Abstract
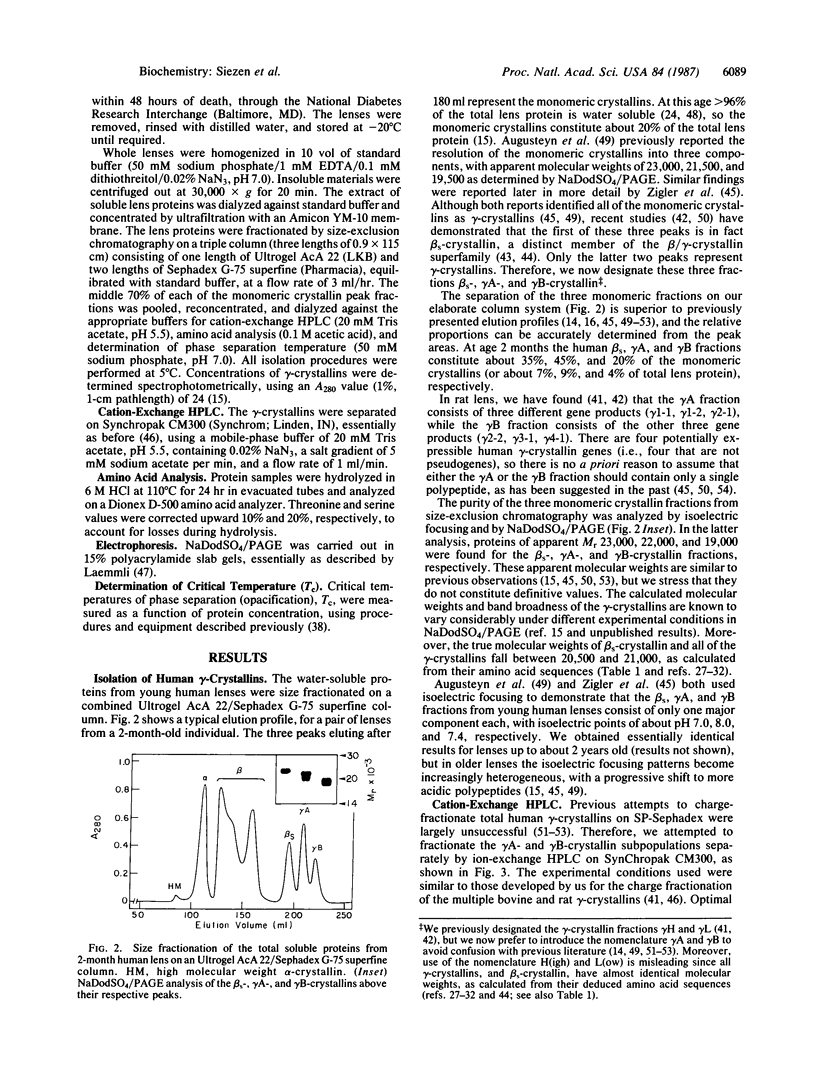
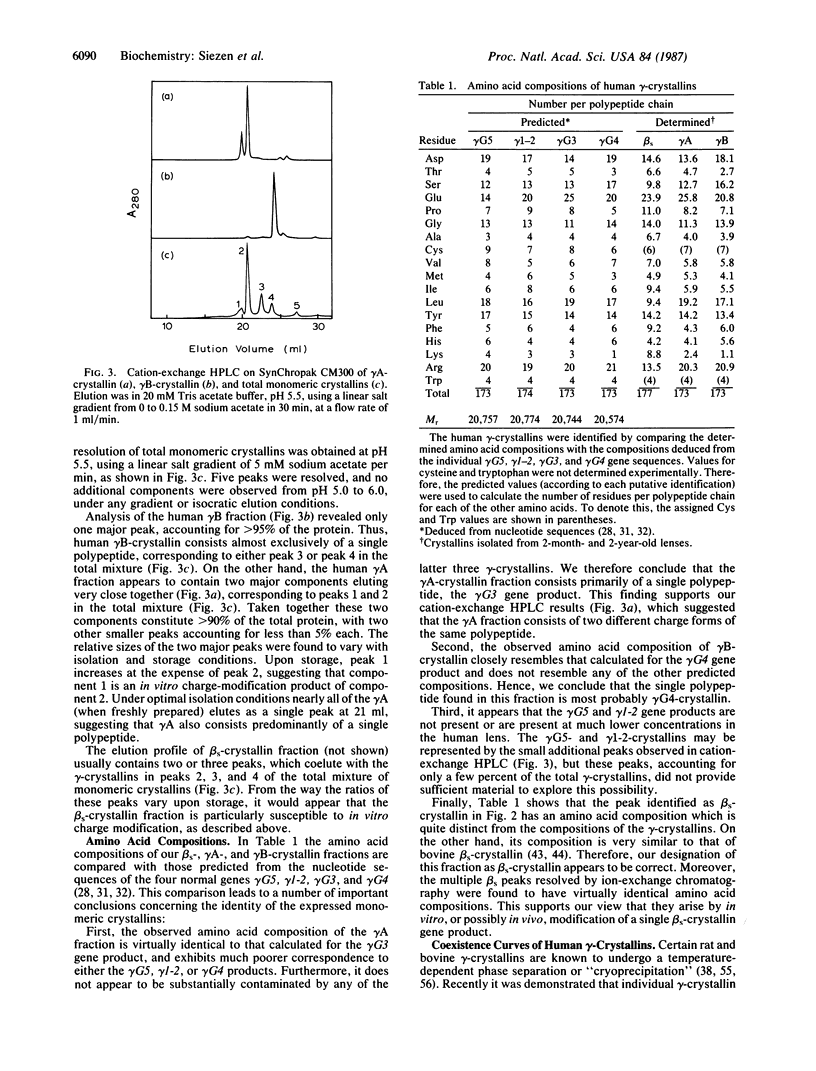
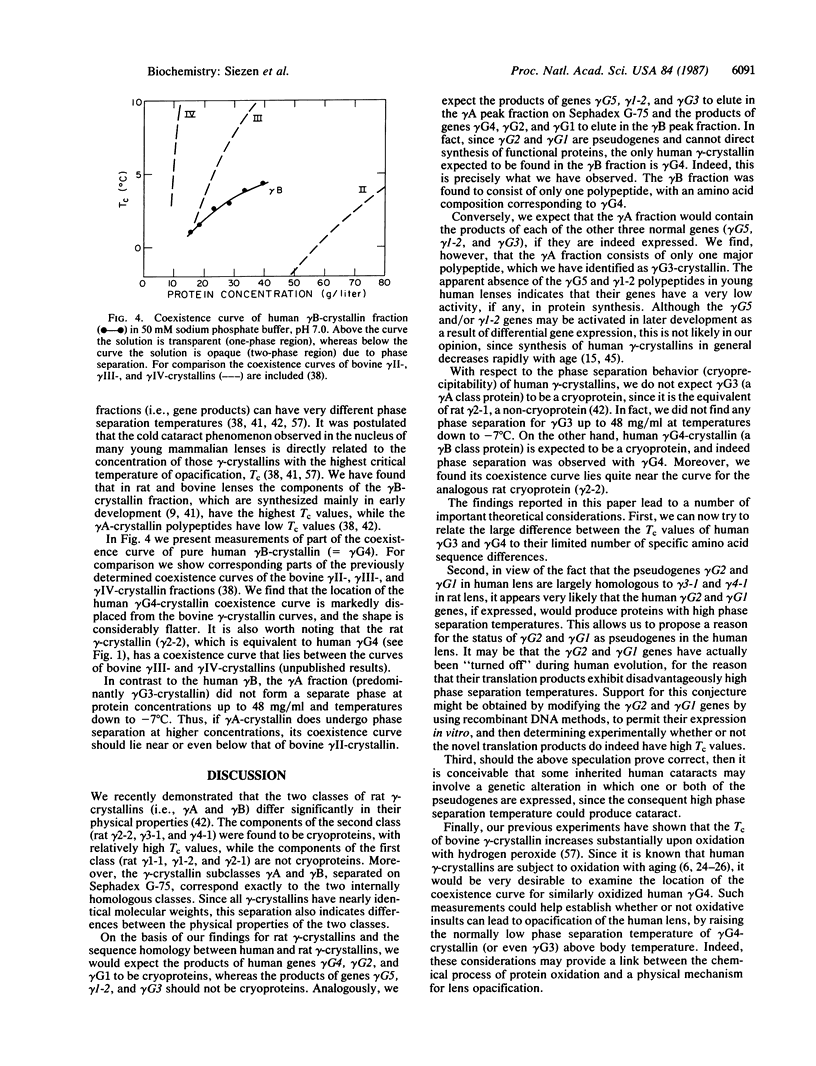
We have isolated the individual gamma-crystallins expressed in young human lenses and identified with which of the six known human gamma-crystallin genes they each correspond. We find that at least 90% of the gamma-crystallins synthesized in the young human lens are the products of genes gamma G3 and gamma G4. We demonstrate that gamma G4-crystallin undergoes a temperature-dependent phase separation, and we have measured the low-concentration branch of its coexistence curve (phase separation temperature vs. concentration) up to about 40 mg/ml. By comparison, we found no evidence of gamma G3-crystallin phase separating, even at lower temperatures and higher concentrations. This is consistent with predictions based on sequence homology between human and rat gamma-crystallins. The implications of these findings for human inherited and senile cataracts are considered.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. I., Spector A. The state of sulfhydryl groups in normal and cataractous human lens proteins. I. Nuclear region. Exp Eye Res. 1978 Apr;26(4):407–417. doi: 10.1016/0014-4835(78)90128-8. [DOI] [PubMed] [Google Scholar]
- Bessems G. J., Hoenders H. J., Wollensak J. Variation in proportion and molecular weight of native crystallins from single human lenses upon aging and formation of nuclear cataract. Exp Eye Res. 1983 Dec;37(6):627–637. doi: 10.1016/0014-4835(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Spector A. Complete nucleotide sequence of a cDNA derived from calf lens gamma-crystallin mRNA: presence of Alu I-like DNA sequences. DNA. 1984 Aug;3(4):287–295. doi: 10.1089/dna.1.1984.3.287. [DOI] [PubMed] [Google Scholar]
- Bindels J. G., Bessems G. J., de Man B. M., Hoenders H. J. Comparative and age-dependent aspects of crystallin size and distribution in human, rabbit, bovine, rat, chicken, duck, frog and dogfish lenses. Comp Biochem Physiol B. 1983;76(1):47–55. doi: 10.1016/0305-0491(83)90169-4. [DOI] [PubMed] [Google Scholar]
