Abstract
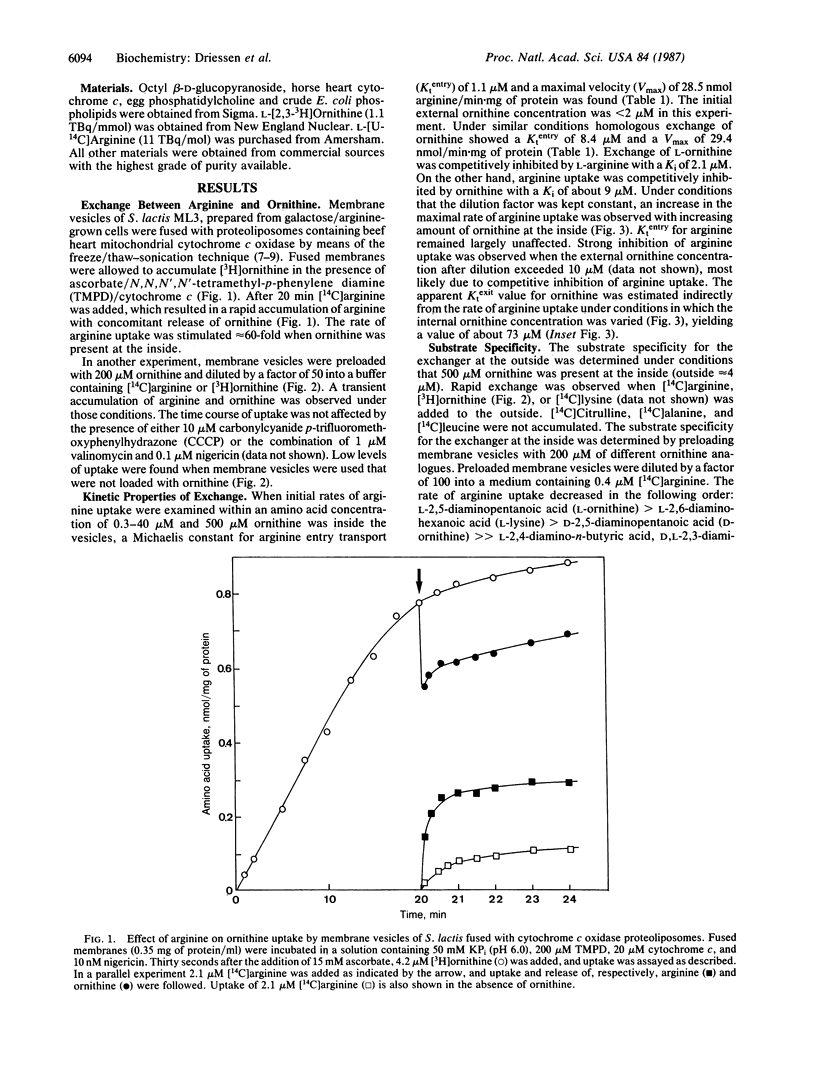
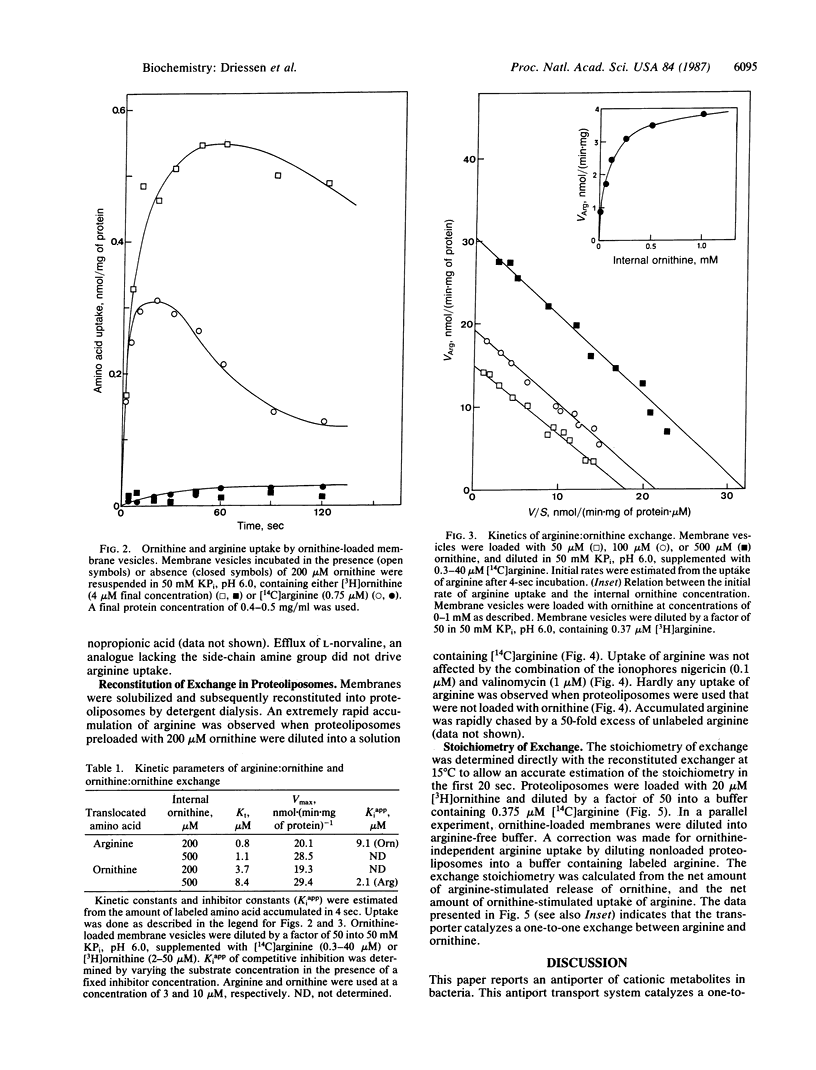
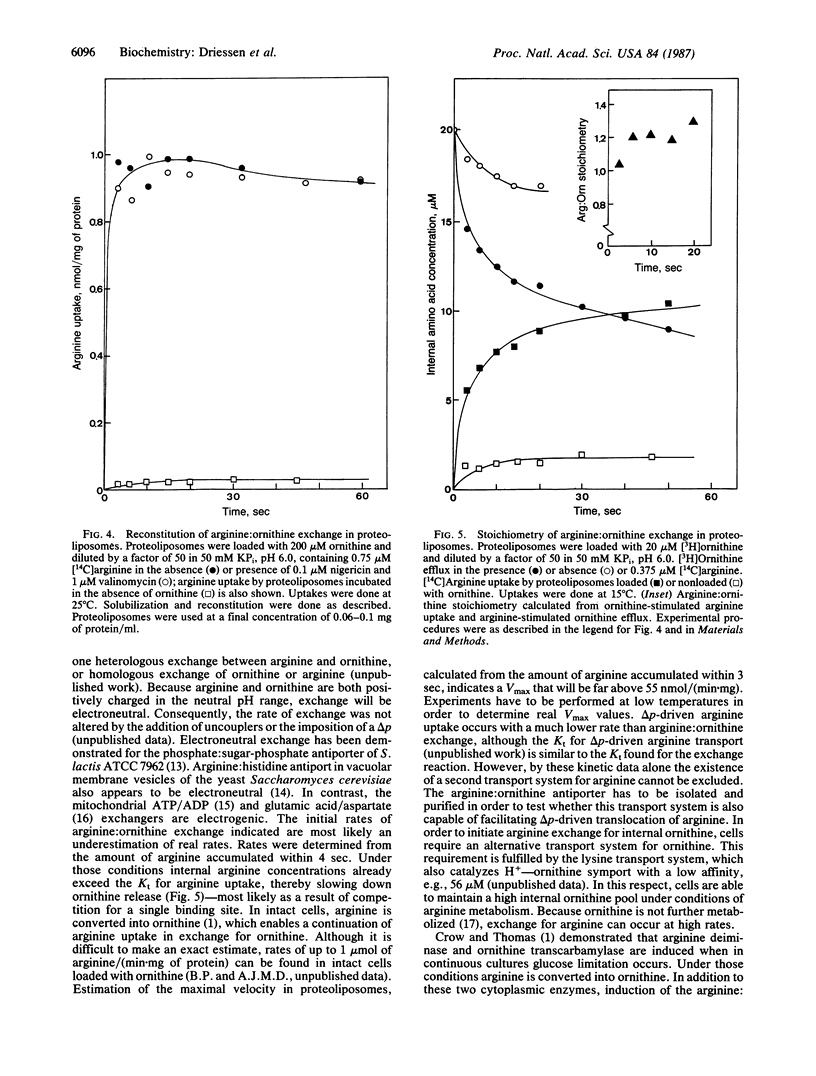
Streptococcus lactis metabolizes arginine via the arginine deiminase pathway to ornithine, CO2, NH3, and ATP. The translocation of arginine and ornithine has been studied using membrane vesicles of galactose/arginine-grown cells of S. lactis fused with cytochrome c oxidase proteoliposomes by the freeze/thaw--sonication procedure earlier described. In the presence of reduced cytochrome c the fused membranes rapidly accumulate ornithine. Addition of arginine releases accumulated ornithine. Rapid uncoupler-insensitive exchange between external arginine and internal ornithine is seen at rates that are at least 60-fold higher than the rate of protonmotive force-driven arginine translocation. This arginine:ornithine exchange activity was reconstituted in proteoliposomes after solubilization of S. lactis membranes with octyl beta-D-glucopyranoside. These proteoliposomes catalyze a one-to-one exchange between arginine and ornithine. The arginine:ornithine exchange system is the first exchange system for cationic metabolites found in bacteria. Translocation of arginine via this system does not require metabolic energy obtained by arginine metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Maloney