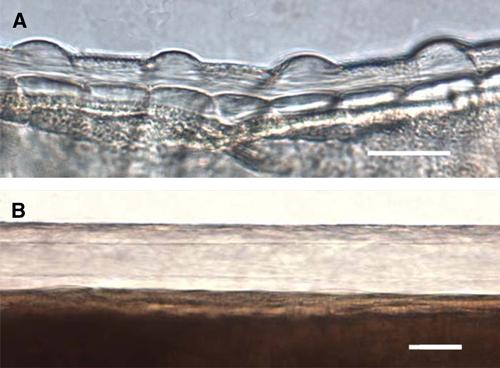
Photomicrographs of the cuticle of female Onchocerca volvulus and Dracunculus medinensis. A, Portion of female Onchocerca volvulus worm removed from case seen in Figure 1. The nature of the cuticle, especially the external ridges and underlying striae, are clearly evident, including one stria under each ridge and one stria between adjacent ridges. Scale bar = 25 μm. B, Portion of D. medinensis female cuticle showing the four-fold greater thickness, and smooth surface devoid of external ridges or internal striae. Scale bar = 50 μm. This figure appears in color at www.ajtmh.org.
The most striking features of these cases was the presence of adult female worms free in the tissues of the dermis or subdermis, rather than being encapsulated in fibrous tissue (an onchocercoma or nodule)6 and the apparent ease of extraction of the worm in toto or in part by a small rent in the skin surface.
A conspicuous feature of human infection with O. volvulus is the formation of subcutaneous nodules in which coiled adult worms may be demonstrated. The parasite was first reported and described in 1893 by Leuckart from nodules under the skin of a person in Ghana. Despite the well-documented occurrence and histologic appearance of such nodules, and the recognition that nodules may occur in deep tissues and not be readily evident nor palpable,7 various authors have noted or speculated on the occurrence of adult worms not being encased in fibrous nodules.8–13 Mohammed speculated that nodule formation was primarily a response to reaction around degenerating worms, or a result of injury to the worm (an explanation of why many nodules were present over bony prominences).8 This theory does not seem to take into account that viable young female worms can often be observed in nodules. However, it is recognized, in general, that nodules can be palpated in fewer than 30% of young microfilaria-positive children (with a presumed younger population of worms), but that this proportion increases to ≥ 60% in microfilaria-positive persons more than 20 years of age.14
Worth noting but of uncertain value is that all worms recovered in this case series were female, and that most appeared to be mature but young, based on size. However, the fact that two of the specimens contained microfilariae indicates that full maturation and mating had occurred, still without the formation of a nodule. Based on personal observation and that of many others working in the field of onchocerciasis, and the nature of nodules extirpated as part of control programs or research protocols, it seems nearly impossible to believe that the worms recovered in the present cases were initially contained within a nodule and then somehow were removed from that nodule during the course of extraction.
Unfortunately, additional information on these cases is lacking and can only be speculated upon. We believe that many of these cases may have come from areas receiving annual ivermectin as part of mass drug administration programs, but we have no reason to suspect drug treatment as a cause of worm expulsion in the present cases, and we do not suspect that ivermectin interferes with nodule formation and therefore accounted for the absence of a nodule. We also do not know if any of the 11 persons from whom these worms were extracted had demonstrable microfilaria in the skin, indicating the presence of other additional adult worms, or whether any palpable nodules were present. Lastly, we cannot say with any certainty whether the worm caused the formation of the skin lesion and opening that enabled the worm to emerge or whether, in some cases, a Guinea worm volunteer or supervisor, another health worker, or possibly the individual himself or herself scratched or cut the skin and created a small fissure through which the worm emerged. However, in this regard several points can be made. The manifestations of cases did not have certain hallmark features of a true case of Guinea worm disease. There was no formation of a classic blister (Figure 5). Because mature female Dracunculus worms secrete or release a substance that creates the formation of the blister, and the worm actively emerges from the ensuing lesion, we suggest that the concept and terminology of a worm emerging be restricted to dracunculiasis/Guinea worm disease, and not be applied to other situations such as those cases in this report. In this regard, a case of dracunculiasis is defined as an person with a skin lesion or lesions with emergence of one or more Guinea worms.15
Figure 5.
