Abstract
Environmental enrichment, i.e., increased intellectual, social, and physical activity makes brain more resilient to subsequent neurological disease. The mechanisms for this effect remain incompletely defined, but evidence shows tumor necrosis factor-alpha (TNF-α) is involved. TNF-α, at acutely high levels, possesses the intrinsic capacity to enhance injury associated with neurological disease. Conversely, the effect of TNF-α at low-levels is nutritive over time, consistent with physiological conditioning hormesis. Evidence shows that neural activity triggers low-level pro-inflammatory signaling involving TNF-α. This low-level TNF-α signaling alters gene expression, resulting in an enhanced resilience to disease. Brain-immune signaling may become maladaptive when increased activity is chronic without sufficient periods of reduced activity necessary for nutritive adaptation. Such tonically increased activity may explain, for example, the transformation of episodic to chronic migraine with related increased susceptibility to spreading depression, the most likely underlying cause of this malady. Thus, TNF-α, whose function is to alter gene expression, and its principal cellular source, microglia, seem powerfully positioned to orchestrate hormetic immune signaling that establishes the phenotype of neurological health and disease from brain activity.
Keywords: migraine, spreading depression, neuro-immune, cytokine, excitotoxicity, environmental enrichment
INTRODUCTION
Brain is unique among organ structures in that it exists to predict (Llinás 2002), a capacity that requires constant “reprogramming.” Importantly, reprogramming increases the brain’s resistance to neurological disease. Neural reprogramming is classically evidenced by Hebbian synaptic plasticity, and extends to environmental enrichment (EE; i.e., increased intellectual, social, and physical activity), which is well-known to be protective (for review see van Praag et al. 2000; Will et al. 2004).
Reprogramming is also an inherent capacity of the immune system (Waldmann 2002; Graca and Waldmann 2006; Waldmann et al. 2008). In addition, immune system reprogramming may be a well-conserved process by which ischemic preconditioning stimuli applied to brain prompt subsequent neuroprotection (Marsh et. al. 2009).
Our focus is to illustrate how immune stimuli within brain, associated with neural activity, similarly reprogram gene function to create the phenotype of increased neurological health through interactive signaling between neurons and glia. Increasing evidence indicates that neural and immune systems interact in response to increased activity to establish the phenotype of enhanced brain health. This activity-dependent cooperative reprogramming may be driven by tumor necrosis factor-alpha (TNF-α), an innate immunity cytokine that emanates from microglia under normal circumstances (Hulse et al. 2008).
Classically, microglia, principal immune signaling cells within brain, have been recognized for their powerful destructive capacities to enhance brain injury via mechanisms that include increased expression of TNF-α (Chao and Hu 1994; Dawson et al. 1996; Barone et al. 1997; Meistrell et al. 1997; Aggarwal et al. 2001; Zou and Crews 2005). Accordingly, why would the brain use lethal signaling systems to enhance its strength? Developing evidence indicates the answer lies in the old saying “…what doesn’t kill you makes you stronger …” (Hadley 2003). This notion termed “physiological conditioning hormesis” (Calabrese et al. 2007) is increasingly recognized as a well-conserved capacity of biological systems, including brain (Mattson et al. 2002; Mattson 2008a, 2008b). Mounting evidence indicates that TNF-α and microglia can be neuroprotective (Cheng et al. 1994; Wilde et al. 2000; Hallenbeck 2002; Stoll et al. 2002; Streit 2005, 2006; Turrin and Rivest 2006; Carson et al. 2007; Hanisch and Kettenmann 2007; Sriram and O’Callaghan 2007; Hulse, et al. 2008; Salmina 2009).
Hormesis is a dose-response pattern involving low-level stimulation and high-level inhibition (Calabrese and Baldwin 2003). Hormesis is seen with neuroprotective treatments for stroke and brain trauma (Calabrese 2008) and also may play a role in the brain’s intrinsic capacity to protect itself against injury. Importantly, such irritative signaling that initiates neuroprotection requires time to develop.
We present original data and related literature to support and extend the suggestion that brain uses TNF-α-dependent signaling (Arumugam et al. 2006) from microglia to initiate subsequent adaptive changes that provide the neuroprotection consistent with physiological conditioning hormesis (Calabrese et al. 2007). Furthermore, we propose that this nutritive scenario may become maladaptive if initiating stimuli (i.e., neural activity) rise to a frequency that precludes sufficient recovery periods needed for adequate adaptive responses. We suggest the latter may exemplify the transformation of episodic to chronic migraine with related increased susceptibility to spreading depression, the most likely underlying cause for this headache disorder (Lauritzen and Kraig 2005). Similar considerations are likely to apply to other neurodegenerative disorders, including temporal lobe epilepsy.
ADAPTIVE POTENTIAL OF ENHANCED BRAIN ACTIVITY
Rationale for study of environmental enrichment-based neuroprotection
The mechanisms of endogenous neuroprotection are commonly studied in reference to “ischemic tolerance,” a phenomenon where a lethal stimulus brought below threshold imparts neuroprotection against subsequent injury (Kirino 2002; Dirnagl et al. 2003, Dirnagl et al. 2009). As noted above, such neuroprotection most often includes the study of stimuli applied to brain. Deciphering the means by which biologically driven mechanisms mitigate brain injury will lead to improved treatment strategies that may result in fewer negative sequelae and greater physiological effects (Kraig and Kunkler 2002). The notion that treatments should follow naturally evolved pathways has considerable merit. Our interest in defining the mechanisms of EE-based neuroprotection extends beyond traditional ischemic tolerance studies. This emphasis has two distinct advantages. First, defining mechanisms will provide important insights for new acute treatment strategies derived from stimuli with no potential for confounding effects associated with lethal stimuli. Second, detailed knowledge of underlying EE mechanisms will establish the physiological bases (and therefore rationale) for neurological preventative health strategies designed to lessen the impact of neurological diseases. The latter would empower patients and healthcare providers with evidence-based strategies to improve their health before the onset of brain disease.
Clinical evidence for the environmental enrichment benefits
Considerable clinical evidence indicates that moderately increased exercise lessens the severity of neurodegenerative disease. For example, Chen and coworkers (2005) noted that higher levels of physical activity may lower the risk of Parkinson’s disease development in men prone to develop this disorder. Curiously, women in the study showed no benefit. Rovio and coworkers (2005) also noted that physical activity at midlife correlated with decreased risk of dementia and development of Alzheimer’s disease in later life. Van Gelder and coworkers reported a similar positive impact of physical activity begun later in life (2004).
Importantly to our thesis, Rovio and colleagues (2005) observed the greatest reduction in disease development, due to increased physical activity, in individuals with a genetic marker for Alzheimer’s disease, APOE4. This finding begins to illustrate the concepts put forward by Silverman (2004) in a recent article entitled, “Rethinking Genetic Determinism.” Silverman notes inadequacies of the traditional assumptions that “…the gene is deterministic in gene expression and can therefore predict disease propensities.” He suggests instead that more attention should be given to how “environment” alters genetic propensity to influence phenotype. While genetic determinism may be valid in specific circumstances (e.g., lethal genetic changes), our research focus is greatly influenced by the therapeutic potential of Silverman’s suggestion, which we believe EE-based neuroprotection powerfully exemplifies.