- Bindels J. G., Bours J., Hoenders H. J. Age-dependent variations in the distribution of rat lens water-soluble crystallins. Size fractionation and molecular weight determination. Mech Ageing Dev. 1983 Jan;21(1):1–13. doi: 10.1016/0047-6374(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Björk I. Studies on gamma-crystallin from calf lens. 3. Comparison of the main protein components by peptide mapping. Exp Eye Res. 1970 Jan;9(1):152–157. doi: 10.1016/s0014-4835(70)80070-7. [DOI] [PubMed] [Google Scholar]
- Bours J., Hockwin O. Biochemistry of the ageing rat lens. II. Isoelectric focusing of water-soluble crystallins. Ophthalmic Res. 1983;15(5):234–239. doi: 10.1159/000265265. [DOI] [PubMed] [Google Scholar]
- Bours J., Vornhagen R., Herlt M., Rink H. Immunological characterization of calf lens gamma-crystallins, separated by preparative isoelectric focusing. Curr Eye Res. 1981;1(11):651–658. doi: 10.3109/02713688109001869. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Lok S., Wistow G., Piatigorsky J., Tréton J. A., Gold R. J., Tsui L. C. Gamma-crystallin family of the mouse lens: structural and evolutionary relationships. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7762–7766. doi: 10.1073/pnas.81.24.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper D., Russell P., Shinohara T., Kinoshita J. H. Differential synthesis of rat lens proteins during development. Exp Eye Res. 1985 Jan;40(1):85–94. doi: 10.1016/0014-4835(85)90110-1. [DOI] [PubMed] [Google Scholar]
- Clark J. I., Benedek G. B. Phase diagram for cell cytoplasm from the calf lens. Biochem Biophys Res Commun. 1980 Jul 16;95(1):482–489. doi: 10.1016/0006-291x(80)90763-9. [DOI] [PubMed] [Google Scholar]
- Coghlan S. D., Augusteyn R. C. Changes in the distribution of proteins in the aging human lens. Exp Eye Res. 1977 Dec;25(6):603–611. doi: 10.1016/0014-4835(77)90139-7. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Fagerholm P. P., Philipson B. T., Lindström B. Normal human lens - the distribution of protein. Exp Eye Res. 1981 Dec;33(6):615–620. doi: 10.1016/s0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- Garner W. H., Garner M. H., Spector A. Gamma-crystallin, a major cytoplasmic polypeptide disulfide linked to membrane proteins in human cataract. Biochem Biophys Res Commun. 1981 Jan 30;98(2):439–447. doi: 10.1016/0006-291x(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Kabasawa I., Kodama T., Kabasawa M., Sakaue E., Watanabe M., Kimura M. Heterogeneity of human cataractous and normal lens gamma-crystallins. Exp Eye Res. 1982 Jul;35(1):1–9. doi: 10.1016/s0014-4835(82)80017-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerman S., Zigman S., Forbes W. F. Properties of a cryoprotein in the ocular lens. Biochem Biophys Res Commun. 1966 Jan 4;22(1):57–61. doi: 10.1016/0006-291x(66)90602-4. [DOI] [PubMed] [Google Scholar]
- Li L. K., Roy D., Spector A. Changes in lens protein in concentric fractions from individual normal human lenses. Curr Eye Res. 1986 Feb;5(2):127–135. doi: 10.3109/02713688609015101. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M. J., Ding L. L., Takemoto L. J., Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985 Dec;41(6):745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Breitman M. L., Tsui L. C. Structural and evolutionary relationships among five members of the human gamma-crystallin gene family. Mol Cell Biol. 1985 Jun;5(6):1408–1414. doi: 10.1128/mcb.5.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer-Orlando M., Paterson R. C., Lok S., Tsui L. C., Breitman M. L. Differential regulation of gamma-crystallin genes during mouse lens development. Dev Biol. 1987 Jan;119(1):260–267. doi: 10.1016/0012-1606(87)90227-2. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Biochemistry of bovine lens proteins. II. The gamma-crystallins of adult bovine, calf and embryonic lenses. Biochim Biophys Acta. 1965 Aug 24;107(1):81–90. doi: 10.1016/0304-4165(65)90390-9. [DOI] [PubMed] [Google Scholar]
- Philipson B. Distribution of protein within the normal rat lens. Invest Ophthalmol. 1969 Jun;8(3):258–270. [PubMed] [Google Scholar]
