Abstract
Angiostrongyliasis is a globally distributed parasitic disease. Early and accurate identification of patients with severe infection is required. In this retrospective study, 81 patients with angiostrongyliasis were divided into two groups: 24 patients with severe disease and 57 with mild disease. Logistic regression analysis was used to determine the factors associated with severe disease. Receiver operating characteristic (ROC) analysis, κ tests, and χ2 tests were performed. The factors analyzed included: headache (P = 0.013), abnormal cerebrospinal fluid pressure (P = 0.013), and abnormal peripheral blood eosinophil count (P = 0.007). The area under the ROC curve for the activation criteria for angiostrongyliasis (ACA) was 0.914, with a score of ≥ 7 points predicting a severe state; the κ value was 0.744. The incidence of severe angiostrongyliasis increased with increasing score. ACA is a useful tool with high accuracy and reliability for predicting the severity of angiostrongyliasis.
Introduction
Angiostrongylus cantonensis is the causative pathogen of angiostrongyliasis. Although the parasite is known to be endemic in the Southeast Asian and Pacific regions,1 the increase in world travel and ship-borne dispersal of infected rat vectors has extended its distribution outside its traditional geographical boundaries. An outbreak in Beijing in 2006 involved 160 people, 100 of whom were hospitalized, which is comparable with the total number of infections recorded in China over the past decade.2 Humans become infected with A. cantonensis by ingesting the larvae in snails or slugs or in contaminated, uncooked vegetables.3,4 The larvae migrate to the brain, spinal cord, and nerve roots, causing eosinophilia in the cerebrospinal fluid (CSF) and peripheral blood.5,6 Infected patients present with severe headache, paresthesia, weakness, and visual disturbances.7 Although most patients make a full recovery, heavy infections can lead to chronic disabling disease and even death.8,9 Severe cases, therefore, need more active treatment to improve their prognosis. However, physicians assess the state of patients mainly by clinical experience and intuition, and there is currently no simple, scientific method for the early identification of patients with severe infections. The purpose of this retrospective study was to identify the factors associated with clinically severe angiostrongyliasis and to establish simple activation criteria for angiostrongyliasis (ACA) that could be used to alert doctors to patients requiring more intensive treatment.
Methods
Patients.
We analyzed the records of 81 patients who contracted angiostrongyliasis during the outbreak in Beijing between June and September 2006. Patients were diagnosed with angiostrongyliasis on the basis of seven factors.10 (1) Epidemiology: history of eating intermediate hosts, such as snails, or transport hosts, such as frogs and fish, or ingestion of contaminated vegetables containing infective larvae. (2) Clinical symptoms: the most common symptom was headache. Other common symptoms included fever, neck stiffness, nausea, vomiting, and skin paresthesia. (3) Peripheral blood investigations: increase in the percentage and absolute count of eosinophils. (4) Cerebrospinal fluid (CSF): raised pressure and eosinophilia. (5) Immunological examinations: A. cantonensis-positive antibody or circulation antigen (CAg). (6) Imaging examinations: possible supporting evidence of angiostrongyliasis from lung X-ray and cranial computed tomography/magnetic resonance imaging. (7) Pathologic examination: larvae or imago of A. cantonensis detected in CSF or eyes. Any patient with larvae would clearly be pathologically positive. Patients meeting criteria 1–4 were considered to be clinically positive, whereas those meeting criteria 5 and/or 6 were considered to display auxiliary signs consistent with angiostrongyliasis. This was a retrospective study using data from medical records, and information on parameters such as age, neck stiffness, skin paresthesia, visual disturbances, visual analogue scale (VAS) scores, intracranial pressure, CSF eosinophil count, and peripheral blood eosinophil count were available for all enrolled patients.
Mild and severe cases were distinguished according to the criteria proposed by the Beijing Tropical Medicine Research Institute.10 Mild cases were patients with fewer, milder clinical symptoms, a VAS score for headache of ≤ 7, intracranial pressure < 250 mmH2O, and hospitalization time ≤ 20 days. Severe cases were patients with a relatively greater number of more severe clinical symptoms, a VAS score for headache of ≥ 7, and intracranial pressure ≥ 250 mmH2O with variable hospitalization time. Based on these criteria, 57 mild cases and 24 severe cases were identified.
Data collection methods.
Clinical data were collected retrospectively using a unified case observation table and included symptoms, signs, and laboratory data for patients. The initial values on admission to hospital were recorded.