A second caveat of EE-based neuroprotection is whether physical activity alone is sufficient to induce the protection. For example, exercise is sufficient to retard development of cardiovascular disease (Thompson et al. 2003). However, physical activity alone may not be adequate for neuroprotection from EE (Sturman et al. 2005). Instead, learning (van Praag et al. 2000) may be the key component by which EE (i.e., increased intellectual, physical, and social activity) triggers neuroprotection.
Experimental evidence for environmental enrichment neuroprotection
Evidence from experimental animal studies supports the neuroprotective capacity of EE (Will et al. 2004). EE initiated after brain trauma (Wagner et al. 2002) or stroke (Dahlqvist et al. 2004) enhances cognitive function involving the hippocampus when compared to non-enriched animals. EE also slows cognitive decline in ageing rodents (Kempermann et al. 2002).
EE is also effective in enhancing brain function after injury to developing brain. Early enrichment in perinatal rats a day after hypoxia/ischemia results in partial recovery of memory deficits (Pereira et al. 2008). However, this effect was only seen in females. A common problem in studies of EE is so-called dominant male effect that stresses other enriched male animals (see below). This prompted many to include only females in enrichment studies. Alternatively, when EE starts two weeks after neonatal ischemia, male rats show significant improvement compared to non-enriched counterparts (Pereira et al. 2007). Furthermore, postnatal EE can ameliorate behavioral effects of fetal alcohol syndrome (Hannigan et al. 2007) and the consequences of other neurological disease models (Nithianantharajah and Hannan 2006). Accordingly, EE and experiential interventions may reduce or ameliorate the neuronal and non-neuronal abnormalities caused by injury to the developing brain (Dong and Greenough 2004). Remarkably, the positive effects of EE are seen when EE is initiated long after perinatal injury (i.e., after weaning).
EE is also effective when initiated before the onset of neurological disease. For example, EE reduces temporal lobe seizures in adult rats and reduces cognitive deficits compared to non-enriched counterparts (Young et al. 1999).
Our studies using in vivo and in vitro models of excitotoxic hippocampal injury confirm and extend the evidence that learning from EE is neuroprotective. First, we did not observe any clinical evidence of a dominant male effect with our enrichment cage conditions (Figure 1), perhaps because the cage was sufficiently large (Marashi et al. 2003). This conclusion is further supported by the fact that we did not find any outlying serum corticosterone values within animal groups (see below). Second, 28-day exposure to EE triggered a significant increase in hippocampal (i.e., contextual) learning compared to non-enriched counterparts (Figure 2). Third, this EE paradigm significantly reduced CA3 area hippocampal pyramidal neuron loss from kainic acid-induced temporal lobe epilepsy, and significantly improved animal survival compared to non-enriched counterparts (Figure 3).
FIGURE 1.
Mouse environmental enrichment cage. Groups of seven mice were housed as shown in an environmental enrichment (EE) cage. This consisted of a cage with a microisolator filter top (A), free access to food and water (B), and an array of toys, running wheel and socialization bowl (C) that allowed increased intellectual, physical and social volitional opportunities. The tunnels (120 cm in length) were changed weekly to a new configuration along with all cage materials. The EE cage size was 60x56x21 cm while the non-enriched (NE) cage, which only contained the microisolator filter top and bedding, was 28x17x13 cm. Animals were housed for 28 days under EE or NE conditions before analyses. Wood chips for chewing were discarded after each experiment and the EE equipment was cleaned by hand-scrubbing with chlorine dioxide (Clydox®) every week while the cages were cleaned by standard automated procedures. Room temperature was controlled to 22 °C with the light-dark cycle beginning at 6 AM and ending at 6 PM. This housing configuration showed no evidence of a dominant male. The cages were manufactured by Ancare.
FIGURE 2.
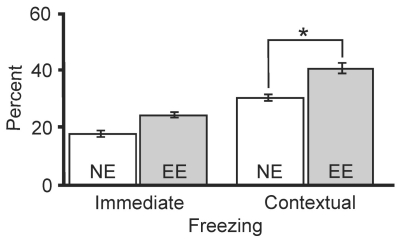
Environmental enrichment increased contextual learning. Emotional memory was examined (n=12 animals/group) by using a fear-conditioning task after 28-day exposure to an enriched environment (EE) or non-enriched environment (NE). NE and EE mice showed immediate freezing response levels (17±3 v. 24±2 %) that were not significantly different, indicating that responses to the unconditioned stimulus of the shock were the same between NE and EE mice. However, when compared to NE mice, the contextual freezing responses in the EE mice (29±3 v. 40±4 %) were significantly (“*”, P > 0.05, Student’s t test) higher, indicating that contextual conditioning memory, a reflection of hippocampal function, was significantly enhanced by EE. Results here and elsewhere are listed as mean ± standard error of mean.
[A fear-conditioning task was used to evaluate the hippocampal-dependent emotional memory in the mice as previously described by Tang and coworkers (1999). Briefly, the apparatus consisted of a shock chamber (25x25x37.5 cm), a multi-tone producer and a speaker, an electrical-shock producer, a photobeam-scanner, and a computerized workstation from TruScan multi-parameter activity monitor system (Coulboun Instruments). The floor of the chamber consisted of 24-bar inescapable shock grids. The walls of the chamber were transparent so that the behavioral responses in the chamber could be automatically recorded with the photobeam-scanner and at the same time recorded by a video camera or experimenters. One day before training, animals were individually habituated to the chamber three times for five min each time. The conditioned stimulus (CS) used for training was an 85 dB sound at 2,800 Hz, and the unconditioned stimulus (US) was a continuous scramble shock at 0.75 mA. During training, mice were individually put into the chamber and allowed to explore for three min before being exposed to the CS for 30 sec. During the last two sec of the CS, the US was delivered to the mouse through the grids for 2 sec. After this CS/US pairing, mice were allowed to stay in the chamber for another 30 sec for an enhancement of the association with the special context (chamber, grids, and cues around the chamber). Mice were then immediately returned to their original cages. Throughout the procedures, freezing responses were recorded with a five sec interval sampling method. Freezing responses were judged as complete immobility of any part of the body, except for respiratory movements. Freezing responses during the 30 sec period after shock were recorded as immediate freezing. Retention tests were carried out 24 hr after the training sessions. During the retention test, each mouse was placed back into the same chamber for contextual freezing measurement and freezing responses were recorded for five min. The floor of the chamber was cleaned with 75% ethanol between tests. At least five min elapsed before the chamber was reused. Throughout all the experiments, the experimenters were blind to the treatment condition. Mice (C57BL/10J) were from Charles River (four-six months old) and both female and male mice were mixed in all experiments.]
FIGURE 3.
