Abstract
Glioblastoma treatment as now constituted offers increased survival measured in months over untreated patients. Because glioblastomas are active in synthesizing a bewildering variety of growth factors, a systematic approach to inhibiting these is being undertaken as treatment adjunct. The serotonin 7 receptor is commonly overexpressed in glioblastoma. Research documentation showing agonists at serotonin receptor 7 cause increased extracellular regulated kinase 1/2 activation, increased interleukin-6 synthesis, increased signal transducer and activator of transcription-3 activation, increased resistance to apoptosis and other growth enhancing changes in glioblastoma is reviewed in this paper. Because three drugs in wide use to treat thought disorders – paliperidone, pimozide and risperidone – are also potent and well-tolerated inhibitors at serotonin receptor 7, these drugs should be studied for growth factor deprivation in an adjunctive role in glioblastoma treatment.
Keywords: 5-HT7; chemotherapy; ERK1/2, glioblastoma; interleukin-6; necrosis; risperidone; paliperidone; pimozide; serotonin receptors
Prelude
All the business of war,
and indeed all the business of life,
is to endeavor to find out
what you don't know by what you do…
Duke of Wellington, Arthur Wellesley 1769–1852…
Introduction
Current glioblastoma treatment options offer limited extension of life over untreated disease course. Because humans are quite prone to madness that proclivity has resulted in much clinical and basic science research on the biochemical and neurophysiological underpinnings of the various forms of psychosis. That research in turn has given us neurophysiological insights that now are applicable to neuro-oncology. This short note reviews one such.
Our knowledge of the group of the 500 similar seven-transmenbrane non-olfactory receptors exceeds our clinical manipulation of these (Alexander et al., 2009); this paper suggests a new use of one of these. Of these seven-transmenbrane receptors, about a dozen are recognized that have serotonin (5-HT) as the primary endogenous ligand (Alexander et al., 2009).
Recent psychiatric research on neurotransmission at serotonin receptor 7 (5-HT7) combined with new understandings of the importance and mechanisms of glioblastoma's use of autocrine and paracrine growth stimulation paths suggest a new and currently available but unused glioblastoma treatment adjunct.
Of all drugs approved for use in humans currently in the EU and USA/Canada, the three exhibiting the most potent inhibition of 5-HT7 are pimozide (Opler and Feinberg, 1991; Tueth and Cheong, 1993), paliperidone (Smith et al., 2006; Marino and Caballero, 2008) and risperidone (Möller, 2005; Smith et al., 2006). This paper will outline evidence for a glioblastoma growth stimulatory system stemming from agonism at 5-HT7, suggesting that antagnonism by these three antipsychotic drugs may be of benefit. Unfortunately 5-HT7 is only one of many growth stimulatory systems. Presumably all must be blocked for cure. Maybe this is a small start on that long path.
Serotonin receptor 7
One of several remarkable features of serotonin as a signalling molecule in brain function generally is that there are over a dozen currently recognized different receptors for it (Hannon and Hoyer, 2008; Alexander et al., 2009). Each serotonin receptor has its own molecular structure, its own array of intracellular second messenger systems engaged, its own unique distribution pattern within brain regions and its own characteristic spatial distribution pattern on individual neurones and glia (Hannon and Hoyer, 2008). 5-HT7 (reviewed in Thomas and Hagan, 2004) is of interest to neuro-oncology and is the subject of this paper because 5-HT7 is the only serotonin receptor about which we have clear data showing activity in stimulating glioblastoma growth and three drugs used in psychiatry happen to potently inhibit it.
In the 16 years since its discovery, a large database on psychiatric effects of agonism or antagonism at 5-HT7 has been generated (Roth et al., 1994; Pittalàet al., 2007; Hannon and Hoyer, 2008). Antidepressant activity of 5-HT7 antagonism is suspected.
5-HT7 like many other G-protein-coupled receptors is positively (Gs) coupled to adenylate cyclase (Hirst et al., 1997; Thomas and Hagan, 2004; Pittalàet al., 2007; Alexander et al., 2009). Thereby agonists at 5-HT7 increase intracellular cyclic adenosine monophosphate, cAMP.
5-HT7 and GLIA
Two core research findings around which this note is built are that of Mahéet al. (2004) where all eight human glioblastoma cell lines tested expressed functioning 5-HT7 (Mahéet al., 2004) and that of Lieb et al. (2005) who documented 5-HT7 on U373MG glioblastoma cell line where agonists stimulated extracellular regulated kinase 1/2 (ERK1/2) and interleukin-6 (IL-6) synthesis (Lieb et al., 2005). This finding assumes particular importance given the prominent role of IL-6 in glioblastoma growth, as outlined below in the section on IL-6.