P. C. Bacterial anion exchange. Use of osmolytes during solubilization and reconstitution of phosphate-linked antiport from Streptococcus lactis. J Biol Chem. 1986 Aug 5;261(22):10079–10086. [PubMed] [Google Scholar]
- Ambudkar S. V., Sonna L. A., Maloney P. C. Variable stoichiometry of phosphate-linked anion exchange in Streptococcus lactis: implications for the mechanism of sugar phosphate transport by bacteria. Proc Natl Acad Sci U S A. 1986 Jan;83(2):280–284. doi: 10.1073/pnas.83.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J Membr Biol. 1980 Sep 30;56(2):97–105. doi: 10.1007/BF01875961. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy E., Coll K. E., Viale R. O., Tischler M. E., Williamson J. R. Kinetics and regulation of the glutamate-aspartate translocator in rat liver mitochondria. J Biol Chem. 1979 Sep 10;254(17):8369–8376. [PubMed] [Google Scholar]
- Newman M. J., Wilson T. H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10583–10586. [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1984 Sep 25;259(18):11509–11511. [PubMed] [Google Scholar]
- Thompson J., Curtis M. A., Miller S. P. N5-(1-carboxyethyl)-ornithine, a new amino acid from the intracellular pool of Streptococcus lactis. J Bacteriol. 1986 Aug;167(2):522–529. doi: 10.1128/jb.167.2.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]