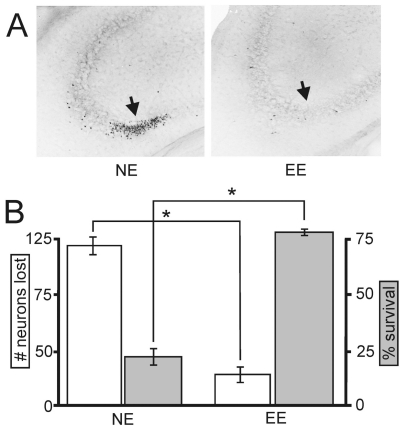
Environmental enrichment protected hippocampus against excitotoxic injury. A well-established model of temporal lobe epilepsy (TLE) was used to determine whether an enriched environment (EE) evoked hippocampal neuroprotection compared to a non-enriched environment (NE). (A) Representative Fluoro-Jade images show caudal CA3 area hippocampal pyramidal neuron death (arrow) four days after kainic acid injections in NE compared to EE mice. (B) Quantification of neurons lost (n=5/group; white) as well as number of surviving animals (n=28/group; gray) showed that EE triggered a significant (“*”, P < 0.05, Student’s t test) reduction in the number of neurons lost and a significant (P<0.01) increase in the percentage of surviving animals. Specific values were 117±17 v. 23±16 neurons lost and 45±8 v. 77±6 % surviving mice (Kraig et al. 2004).
[TLE was induced by a subcutaneous injection of freshly prepared kainic acid (30 mg/kg (Calbiochem) dissolved in phosphate buffered saline (PBS; at 1mg/mL; Avanzini et al. 1998)) in C57BL/10J mice. Sham controls received similar volume subcutaneous injections of PBS. Animals were briefly restrained in a holding device for injections. After injections, animals were placed in individual cages without lids for continuous observation. Seizure severity was quantitated (every 20 min during continuous monitoring) for the next four hrs according to a scale proposed by Racine (1972) and modified to include death (Hulse et al. 2004). This rating was used to ensure that all animals included for study reached the same general level of seizure severity. Those that did not were excluded from study. In the event that mice experienced continuous seizures for 20 min, they would have been euthanized. No animals had to be removed or euthanized in this study. At the conclusion of observation periods, animals were kept in individual cages with standard housing until their brains were serially sectioned (40 μm) through the hippocampus. TLE injury was measured using Fluoro-Jade staining (Schmued and Hopkins 2000) to count dying neurons using stereological techniques (Howard and Reed 1998; West 1999) for the CA3 area of hippocampus (from sections taken every 240 μm).]
Initially, serum corticosterone levels were measured to probe for potential stress reaction from exposure to EE. Our results indicate what may be an important signaling pattern associated with increased learning from EE. We, like others (Kempermann et al. 2002), found that morning corticosterone levels from EE animals were modestly elevated compared to non-enriched counterparts (Figure 4). However, we also found that evening levels were significantly higher in EE animals compared to non-enriched mice with nearly two-fold increase in circadian rhythm change (Figure 4). A similar effect of EE on circadian corticosterone was reported by others studying growing pigs (de Jong et al. 2000).
FIGURE 4.
Environmental enrichment and enhanced circadian corticosterone changes. Measurement of mouse (C57BL/10J) serum circadian corticosterone levels 28 days after housing in an enriched environment (EE) v. a non-enriched environment (NE) add further support to the suggestion that low-level phasic stress (or irritative signaling) is associated with enhanced brain function. For example, mice (n=14/group) housed in an NE showed AM-PM serum corticosterone levels of 14±2 and 70±9 ng/mL, and mice housed in an EE showed AM-PM levels of 18±3 and 122±18 ng/mL. While AM levels were not significantly different between housing types, PM levels were significantly different (“*”, P<0.001, Student’s t test) between housing types and significantly different (P=0.01) from AM levels within housing types. (Kraig et al. 2004).
[Serum was harvested following methods described by Hulse and coworkers (2004) to minimize acute stress and control for circadian variations in samples. Briefly, anesthesia was induced within cages using a 10% halothane (90% air). Animals were then quickly transferred to a new chamber with 3% halothane (97% air) until harvest, followed by rapid decapitation and collection of truncal blood for animals within a cage. Collections were performed at 10 AM and 10 PM. Serum corticosterone measurements were performed by Diagnostic Systems Laboratories (Texas).]
Phasically elevated corticosterone can initially enhance hippocampal function, whereas chronically elevated corticosterone inhibits hippocampal function (de Kloet et al. 1999; Zoladz and Diamond 2008). Diamond and coworkers showed that corticosterone enhances primed burst electrical potentiation of hippocampal pyramidal neurons (Diamond et al. 1992; Kim and Diamond 2002). Moderate and intermittent corticosterone levels enhance electrical activity, while extreme and chronically elevated levels (e.g., consistent with clinical depression) inhibit electrical responses. These effects are consistent with physiological hormesis.
While corticosterone is a peripheral, and potentially systems-wide, modulator of EE-based neuroprotection, our research focuses to intrinsic brain signaling. There, TNF-α, and the cellular source of this innate cytokine, microglia, are likely key mediators of EE-based neuroprotection. This focus stems from data indicating that learning from EE initiates neuroprotective and hormetic TNF-α signaling from microglia.
First, EE (i.e., increased neural activity) triggered a significant increase in expression of TNF-α within hippocampus, the site of excitotoxic neuroprotection (Figure 5). Prior evidence shows that brain TNF-α rises in response to treadmill activity (Ding et al. 2005).
FIGURE 5.
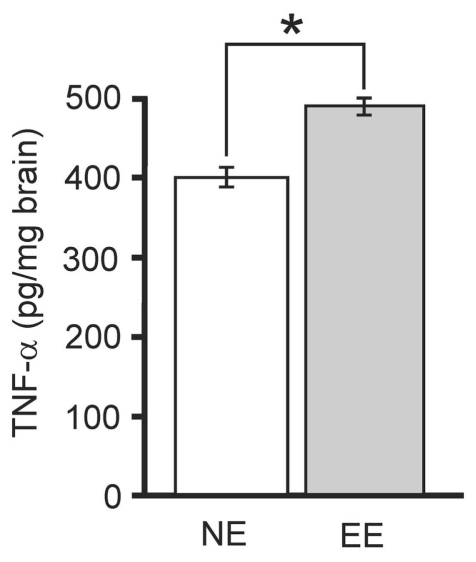
Environmental enrichment triggered hippocampal TNF-α expression. Increasing evidence shows that brain may employ immune signaling to effect physiological conditioning hormesis of environmental enrichment (EE) from increased “thinking.” For example, the innate cytokine TNF-α, which can be lethal when associated with disease, may be a principal signal that initiates neuroprotection from EE. TNF-α is one of a group of innate immune cytokines that are highly interactive with one another and can variably stimulate and/or inhibit each other’s expression. Accordingly, multiplexed measurements of innate cytokines from hippocampus after non-enriched (NE) or EE housing were completed. Notably, only TNF-α showed a significant (“*”, P<0.02, Student’s t test) change from 404±12 to 488±22 pg/mg brain (n=6/group). A principal function of TNF-α is to alter gene expression. Additionally brain TNF-α expression is activity-dependent. Therefore, enhanced neural activity from EE may alter brain gene expression to create the phenotype of neuroprotection via TNF-α. We tested for this possibility in the experiments of Figure 6.
[Cytokine measurements were performed as previously described (Hulse et al. 2004; Kunkler et al. 2004) using microsphere flow cytometric assays (Bio-Rad) run on a Bio-Plex analyzer with associated software. After 28 days of NE or EE housing, mouse (C57BL/10J) hippocampi were harvested via group anesthesia as described in Figure 4 and detailed elsewhere (Hulse et al. 2004). While hippocampal TNF-α measurements showed a significant change as described above, no significant changes were seen between NE and EE mice (n=6/group) for IL-1α (34±2 v. 37±1), IL-1β (34±3 v. 39±1), IL-2 (77± v. 78±3), IL-4 (none detected), IL-6 (66±4 v. 64±4), IL-10 (10±1 v. 10±1), IFN-γ (none detected), and GM-CSF (none detected) (Kraig et al. 2003).]