Classic blister formation on the ankle at pre-emergence of a female Guinea worm. This figure appears in color at www.ajtmh.org.
The Onchocerca worms were decidedly less stout in their gross appearance than female Dracunculus worms (Figure 6). In the present cases, size was helpful, but not always definitive as noted above. In a recent study of broken worms in Ghana, slightly thinner Guinea worms were noted but these worms are still substantially larger than the Onchocerca worms reported here.16 The caveat is that in the case of Dracunculus worms, one or both of the uterine tubes can, in the absence of the body of the worm, protrude out of the lesion, creating the appearance of a much thinner worm (Figure 7). This finding can lead to confusion on the part of program staff in the field who often do not understand the intricate worm morphology. However, because size in a clinical setting is a subjective measure, further evaluation is necessary. The ability to readily distinguish Onchocerca worms from Dracunculus worms based on a single microscopic examination to reveal the morphology of the cuticle makes this a rapid and low-tech procedure.
Figure 6.
Emergence of Guinea worm from the foot showing the typical presentation, including relative size of the worm (compare with Figures 1–3). This figure appears in color at www.ajtmh.org.
Figure 7.

Guinea worm lesion from which only a single uterine tube is protruding. Note the small diameter and transparent appearance. This figure appears in color at www.ajtmh.org.
Although the presentation of worms through the skin is unusual for human onchocerciasis, in our experience it is being reported increasingly over the past decade, but it is not clear whether this presentation is caused by the decreasing incidence of Guinea worm disease, and was simply lumped within that disease and all worms that emerged from the skin were considered Guinea worms. With the heightened interest in documenting and containing all cases of Guinea worm disease to suppress spread and assure interruption of transmission, this rare presentation of onchocerciasis may just now be coming to our attention. It is clear that Guinea worm disease program managers and others need to be aware that all worms that are removed from the skin are not Guinea worms.
As cases of Guinea worm disease continue to decrease and final eradication is achieved, focus for a few years will remain high on any worms emerging from the skin to ensure that no reemergence of transmission of Guinea worm disease has occurred. It would appear that Onchocerca worms will be a most common confounding presentation, although other conditions such as cutaneous larva migrans, bot fly larva, or Tunga penetrans have on occasion been confused with dracunculiasis. Of the 58 specimens submitted to CDC for confirmation during 2000–2010, 14 (24%) have been determined to be Onchocerca worms. Of the remaining cases, one was a fly larva (2%), two were unidentifiable nematodes (4%), and 11 (20%) were not worms but some other tissue presumed to be of human origin (e.g., connective tissue, blood vessel).
It remains unclear whether this manifestation of human onchocerciasis represents a normal occurrence, albeit, not common, and opens for some debate again, the question of how frequently adult Onchocerca worms are free in the tissues versus being encased in dense connective tissue nodules. We are not able to address either of these questions, other than to speculate, but the documented occurrence of the cases does provide additional information regarding what may or may not be an unusual aspect of onchocerciasis.
Acknowledgments
We thank all Ministry of Health, the Carter Center, and the World Health Organization staff for facilitating collection and transport of specimens to the World Health Organization Collaborating Center at CDC.
Footnotes
Authors' addresses: Mark L. Eberhard, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, E-mail: mle1@cdc.gov. Ernesto Ruiz-Tiben, Dracunculiasis Eradication Program, The Carter Center, Atlanta GA, E-mail: eruizti@emory.edu. Andrew S. Korkor, Ghana Guinea Worm Eradication Programme, Ghana Health Service, Tamale, Ghana, E-mail: seidu.korkor@ghsmail.org. Sharon L. Roy, Division of Foodborne, Waterborne, and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: str2@cdc.gov. Philip Downs, Neglected Tropical Diseases Program, Center for International Health/International Development Group, RTI International, Washington, DC, E-mail: pdowns@rti.org.