- Quax-Jeuken Y., Driessen H., Leunissen J., Quax W., de Jong W., Bloemendal H. beta s-Crystallin: structure and evolution of a distinct member of the beta gamma-superfamily. EMBO J. 1985 Oct;4(10):2597–2602. doi: 10.1002/j.1460-2075.1985.tb03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers J. G., den Dunnen J. T., Moormann R. J., Jongbloed R., van Leen R. W., Lubsen N. H. The crystallin gene families. Ciba Found Symp. 1984;106:208–218. doi: 10.1002/9780470720875.ch12. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Benedek G. B. Controlled modulation of the phase separation and opacification temperature of purified bovine gamma IV-crystallin. Curr Eye Res. 1985 Oct;4(10):1077–1085. doi: 10.3109/02713688509003352. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Fisch M. R., Slingsby C., Benedek G. B. Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1701–1705. doi: 10.1073/pnas.82.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R. J., Kaplan E. D., Anello R. D. Superior resolution of gamma-crystallins from microdissected eye lens by cation-exchange high-performance liquid chromatography. Biochem Biophys Res Commun. 1985 Feb 28;127(1):153–160. doi: 10.1016/s0006-291x(85)80138-8. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Croft L. R. Developmental changes in the low molecular weight proteins of the bovine lens. Exp Eye Res. 1973 Nov 25;17(4):369–376. doi: 10.1016/0014-4835(73)90246-7. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Miller L. R. Purification and crystallization of mammalian lens gamma-crystallins. Exp Eye Res. 1983 Nov;37(5):517–530. doi: 10.1016/0014-4835(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Summers L. J., Slingsby C., Blundell T. L., den Dunnen J. T., Moormann R. J., Schoenmakers J. G. Structural variation in mammalian gamma-crystallins based on computer graphics analyses of human, rat and calf sequences. 1. Core packing and surface properties. Exp Eye Res. 1986 Jul;43(1):77–92. doi: 10.1016/s0014-4835(86)80047-1. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Augusteyn R. C. Ontogeny of human lens crystallins. Exp Eye Res. 1985 Mar;40(3):393–410. doi: 10.1016/0014-4835(85)90152-6. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Meakin S. O., Tsui L. C., Breitman M. L. Assignment of human gamma crystallin multigene family to chromosome 2. Somat Cell Mol Genet. 1985 Sep;11(5):511–516. doi: 10.1007/BF01534846. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Horwitz J., Kinoshita J. H. Studies on the low molecular weight proteins of human lens. Exp Eye Res. 1981 Jan;32(1):21–30. doi: 10.1016/s0014-4835(81)80035-8. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Russell P., Horwitz J., Reddy V. N., Kinoshita J. H. Further studies on low molecular weight crystallins: relationship between the bovine beta s, the human 24kD protein and the gamma-crystallins. Curr Eye Res. 1986 May;5(5):395–401. doi: 10.3109/02713688609025179. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Russell P., Takemoto L. J., Schwab S. J., Hansen J. S., Horwitz J., Kinoshita J. H. Partial characterization of three distinct populations of human gamma-crystallins. Invest Ophthalmol Vis Sci. 1985 Apr;26(4):525–531. [PubMed] [Google Scholar]
- den Dunnen J. T., Jongbloed R. J., Geurts van Kessel A. H., Schoenmakers J. G. Human lens gamma-crystallin sequences are located in the p12-qter region of chromosome 2. Hum Genet. 1985;70(3):217–221. doi: 10.1007/BF00273445. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Moormann R. J., Lubsen N. H., Schoenmakers J. G. Concerted and divergent evolution within the rat gamma-crystallin gene family. J Mol Biol. 1986 May 5;189(1):37–46. doi: 10.1016/0022-2836(86)90379-7. [DOI] [PubMed] [Google Scholar]
- van Dam A. F. Purification and composition of beta-s-crystallin. Exp Eye Res. 1966 Oct;5(4):255–266. doi: 10.1016/s0014-4835(66)80035-0. [DOI] [PubMed] [Google Scholar]
- van Leen R. W., van Roozendaal K. E., Lubsen N. H., Schoenmakers J. G. Differential expression of crystallin genes during development of the rat eye lens. Dev Biol. 1987 Apr;120(2):457–464. doi: 10.1016/0012-1606(87)90249-1. [DOI] [PubMed] [Google Scholar]