Second, TNF-α modulates synaptic activity. Within minutes of exposure, TNF-α enhances synaptic efficacy by increasing the exocytosis of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid) receptors in cultured neurons (Beattie et al. 2002) as well as the endocytosis of inhibitory GABAA (γ-amino butyric acid type A) receptors (Stellwagen et al. 2005). TNF-α also triggers increased expression of AMPA receptors as a synaptic scaling response to activity deprivation by tetrodotoxin (Stellwagen and Malenka 2006). Increased AMPA receptor expression is seen with long-term potentiation (LTP) (Park et al. 2004), a well-accepted cellular model of learning, and EE (Naka et al. 2005). While TNF-α may not be necessary for LTP induction (Albensi and Mattson 2000) and may retard it (Cunningham et al. 1996), TNF-α does play a role in related homeostatic plasticity changes (Kaneko et al. 2008). This non-Hebbian capacity of neurons (and neural tissue) to modulate their overall sensitivity to activity may play a critical role in maintaining network stability during development and in the mature, learning brain (Turrigiano et al. 1998; Goldberg et al. 2002).
Recent work from Wheeler and coworkers (2009) shows that acute exposure to TNF-α also leads to prompt increased neuronal excitability that involves NMDA (N-methyl-d-aspartate) receptor expression. Importantly, these workers studied in detail the molecular events that lead to fusion of NMDA containing vesicles with the plasma membrane. Their work demonstrates that TNF-α-induced activation of neutral sphingomyelinase-2 and increased expression of ceramide are essential for increased surface membrane expression of NMDA receptors and increased synaptic efficacy. This is an important step forward for defining the signaling syntax by which TNF-α from glia can acutely alter neuronal excitability.
Third, neuroprotection from increased neuronal activity involves TNF-α. Reduced synaptic efficacy from long-term depression has no impact on CA1 area excitotoxic injury in hippocampal slices. On the other hand, increased synaptic efficacy from LTP triggers a significant reduction in injury that requires TNF-α (Kraig et al. 2006) (Figure 6). A similar neuroprotective effect requiring TNF-α is seen with spreading depression (Kraig et al. 2005). Spreading depression is non-injurious and has many cellular and molecular features similar to learning and memory (Kraig and Kunkler 2002; Kunkler et al. 2005), including transient elevation of TNF-α (Kunkler et al. 2004). Finally, Lambertsen and coworkers (2009) used traditional knockout animals for TNF-α (and its cognate receptors) to show that stroke injury and behavioral deficits are increased in the absence of TNF-α signaling. Potentially confounding adaptive effects may obscure observations using traditional knockout/in animals (Gao et al. 1999); however, here TNF-α related adaptive changes may account for the observations.
FIGURE 6.
Neuroprotection from long-term potentiation depends on TNF-α. Long-term potentiation (LTP), a cellular model of learning, can be elicited in vitro using electrical or chemical stimulation of susceptible brain regions. Chemical LTP (cLTP) maximizes the number of synapses and adjacent cells activated to undergo these plasticity changes. Accordingly, cLTP was used to show that physiologically increased neural activity triggered neuroprotection from excitotoxic injury like that seen in vivo and, importantly, the protection depended on TNF-α. (A) Specifically, NMDA irreversible injury of CA1 in rat hippocampal slice cultures is significantly (“*”, P<0.001, ANOVA using Holm-Sidak post hoc testing) reduced from control (normalized to 1.00±0.05, n=9) compared to cLTP (0.59±0.02, n=6). Furthermore, chemical long-term depression (cLTD), a method that mimics electrically induced reduction in synaptic efficacy, has no protective effect with a relative injury level of 0.95±0.05 (n=5). (B) Similarly, excitotoxic injury of the CA1 area produced by oxygen glucose deprivation (OGD) is significantly (P<0.001) reduced to 0.53±0.05 (n=6) by cLTP compared to control (1.00±0.06; n=9). (C) Furthermore, neuroprotection from cLTP is significantly returned toward control levels of injury due to abrogation of TNF-α signaling using soluble TNF receptor 1 (sTNFR1). Specific injury levels (n=5–6/group) were 1.00±0.06 for control, 0.51±0.05 for cLTP and 0.77±0.05 for cLTP plus sTNFR1. Taken together, these experiments provide direct support for the suggestion that low-level elevation in TNF-α from EE initiates activity-dependent neuroprotection.
[cLTP was induced by 12 min exposure to a magnesium-free Ringer’s solution (37 °C) that contained 100 nM Rolipram and 50 μM forskolin (Otmakhov et al. 2004). cLTD was evoked by 20 min exposure to Ringer’s solution containing 100 μM of RS-3,5-dihydroxyphenyl-glycine at 37 °C (Volk et al. 2006). sTNFR1 (200 ng/mL) was applied to cultures 30 min before cLTP and after cLTP induction but was excluded from maximal injury aspects of the excitotoxicity paradigms which followed previous descriptions (Hulse et al. 2008).]
Fourth, monomeric immunoglobin G (IgG) may help interlink neuronal activity to TNF-α production (Hulse et al. 2008) (Figure 7). EE activates microglia to a pro-inflammatory state (Ziv et al. 2006). While all brain cell types plus adjacent endothelial cells are capable of releasing TNF-α, microglia are recognized as the likely predominant source of this pro-inflammatory cytokine, especially in response to disease (Hopkins and Rothwell, 1995; Allan and Rothwell, 2001). Microglia are also likely the predominant, if not sole, source of TNF-α under physiological conditions including EE (Hulse et al. 2008). Monomeric IgG is commonly regarded as a quiescent signaling molecule poised only to initiate potentially injurious inflammatory reactions in response to disease via immune complex formation and associated phagocytosis and TNF-α production. Instead, IgG signaling within non-injured brain results in neuroprotection when it follows a physiological conditioning hormesis dose-response pattern and takes time to develop. This IgG-mediated neuroprotection involves enhanced microglial recycling endocytosis and TNF-α production—changes that are quickly evident after IgG exposure. Importantly, minocycline, known for its anti-inflammatory and neuroprotective effects when administered after the onset of brain disease, abrogates IgG-mediated neuroprotection and related microglial enhanced recycling endocytosis and TNF-α production. Furthermore, E-prostanoid receptor subtype 2 activation by prostaglandin E2, which is released by hippocampal pyramidal neurons proportionate to their electrical activity, amplifies IgG-mediated effects on microglia. This suggests paracrine mediators released from neurons, due to their increased activity, can affect microglial TNF-α release via amplification of recycling endocytosis. These results confirm and extend evidence indicating that microglial pro-inflammatory activation over time is nutritive. Furthermore, they begin to illustrate specific immune signaling mechanisms by which neurons and microglia interact to enhance brain function.
FIGURE 7.