Statistical analysis.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 16.0). The factors were initially identified from detailed retrospective information on severe cases. Abnormal symptoms, signs, and laboratory data for patients with severe disease were compared with those for patients with mild disease, who were considered to be the control sample. The relationships between the mild and severe state were assessed using Student t test or the non-parametric Mann–Whitney U test for measured data and the χ2 test for counted data. The factors in the severe and control group were further compared using binary logistic regression analysis. Odds ratios (OR), including 95% confidence intervals (CI), were calculated. Then, the factors for severe disease were formulated into a table of ACA. Factors were weighted, with a higher weighting given to deteriorating symptoms and signs and laboratory data. The weighted factors were applied to patients with both mild and severe disease to evaluate the diagnostic accuracy and reliability of cumulative ACA. Finally, the diagnostic accuracy was calculated using receiver operating characteristics (ROCs). The κ test was used to evaluate the diagnostic reliability of ACA. A χ2 test for trends was used to identify any relationships between ACA and the severity of the illness.
Results
Identification of factors associated with clinically severe angiostrongyliasis.
The following indicators were identified as factors associated with the development of severe, rather than mild, angiostrongyliasis: headache (P = 0.013), abnormal CSF pressure (CSFP; P = 0.013), and abnormal peripheral blood eosinophil count (EOS; P = 0.007) (Table 1).
Table 1.
Predictors of severe angiostrongyliasis
| B* | SE | df | P | OR (95% CI) | B† | Max score | |
|---|---|---|---|---|---|---|---|
| Headache | 1.44 | 0.58 | 1 | 0.013 | 4.24 (1.35–13.33) | 0.47 | 3 |
| CSFP | 2.11 | 0.85 | 1 | 0.013 | 8.27 (1.55–44.02) | 0.99 | 5 |
| Peripheral EOS | 1.73 | 0.64 | 1 | 0.007 | 5.66 (1.62–19.81) | 0.61 | 3 |
SE = standard error; df = degrees of freedom; OR = odds ratio; 95% CI = 95% confidence interval; CSFP = cerebrospinal fluid pressure; EOS = eosinophil count.
Partial regression coefficient.
Standard regression coefficient.
Formulation of ACA.
The standard regression coefficient and an expert panel decided the weighting criteria for each parameter (Tables 1 and 2). The cumulative scoring system was applied to 77 patients with either mild or severe disease, after excluding four cases with incomplete data. The accuracy and reliability of ACA were assessed. The area under the ROC curve (AUC) for ACA was 0.914 (Figure 1), the standard error (SE) was 0.04, and 95% CI was 0.84–0.99. A score of 7 alerted doctors to patients with severe angiostrongyliasis (94.3% specificity and 78.3% sensitivity); a score of ≥ 7, therefore, identified 78.3% of patients with severe disease, whereas 21.7% of patients with severe disease would have scores < 7. Only 5.7% of patients with mild disease scored ≥ 7. A κ value of 0.744 represented the degree of consistency between ACA and the actual severity.
Table 2.
Activation criteria for angiostrongyliasis (ACA)
| Item | Score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Headache | None | Mild | Moderate | Severe | ||
| CSFP (mmH2O) | ≤ 180 | 181–249 | 250–300 | > 300 | ||
| Peripheral EOS (109/L) | ≤ 0.60 | 0.61–1.00 | > 1.00 | |||
CSFP = cerebrospinal fluid pressure; EOS = eosinophil count.
Figure 1.
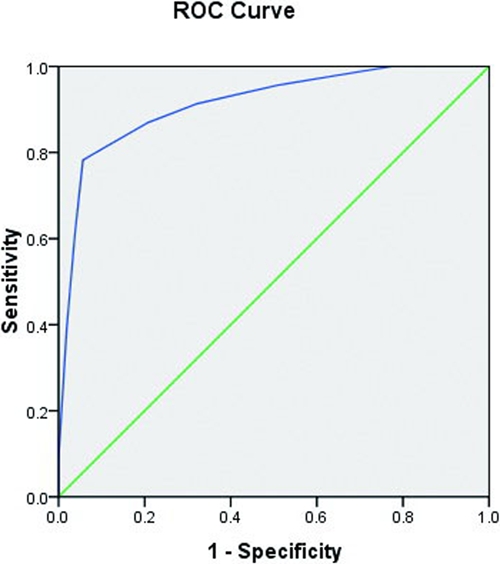
ROC of ACA predicting severe human angiostrongyliasis. This figure appears in color at www.ajtmh.org.
Population distribution of patients infected with angiostrongyliasis in relation to ACA.
Patients' scores ranged from 1 to 11 (Figure 2). The majority of patients with mild disease had scores of 1–6, whereas patients with severe disease scored 7–11. The population of patients with severe disease with an ACA of ≥ 7 was significantly greater than the population with severe disease with an ACA of ≤ 6 (0–6 points, 21.7%; ≥ 7 points, 78.3%; P < 0.001).
Figure 2.