References
- 1.Hopkins DR, Foege WH. Guinea worm disease. Science. 1981;12:495. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Progress toward global eradication of dracunculiasis, January 2008–June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1123–1125. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Guinea Worm Disease. www.cdc/guineaworm/resources/wrap-up/gw195www.cdc/guineaworm/resources/wrap-up/gw198 Available at. Accessed September 22, 2010.
- 4.Bimi L, Freeman AR, Eberhard ML, Ruiz-Tiben E, Pieniazek NJ. Differentiating Dracunculus medinensis from D. insignis by the sequence analysis of the 18S rRNA gene. Ann Trop Med Parasitol. 2005;99:511–517. doi: 10.1179/136485905X51355. [DOI] [PubMed] [Google Scholar]
- 5.Eberhard ML, Melemoko G, Zee AK, Weisskopf MG, Ruiz-Tiben E. Misidentification of Onchocerca volvulus as guinea worm. Ann Trop Med Parasitol. 2001;95:821–826. doi: 10.1080/00034980120103397. [DOI] [PubMed] [Google Scholar]
- 6.Albiez EJ, Buttner DW, Duke BO. Diagnosis and extirpation of nodules in human onchocerciasis. Trop Med Parasitol. 1988;39:331–346. [PubMed] [Google Scholar]
- 7.Duke BO. Onchocerciasis: deep worm bundles close to hip joints. Trans R Soc Trop Med Hyg. 1970;64:791–792. doi: 10.1016/0035-9203(70)90030-1. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed AS. Contribution to the study of the pathology and morbid histology of human and bovine onchocerciasis. Ann Trop Med Parasitol. 1931;25:215–298. [Google Scholar]
- 9.Van den Berghe L. Recherches sur l'onchocercose au Congo Belge. II: les vers adultes et leur localization chez l'homme. Ann Soc Belg Med Trop. 1941;21:167–187. [Google Scholar]
- 10.Becker CK. Adult filaria (Onchocerca volvulus) free in tissue. Ann Soc Belg Med Trop. 1950;30:9–10. [PubMed] [Google Scholar]
- 11.Duke BO. The reappearance, rate of increase and distribution of the microfilariae of Onchocerca volvulus following treatment with diethylcarbamazine. Trans R Soc Trop Med Hyg. 1957;51:37–44. doi: 10.1016/0035-9203(57)90004-4. [DOI] [PubMed] [Google Scholar]
- 12.Israel MS. The nodule in onchocerciasis. Trans R Soc Trop Med Hyg. 1959;53:142–147. doi: 10.1016/0035-9203(59)90063-x. [DOI] [PubMed] [Google Scholar]
- 13.Nnochiri E. Observations on onchocercal lesions seen in autopsy specimens in western Nigeria. Ann Trop Med Parasitol. 1964;58:89–93. doi: 10.1080/00034983.1964.11686218. [DOI] [PubMed] [Google Scholar]
- 14.Buck AA. Onchocerciasis: Symptomatology, Pathology, Diagnosis. Geneva: World Health Organization; 1967. [Google Scholar]
- 15.World Health Organization 1974: dracunculiasis eradication: case definition, surveillance and performance indicators. Wkly Epidemiol Rec. 2003;37:323–328. [PubMed] [Google Scholar]
- 16.Glenshaw MT, Roy S, Ruiz-Tiben E, Downs P, Williamson J, Eberhard ML. Guinea worm disease outcomes in Ghana: determinants of broken worms. Am J Trop Med Hyg. 81. 2009:305–312. [PubMed] [Google Scholar]