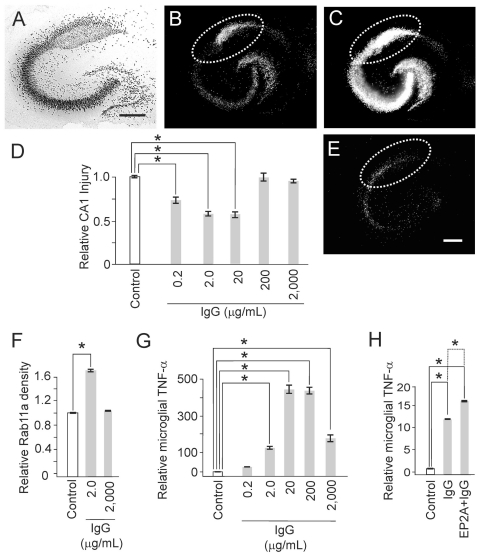
Monomeric immunoglobin G may link neuronal activity to TNF-α expression. Monomeric immunoglobin G (IgG) is not known to have signaling function in normal brain. Instead, monomeric IgG triggers physiological hormetic signaling involving microglia that includes increased recycling endocytosis and TNF-α secretion. Over time, these activating microglial changes are associated with neuroprotection at physiological but not elevated levels of IgG. E-prostanoid receptor subtype 2 activation (EP2A), used as an exemplary paracrine signal proportionate to hippocampal pyramidal neuron activity, amplifies IgG-mediated effects on microglia. The following data illustrate these conclusions. (A) Immunostaining with NeuN shows the principal neuron cytoarchitecture for the pyramidal and dentate areas. Calibration bar is 250 μm. (B–D) Excitotoxic injury from NMDA, using a fluorescent marker for cell death, was quantified in the CA1 area (dotted lines) and expressed as a ratio in injury (B) over maximal injury (C) relative to control conditions (D). (E) Exposure to IgG evokes significant (“*”, P<0.001) neuroprotection against NMDA injury, with ~20 μg/mL as the level expected for mammalian brain interstitial fluid under normal conditions. Microglial recycling endocytosis (F) and TNF-α production (G) also respond to IgG in a physiological hormetic dose-response pattern. (H) Finally, neuroprotection from IgG is amplified by co-incubation of IgG with Butaprost, an EP2 receptor agonist, which acted as a surrogate of prostaglandin E2 release from increased neuronal activity. This amplification was removed by abrogation of TNF-α signaling via sTNFR1 inclusion (not shown). Results adapted with permission from Hulse et al. 2008.
Adaptive nature of activity-dependent neuroprotection
Neuroprotection from EE requires time to develop. This suggests that EE, like other preconditioning stimuli (Barone et al. 1998; Nishio et al. 2000), requires new protein synthesis. The predominant effect of TNF-α is to alter gene expression (Abbas and Lichtman 2003). Thus, TNF-α is well positioned to orchestrate the activity-dependent structural and functional adaptation necessary for EE-based neuroprotection. However, the precise nature of these adaptive processes and their relation to TNF-α and microglial activation remain undefined. Nonetheless, evidence from physical activity, dietary restriction, and ageing research provide provocative clues.
Radak and colleagues (2008) review evidence that moderate physical activity improves general health by triggering adaptive responses to reactive oxygen species (ROS). They note that physical activity extremes (i.e., inactivity and excessive activity) lead to deterioration in health, consistent with the basic tenet of hormesis. Exercise must be sufficiently high to initiate “stress” so that adaptive responses will occur. Importantly, exercise needs to be intermittent with sufficient periods of rest to allow for the development of adaptive responses associated with improved muscle (and organismal) health. We suggest this requirement not only applies to brain and EE (Figure 8), but may also be involved in the maladaptive effects of excessively increased brain activity (see below). The authors point to literature (Gomez-Cabrera et al. 2005) indicating that ROS inhibition via exogenous scavengers abrogates the positive effects of physical exercise, a result now extended to humans (Ristow et al. 2009).
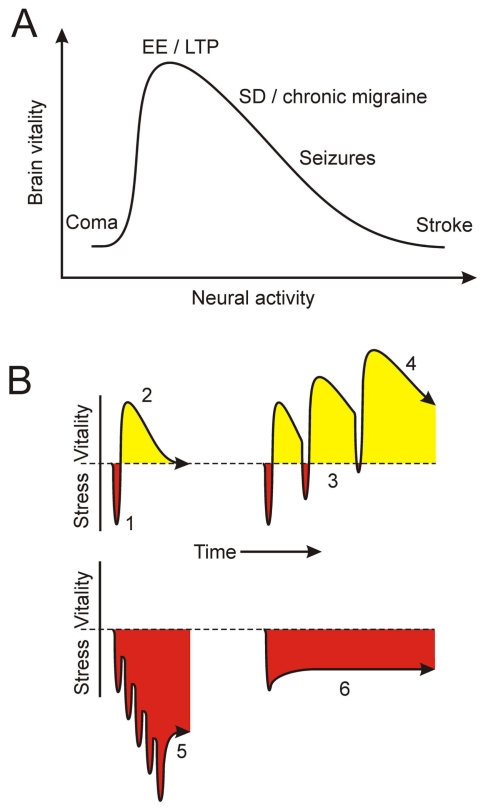
FIGURE 8.
Sufficient and phasic signaling of activity-dependent neuroprotection. (A) Brain vitality and neural activity (i.e., the degree and duration of brain cellular depolarization) follow a hormetic dose response relationship. At the extremes, severely reduced (e.g., coma) and excessive activity (e.g., stroke) are lethal. On the other hand, increased brain cell activity from environmental enrichment (EE) or long-term potentiation, a cellular model of learning, is maximally nutritive. Episodic spreading depression, the underlying cause of migraine, can be neuroprotective. However, when spreading depression becomes too frequent, episodic migraine may be transformed into chronic migraine because periods for adaptive signaling are too short. (B) The principal tenets of physiological conditioning hormesis are illustrated as the need for an initial irritative stimulus (1) which over time results in a period of increased vitality via anabolic structural and functional changes (2). Repetitive phasic episodes of initiating stimuli (3) further enhance brain vitality (4). However, when the frequency of initiating stimuli precludes adequate periods of adaptation (5), maladaptive structural and functional changes ensue that include the development of disease (e.g., chronic migraine). In the extreme (6), initiating stimuli are so overwhelming that associated irritative signals (i.e., microglial activation and production of TNF-α and oxidants) only enhance neurodegeneration.
Dietary restriction may also improve organismal health. Schulz and coworkers (2007) show that glucose restriction increases the lifespan of Caenorhabditis elegans. This caloric restriction paradigm triggers increased ROS formation, and importantly, increased ROS scavenging via heightened catalase activity. Similar to physical activity, exogenous antioxidants and vitamins that scavenge ROS may reduce the worm lifespan initially increased by dietary restriction.
The Mattson laboratory emphasizes that these peripheral preconditioning strategies for exercise and dietary restriction hormesis involving ROS are likely to apply to the ageing brain (Arumugam et al. 2006). This suggestion is supported by data showing that NMDA receptor activation enhances neuronal antioxidant defenses (Papadia et al. 2008).