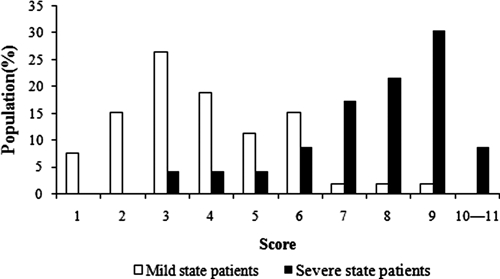
Population distribution of patients with mild and severe angiostrongyliasis in relation to ACA score.
Relationship between ACA and actual severity.
There was a significant positive relationship between the actual severity and ACA. As the score increased, the percentage of patients with severe angiostrongyliasis also increased (Figure 3). The percentage of patients with severe disease was increased > 9-fold for a score of ≥ 7 compared with a score of 0–6 (0–6 points, 9.1%; ≥ 7 points, 85.7%; P < 0.001).
Figure 3.
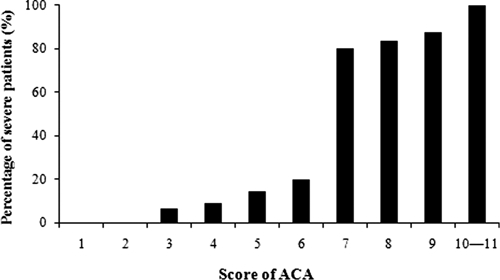
Actual percentages of patients with severe disease in relation to ACA.
Comparison of predictive value of ACA with single scores for headache, CSFP, or peripheral EOS.
As seen in Figure 4, the predictive value of ACA for severe angiostrongyliasis was superior to those of single scores for headache, CSFP, or peripheral EOS. The AUCs (±SE) were 0.914 (±0.04), 0.691 (±0.06), 0.837 (±0.06), and 0.697 (±0.07), respectively.
Figure 4.
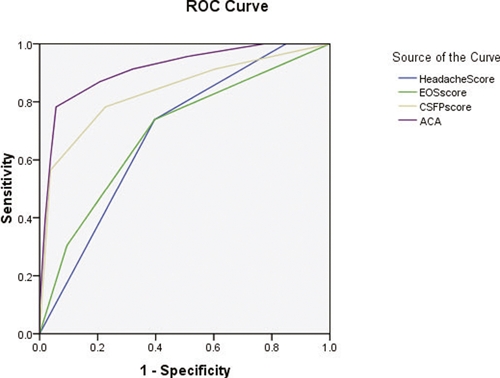
Predictive values of ROCs for ACA, headache, CSFP, and peripheral EOS for severe angiostrongyliasis. This figure appears in color at www.ajtmh.org.
Discussion
The aim of the current study was to identify the factors associated with the development of clinically severe angiostrongyliasis and to establish an ACA to alert doctors to patients requiring more intensive treatment. The results suggested that a score of ≥ 7 represented the trigger level at which doctors should be alerted to the severity of a patient's disease and therefore, implement more rigorous prevention and intervention measures. We suggest that patients with scores ≥ 7 should be hospitalized and treated with corticosteroids.11 The patients with high cranial pressure should be given intravenous mannitol or glycerol/fructose according to the extent of the actual illness. Ibuprofen can be administered to relieve severe headache and fever. In addition, some patients can be given neurotrophic drugs, such as vitamin B1, mecobalamin, and piracetam, to promote recovery, whereas patients with scores of 1–6 should be treated with corticosteroid as outpatients. However, a 100% accurate predictive scoring system is unlikely, given the natural variation in physiology, and doctors should be vigilant, even in patients with scores of 3–6. However, disease in patients with scores of 1 or 2 is sufficiently mild to be treated in an outpatient clinic. In addition, the ACA, which comprises indicators of headache, CSFP, and peripheral EOS, could help to differentiate patients infected with angiostrongyliasis from those with other diseases.
AUC reflects the diagnostic accuracy of a new diagnostic method. It is generally believed that an AUC of 0.5–0.7 represents low diagnostic accuracy, an AUC of 0.7–0.9 indicates moderate diagnostic accuracy, and a high diagnostic accuracy requires an AUC value > 0.9. The scoring system used in this study showed a high degree of accuracy, with an AUC of 0.914, 94.3% specificity, and 78.3% sensitivity. The κ test was used to evaluate the consistency of ACA in practice: the higher the value of κ, the better the consistency. Consistency is generally good when the κ value is ≥ 0.75, whereas a κ value < 0.40 reflects unsatisfactory consistency. In this study, the κ value approached 0.75, indicating good consistency and reliability of the scoring system. In conclusion, ACA is a valuable tool with high specificity and sensitivity for the early prediction of severe angiostrongyliasis.