Modestly increased ROS production and the pronounced impact of intrinsic antioxidants on neurological preventative health may be linked to upstream microglial activation that includes production of TNF-α and oxidants. Several lines of evidence support this suggestion. First, TNF-α stimulates glucose uptake into astrocytes (Yu et al. 1995; Véga et al. 2002), which in turn provide high-energy substrates to neurons (Barros and Deitmer 2009). Second, learning increases brain oxidative metabolism (Heiss et al. 1992). Third, microglia are activated by synaptic activity (Ziv et al. 2006; Hulse et al. 2008) and activated microglia release ROS (Block et al. 2007; Innamorato et al. 2009), which can prompt increased generation of neural tissue antioxidants. Fourth, oxidant stress plus antioxidant responses can modulate synthesis of proteins involved in activity-dependent neuroprotection, most likely via the “translational switch,” eukaryotic translational initiation factor 2α (Costa-Mattiolo et al. 2009; Tan et al. 2009).
Fifth, Radak and colleagues (2008) emphasize that not only must exercise be sufficiently stressful, but adequate rest periods are also essential to improve health. While sufficiently increased brain activity (i.e., EE) is necessary to initiate improved health, sleep may provide the essential rest period required for adaptive changes. Vyazovskiy and colleagues (2008) note that while the wake state is associated with potentiation of synaptic function, sleep reverses this to depotentiation. This scenario is schematized in Figure 8. Importantly, although synaptic potentiation falls with sleep, protein synthesis rises (Ramm and Smith, 1990). Seemingly paradoxical to our overall thesis involving neural activity and TNF-α, hippocampal TNF-α mRNA rises with sleep (Cearley et al. 2003). However, TNF-α changes associated with LTP in the wake state may be localized to only the synaptic regions activated by learning and thus changes would not be detectable in whole brain regions used for the circadian mRNA measurements. Finally, brain catalase activity (and possibly the activities of other antioxidants which collectively can influence protein synthesis), shows phasic behavior (Sani et al. 2006).
In summary, mounting evidence indicates that physiologically increased neural activity begets increased neuronal excitability via mechanisms that include TNF-α from microglia (Figure 9). When this increased neural activity is sufficiently phasic, brain becomes more resilient. In contrast, heightened neural activity and associated glial activation, without adequate periods for adaptation, may leave brain more susceptible to disease (see below).
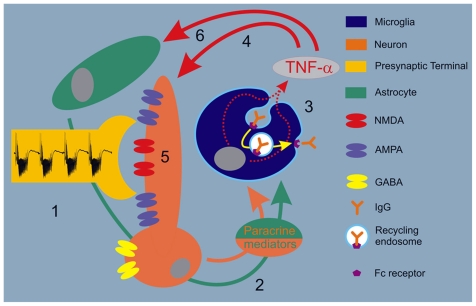
FIGURE 9.
Schematic of neuro-immune initiating signaling of activity-dependent neuroprotection. (1) Physiologically enhanced neuronal activity, if sufficiently phasic, becomes neuroprotective. (2) This nutritive process begins with activity-dependent release of neuronal paracrine mediators including high energy phosphates, glutamate, potassium and especially prostaglandins, which can enhance (3) recycling endocytosis associated with TNF-α production from microglia. Astrocytes are also likely involved in activity-dependent release of paracrine mediators, which activate microglia. (4) As a result, TNF-α released from microglia further triggers an increase in neuronal activity by (5) increasing the surface expression of NMDA and AMPA receptors while decreasing expression of GABA receptors. (6) At the same time, TNF-α from microglia likely influence astrocytes. The choreography between this neuron-glial interactive behavior from heightened neuronal activity involves two important tenets. First, it must be sufficiently robust to trigger “irritative” signaling that initiates subsequent anabolic adaptive processes. Second, it must be sufficiently phasic to allow for homeostatic plastic processes to return activity levels toward normal. Furthermore, but in contrast to a return toward normal, heightened physiological activity must be sufficiently phasic to allow anabolic processes sufficient time to build a stronger resilience to neurological disease. While these latter “processes” remain undefined, evidence from physical activity, dietary restriction, and ageing research suggest that antioxidant defenses and anabolism in response to oxidant stress may be involved.
MALADAPTIVE POTENTIAL OF ENHANCED BRAIN ACTIVITY
Considerations of brain physiological conditioning hormesis have largely focused on the nourishing and restorative capacities of this signaling response pattern. However, examination of mechanisms by which increased brain activity can become maladaptive may help explain select brain diseases. For example, spreading depression is a non injurious and transient perturbation of brain that, like EE, triggers microglial activation (Caggiano and Kraig 1996), increased TNF-α (Kunkler et al. 2004) (Figure 10), and neuroprotection that depends on TNF-α (Kraig et al. 2005). Spreading depression is also the most likely underlying cause for episodic migraine aura and pain (Moskowitz et al. 1993; Kunkler and Kraig 2003; Lauritzen and Kraig 2005).
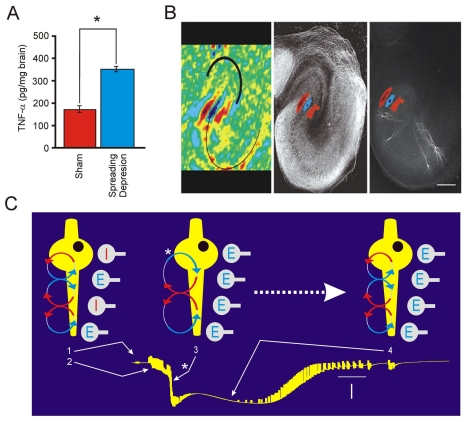
FIGURE 10.
Spreading depression in hippocampus begins with a reversal of somatic pyramidal neuron inhibition. (A) Like environmental enrichment (EE), spreading depression (SD) triggers a significant (“*”, P<0.05, Student’s t test) rise in TNF-α expression from 171±31 pg/mg brain of sham controls (n=5) to 340±23 pg/mg brain (n=9) six hrs after one hr of recurrent SD (Kunkler et al. 2004). This rise likely comes from microglia and is involved in SD-dependent neuroprotection (Kraig et al. 2005). (B) Optical current source density analyses (Kunkler et al. 2005) begin to allude to mechanisms by which episodic migraine, which is neuroprotective, may become maladaptive and account for chronic migraine. For example, estimation of current flow from principal neurons (i.e., pyramidal neurons) shows that SD begins with a massive current sink (blue) at pyramidal neuron cell bodies (i.e., inward current) and is surrounded by current sources (red; i.e., outward current flow) in the adjacent dendrites. This is illustrated in the pseudo-colored left hand image where a thick black line marks the dentate gyrus and a thin black line marks the pyramidal neuron area. This current flow pattern is opposite to that seen after normal electrical activity, where the soma is a sink (see below) and is further emphasized here by superimposition of the SD-initiating current flow pattern on representative images of pyramidal axons (using neurofilament immunostaining (middle image)) and pyramidal neuron dendrites (using cellular injections of Biocytin (right hand image)). Calibration bar is 250 μm. (C) Under normal conditions, current flow in pyramidal neurons from evoked or spontaneous activity shows the typical current sink-source-sink-source pattern moving from the distal dendrites to cell body area (left, “1”). Then with increased activity due to an initiating stimulus, neuronal activity increases (bursting; left, “2”) followed by the onset of SD, where currents at the soma reverse (middle, “3”, “*”). This is followed by the massive interstitial DC potential change of SD (i.e., nearly complete cellular depolarization (right, “4”)), where the current flow pattern returns to a normal orientation but is markedly prolonged to seconds instead of the milliseconds seen with normal activity. Calibration bars are 10 sec (horizontal) and 10 mV (vertical, negative downward). Perhaps, when SD becomes too frequent, tonic somatic inhibition is enhanced by the lack of adaptation to initiating irritative stimuli from microglia that include TNF-α and oxidants, which can reduce or reverse inhibitory synaptic drive. As a result, pyramidal neuron excitability is enhanced and brain becomes more susceptible to SD, resulting in recurrent SD (i.e., chronic migraine). Optical current source density images were adapted with permission from Kunkler et al. 2005.
Reduced inhibitory synaptic function plays a key role in the mechanisms of spreading depression. Our work, and that from the Somjen laboratory (for review see Kunkler et al. 2005), show that a synchronous reduction in synaptic inhibitory drive occurring over a sufficiently large brain volume initiates spreading depression (Figure 10). In addition, as spreading depression subsides, inhibitory synaptic function is last to recover. We speculate that as spreading depression (i.e., migraine) becomes more frequent, the lack of sufficient periods for compensatory adaptive recovery leads to increased brain excitability from reduced inhibition, which may account for the transformation of episodic to chronic migraine (Figure 8).
Chronic migraine is a prevalent healthcare burden (Lipton et al. 2004) whose pathogenesis remains incompletely defined. Evidence suggests that chronic migraine occurs with central sensitization of sensory pathways that involves increased expression of pro-inflammatory mediators and alterations in the periaqueductal gray (Aurora 2009). However, increased migraine frequency also correlates with the transformation of episodic migraine to chronic migraine (Silberstein and Olesen 2005), suggesting the frequency of spreading depression may be an initiating cause of chronic migraine pain. Accordingly, central sensitization of sensory pathways and alterations of the periaqueductal gray may be “downstream” signaling phenomena of chronic migraine while recurrent spreading depression is the “upstream” neural signaling causal change.
Spreading depression initiates pro-inflammatory changes. Astrocytes (Kraig et al. 1991) and microglia (Caggiano and Kraig 1996) show reactive changes for weeks after spreading depression. Importantly, spreading depression also triggers increased TNF-α production (Kunkler et al. 2004) and microglial activation (Caggiano and Kraig 1996) which would include increased production of tissue oxidants. Together these factors, as well as release of other factors such as brain derived neurotrophic factor from microglia (Coull et al. 2005), can reduce neural network inhibitory synaptic drive, which would promote recurrent spreading depression (i.e., chronic migraine). Evidence supports this maladaptive potential of physiological conditioning hormesis. Repetitive spreading depression triggers a selective suppression of inhibitory function (Kruger et al. 1996) and cortical hyperexcitability in migraineurs stems from reduced inhibition (Palmer et al. 2000). Accordingly, microglial activation from increased brain activity that occurs without sufficient time to permit adequate adaptation, may initiate maladaptive consequences to brain.
SUMMARY
Physiological conditioning hormesis characterizes the signaling response patterns by which increased brain activity from EE generates enhanced resilience to brain disease. Two basic tenets of hormesis require that initiating irritative, but not injurious, stimuli are sufficiently robust and that adequate periods of recovery from stressful initiating stimuli occur to allow adequate time for adaptive processes to take effect. Microglia, because of their activation from neural activity and their associated production of TNF-α and oxidant irritants, are well-positioned to orchestrate hormetic immune signaling that establishes the phenotype of neurological health and disease from brain activity.
ACKNOWLEDGEMENT
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS-19108), the National Institute of Child Health and Human Disorders (5 PO1 HD 09402), the Migraine Research Foundation and the White Foundation. BC-P was supported by a Campbell McConnell Fellowship from Cornell College. Ms. Marcia P. Kraig assisted in the preparation and maintenance of culture systems and in the mouse enrichment studies. We thank Yelena Grinberg for reading and commenting on a final version of the manuscript.
REFERENCES
- Abbas AK, Lichtman AH. Cellular and Molecular Immunology. Saunders; Philadelphia: 2003. [Google Scholar]
- Aggarwal BB, Samanta A, Feldmann M. TNFα. In: Openheim JJ, Feldmann M, editors. Cytokine Reference, Volume 1: Ligands. Academic Press; San Diego: 2001. pp. 413–434. [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF B in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–78. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Aurora SK. Spectrum of Illness: understanding biological patterns and relationships in chronic migraine. Neurology. 2009;72(Suppl 1):S8–13. doi: 10.1212/WNL.0b013e31819749fd. [DOI] [PubMed] [Google Scholar]
- Avanzini G, Moshé SL, Schwartzkroin PA, Engel J., Jr . Animal models of localization-related epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven; Philadelphia: 1998. pp. 271–310. [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemia brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFα. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Kraig RP. Eicosanoids & nitric oxide influence induction of reactive gliosis from spreading depression in microglia but not astrocytes. J Comp Neurol. 1996;369:93–108. doi: 10.1002/(SICI)1096-9861(19960520)369:1<93::AID-CNE7>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Drug therapies for stroke and traumatic brain injury often display U-shaped dose responses: occurrence, mechanisms, and clinical implications. Crit Rev Toxicol. 2008;38:557–577. doi: 10.1080/10408440802014287. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics. 2007;4:571–579. doi: 10.1016/j.nurt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley C, Churchill L, Krueger JM. Time of day differences in IL1beta and TNFalpha mRNA levels in specific regions of the rat brain. Neurosci Lett. 2003;352:61–63. doi: 10.1016/j.neulet.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu SS. Tumor necrosis factor-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci. 1994;16:172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, DeKoninck YD. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:923–935. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LAJ, Lynch MA, O’Connor JJ. Interleukin-1β (IL-1β) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Rönnback A, Bergstrom SA, Söderstrom I, Olsson T. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur J Neurosci. 2004;19:2288–2298. doi: 10.1111/j.0953-816X.2004.03248.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- de Jong IC, Prelle IT, van de Burgwal JA, Lambooij E, Korte SM, Blokhuis HJ, Koolhaas JM. Effects of environmental enrichment on behavioral responses to novelty, learning and memory, and the circadian rhythm in cortisol in growing pigs. Physiol Behav. 2000;68:571–578. doi: 10.1016/s0031-9384(99)00212-7. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP, Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends in Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurology. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Mental Ret Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- Gao X, Kemper A, Popko B. Advanced transgenic and gene-targeting approaches. Neurochem Res. 1999;24:1181–1188. doi: 10.1023/a:1020772706279. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Holthoff K, Yuste R. A problem with Hebb and local spikes. Trends Neurosci. 2002;25:433–435. doi: 10.1016/s0166-2236(02)02200-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Borrás C, Pallardó FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca L, Waldmann H. Reprogramming the immune system using antibodies. Methods Mol Biol. 2006;333:247–268. doi: 10.1385/1-59745-049-9:247. [DOI] [PubMed] [Google Scholar]
- Hadley C. What doesn’t kill you makes you stronger. EMBO Reports. 2003;4:924–926. doi: 10.1038/sj.embor.embor953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, O’Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci BioBehav Rev. 2007;31:202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Pawlik G, Holthoff V, Kessler J, Szelies B. PET correlates of normal and impaired memory functions. Cerebrovasc Brain Metab Rev. 1992;4:1–27. [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology – Three Dimensional Measurement in Microscopy Stereology. BIOS Scientific Publishers; Oxford; 1998. [Google Scholar]