The scoring system was characterized by several factors. First, it was significantly correlated with disease severity; the percentage of patients with severe angiostrongyliasis increased with increasing score. Second, the three factors included in the ACA for severe angiostrongyliasis (headache, CSFP, and peripheral EOS) were objective, and the predictive value of ACA was superior to that of individual scores for either headache, CSFP, or peripheral EOS. Third, our effective evaluation and appropriate treatment of patients meant there were no deaths in our study. However, angiostrongyliasis generally has a very low mortality, and this scoring system would, therefore, be appropriate. Fourth, the scoring system can be applied not only to outbreak cases but also to sporadic cases. Finally, the parameters of ACA are simple, objective, and convenient. This scoring system could, therefore, help doctors to make a rapid, early, and accurate diagnosis in patients infected with angiostrongyliasis and additionally, allow them to take referral and isolation measures to provide timely interventions. The system is suitable not only for inpatients but also in outpatient clinics, and it can take account of local needs and available resources.
Acknowledgments
The authors thank the doctors at Beijing Tropical Medicine Research Institute, Beijing Friendship Hospital for their assistance.
Footnotes
Financial support: This study was supported by the Beijing New Century Millions of Talents Project of China (No. B2006003). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Zongli Diao, Hongli Xiao, Jing Wang, Haiyu Qi, Xiaoli Li, and Chenghong Yin, Beijing Tropical Medicine Research Institute, Beijing Friendship Hospital, Capital Medical University, Beijing, China, E-mails: diaozongli@yahoo.com.cn, xiaohongli@126.com, wangjing_jakee@yahoo.com.cn, qihaiyu789@126.com, lixl_beijing@yahoo.com.cn, and modscn@yahoo.com.cn.
Reprint requests: Chenghong Yin, Beijing Tropical Medicine Research Institute, Beijing Friendship Hospital, Capital Medical University, 95 Yong-An Road, Beijing 100050, China, E-mail: modscn@yahoo.com.cn.
References
- 1.Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- 2.Diao Z, Chen X, Yin C, Wang J, Qi H, Ji A. Angiostrongylus cantonensis: effect of combination therapy with albendazole and dexamethasone on Th cytokine gene expression in PBMC from patients with eosinophilic meningitis. Exp Parasitol. 2009;123:1–5. doi: 10.1016/j.exppara.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Chau TT, Thwaites GE, Chuong LV, Sinh DX, Farrar JJ. Headache and confusion: the dangers of a raw snail supper. Lancet. 2003;361:1866. doi: 10.1016/s0140-6736(03)13506-4. [DOI] [PubMed] [Google Scholar]
- 4.Lai CH, Yen CM, Chin C, Chung HC, Kuo HC, Kuo HC, Lin HH. Eosinophilic meningitis caused by Angiostrongylus cantonensis after ingestion of raw frogs. Am J Trop Med Hyg. 2007;76:399–402. [PubMed] [Google Scholar]
- 5.Baheti NN, Sreedharan M, Krishnamoorthy T, Nair MD, Radhakrishnan K. Eosinophilic meningitis and an ocular worm in a patient from Kerala, south India. J Neurol Neurosurg Psychiatry. 2008;79:271. doi: 10.1136/jnnp.2007.122093. [DOI] [PubMed] [Google Scholar]
- 6.Furugen M, Yamashiro S, Tamayose M, Naha Y, Miyagi K, Miyagi K, Nakasone C, Uchihara T, Haranaga S, Azuma M, Yara S, Shinzato T, Higa F, Toma H, Tateyama M, Fujita J. Elsberg syndrome with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. Intern Med. 2006;45:1333–1336. doi: 10.2169/internalmedicine.45.1871. [DOI] [PubMed] [Google Scholar]
- 7.Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med. 2001;111:109–114. doi: 10.1016/s0002-9343(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Xu F, Gu JB, Chen XG. A severe eosinophilic meningoencephalitis caused by infection of Angiostrongylus cantonensis. Am J Trop Med Hyg. 2008;79:568–570. [PubMed] [Google Scholar]
- 9.Chotmongkol V, Sawanyawisuth K. Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health. 2002;33:231–234. [PubMed] [Google Scholar]
- 10.Yin C, Gan S, Liu J, Han X, Feng M, Ji A, Guo Z. The criteria for diagnosis and treatment of angiostrongyliasis. Chin J Integr Med. 2006;45:1051–1052. [Google Scholar]
- 11.Chotmongkol V, Kittimongkolma S, Niwattayakul K, Intapan PM, Thavornpitak Y. Comparison of prednisolone plus albendazole with prednisolone alone for treatment of patients with eosinophilic meningitis. Am J Trop Med Hyg. 2009;81:443–445. [PubMed] [Google Scholar]