- Hulse RE, Kunkler PE, Fedynyshyn J, Kraig RP. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J Neurosci Methods. 2004;136:87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato NG, Lastres-Becker I, Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr Opin Neurol. 2009;22:308–314. doi: 10.1097/WCO.0b013e32832a3225. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-α mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained five-fold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Met. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Dong LM, Thisted R, Jaeger CB. Recurrent spreading depression increases immunohistochemical evidence of glial fibrillary acidic protein. J Neurosci. 1991;11:2187–2198. doi: 10.1523/JNEUROSCI.11-07-02187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig RP, Kunkler PE. Spreading depression - a teleological means for self-protection from brain ischemia. In: Chan P, editor. Cerebrovascular Disease (22nd Princeton Research Conference) Cambridge University Press; New York: 2002. pp. 142–157. [Google Scholar]
- Kraig RP, Hulse RE, Kunkler PE, Langan G. Environmental enrichment (EE) may be neuroprotective by modulating synaptic activity (SA) via pro- & anti-inflammatory mediators (IMs) Soc Neurosci. 2003;29 Prog # 737.15. [Google Scholar]
- Kraig RP, Hulse RE, Kunkler PE, Nakajima A, Tang Y. Increased learning and diurnal corticosterone changes may trigger environmental enrichment (EE)-based neuroprotection (NP) Soc Neurosci. 2004;30 Prog #681.16. [Google Scholar]
- Kraig RP, Hulse RE, Kunkler PE. Spreading depression (SD)-induced neuroprotection depends on TNF-α. Soc Neurosci. 2005;31 Prog # 97.12. [Google Scholar]
- Kraig RP, Hulse RE, Kunkler PE. LTP-induced neuroprotection depends on TNF-α. Soc Neurosci. 2006;32 Prog #87.4. [Google Scholar]
- Kruger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996;7:2733–2736. doi: 10.1097/00001756-199611040-00065. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus. 2003;13:835–844. doi: 10.1002/hipo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Kraig RP. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. J Cereb Blood Flow Metab. 2004;24:829–839. doi: 10.1097/01.WCB.0000126566.34753.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen MC, Kraig RP. Spreading depression and migraine. In: Olesen J, Goadsby P, Ramadan N, Tfelt-Hansen, Welch KMA, editors. The Headaches. 3rd Edition. Lippincott-Raven; Philadelphia: 2005. pp. 269–276. [Google Scholar]
- Llinás RR. i Of The Vortex. MIT Press; Westwood, MA: 2002. [Google Scholar]
- Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004;63:427–435. doi: 10.1212/01.wnl.0000133301.66364.9b. [DOI] [PubMed] [Google Scholar]
- Marashi V, Barnekow A, Ossendorf E, Sachser N. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav. 2003;43:281–292. doi: 10.1016/s0018-506x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008a;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008b;27:155–162. doi: 10.1177/0960327107083417. [DOI] [PubMed] [Google Scholar]
- Meistrell ME, 3rd, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor alpha is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–3348. [PubMed] [Google Scholar]
- Moskowitz M, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity with trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka F, Narita N, Okado N, Narita M. Modification of AMPA receptor properties following environmental enrichment. Brain & Dev. 2005;27:275–278. doi: 10.1016/j.braindev.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Nishio S, Yunoki M, Chen ZF, Anzivino M, Lee KS. Ischemic tolerance in the rat neocortex following hypothermic preconditioning. J Neurosurg. 2000;93:845–851. doi: 10.3171/jns.2000.93.5.0845. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Khibnik L, Otmakhova N, Carpenter S, Riahi S, Asrican B, Lisman J. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol. 2004;91:1955–1962. doi: 10.1152/jn.00941.2003. [DOI] [PubMed] [Google Scholar]
- Palmer JE, Chronicle EP, Rolan P, Mulleners WM. Cortical hyperexcitability is cortical under-inhibition: evidence from a novel functional test of migraine patients. Cephalgia. 2000;20:525–532. doi: 10.1046/j.1468-2982.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer KA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Arteni NS, Petersen RC, da Rocha AP, Achaval M, Netto CA. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol Learn Mem. 2007;87:101–108. doi: 10.1016/j.nlm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Strapasson AC, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–266. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroenceph Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48:749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Salmina AB. Neuron-glia interactions as therapeutic targets in neurodegeneration. J Alzheimers Dis. 2009;16:485–502. doi: 10.3233/JAD-2009-0988. [DOI] [PubMed] [Google Scholar]
- Sani M, Sebai H, Gadacha W, Boughattas NA, Reinberg A, Mossadok BA. Catalase activity and rhythmic patterns in mouse brain, kidney and liver. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:331–337. doi: 10.1016/j.cbpb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Met. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Olesen J. Chronic migraines. In: Olesen J, Goadsby P, Ramadan N, Tfelt-Hansen, Welch KMA, editors. The Headaches. 3rd Edition. Lippincott-Raven; Philadelphia: 2005. pp. 613–617. [Google Scholar]
- Silverman PH. Rethinking genetic determinism. The Scientist. 2004;18:32–33. [Google Scholar]
- Sriram K, O’Callaghan JP. Divergent roles for tumor necrosis factor-α in the brain. J Neuroimmune Pharmacol. 2007;2:140–153. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol. 2005;62:1750–1754. doi: 10.1001/archneur.62.11.1750. [DOI] [PubMed] [Google Scholar]
- Tan S, Somia N, Maher P, Schubert D. Regulation of antioxidant metabolism by translation initiation factor 2alpha. J Cell Biol. 2009;152:997–1006. doi: 10.1083/jcb.152.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol Neurobiol. 2006;34:221–242. doi: 10.1385/MN:34:3:221. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Véga C, Pellerin L, Dantzer R, Magistretti PJ. Long-term modulation of glucose utilization by IL-1 alpha and TNF-alpha in astrocytes: Na+ pump activity as a potential target via distinct signaling mechanisms. Glia. 2002;39:10–18. doi: 10.1002/glia.10080. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Waldmann H. Reprogramming the immune system. Immunol Rev. 2002;185:227–235. doi: 10.1034/j.1600-065x.2002.18519.x. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Adams E, Cobbold S. Reprogramming the immune system: co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immnol Rev. 2008;223:361–370. doi: 10.1111/j.1600-065X.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VVR, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-α-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde GJC, Pringle AK, Sundstrom LE, Mann DA, Ianotti F. Attenuation and augmentation of ischaemia-related neuronal death by tumour necrosis factor-α in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Prog Brain Res. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Yu N, Maciejewski-Lenoir D, Bloom FE, Magistretti PJ. Tumor necrosis factor-alpha and interleukin-1 alpha enhance glucose utilization by astrocytes: involvement of phospholipase A2. Mol Pharmacol. 1995;48:550–558. [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zoladz P, Diamond DM. Linear and non-linear dose-response functions reveal a hormetic relationship between stress and learning. Dose-Response. 2008;7:132–148. doi: 10.2203/dose-response.08-015.Zoladz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNFα potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection mediated by NF B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]