Taken alone the data of Mahéet al. (2004) and Lieb et al. (2005) would be weaker than it is. With supporting data below, their findings make inhibiting 5-HT7 function an attractive target in adjunctive glioblastoma treatment. The various signalling paths discussed and documented in this paper that connect 5-HT7 to growth enhancing changes in glioblastoma are depicted in Figure 1.
Figure 1.
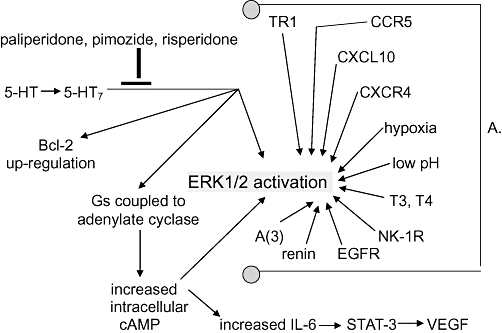
A schema drawn from data presented in text. Some of these relationships are putative, empirically demonstrated not causally established. For example it has not been shown that Bcl-2 up-regulation after 5-HT7 ligation of serotonin is not secondary to adenylate cyclase activation, or to STAT-3 activation, or to ERK1/2 activation, or to none of these, or to some combination of these. Which of the three suggested drugs is best at 5-HT7 signalling inhibition is unknown. Note the crowd of arrows pointing to ERK1/2 activation (phosphorylation). That's a problem. In all likelihood they can to at least some degree cross-cover for each other so that each of these and more must be inhibited for cure. A. Some routes to ERK1/2 in glioblastoma. CCR5 is the chemokine receptor (synonomous with CD195) for RANTES (regulated upon activation, normal T-cell expressed and secreted, synonomous with CCL5). CXCL10 is the 10 kDa ligand for CXCR3. CXCR4 is the receptor for CXCL12. T3 is liothyronine, and T4 is levothyroxine. NK-1R is the receptor for the 11-amino-acid peptide neurotransmitter substance P. EGFR is epidermal growth factor receptor (synonomous with HER-1). A(3) is the adenosine receptor 3. TR1 is the transferring receptor 1. 5-HT, serotonin; 5-HT7, the serotonin receptor 7; cAMP, cyclic ademosine monophosphate; ERK1/2, extracellular regulated kinase 1/2; IL-6, interleukin-6; STAT-3, signal transducer and activator of transcription-3; VEGF, vascular endothelial growth factor.
| Condition or receptor system | References |
|---|---|
| CCR5 | Kast (2010) |
| CXCL10 | Maru et al. (2008) |
| CXCR4 | Porcile et al. (2005) |
| Hypoxia | Kim et al. (2009) |
| Low pH | Xu et al. (2002) |
| T3, T4 | Lin et al. (2009) |
| NK-1R | Kast (2009) |
| EGFR | Loew et al. (2009) |
| Renin/prorenin | Juillerat-Jeanneret et al. (2009) |
| A(3) | Gessi et al. (2010) |
| TR1 | Calzolari et al. (2010) |
Extracellular regulated kinase 1/2 forms one of several signalling hubs through which diverse outer cell membrane receptors exert their effects. The critical role of ERK1/2 specifically in glioblastoma (Lopez-Gines et al., 2008; Kim et al., 2009) and the array of stimuli by which glioblastomas achieve a highly activated ERK1/2 state was reviewed in Lopez-Gines et al., 2008; Kast, 2009; Samaras et al., 2009; Zohrabian et al., 2009.
Extracellular regulated kinase 1/2 is activated (phosphorylated) by 5-HT via activation of 5-HT7 in monocytes (Soga et al., 2007), T lymphocytes (Angileri et al., 2008), microglia (Mahéet al., 2004) and normal hippocampal neurones (Lin et al., 2009). These findings compel us to test if 5-HT7 agonism can/does stimulate ERK1/2 in human glioblastoma too.
Serotonin agonism at 5-HT7 reduces monocytes' apoptosis rate in vitro and up-regulates the anti-apoptosis 26 kDa protein Bcl-2 (Soga et al., 2007). Bcl-2 itself (Angileri et al., 2008; Tagscherer et al., 2008) and Bcl-2-related proteins (Stegh et al., 2007; Degterev and Yuan, 2008; Stegh et al., 2008; Yip and Reed, 2008) are up-regulated in glioblastomas and form one of several core anti-apoptosis changes in glioblastoma not seen in normal glia.
A caveat: the work of Lopez-Gines et al. (2008) showed that there are many paths to ERK1/2 activation that lead to apoptosis resistance and mitosis as shown in Figure 1, but also that there are paths active in glioblastoma obviating an obligatory ERK1/2 activation step (Lopez-Gines et al., 2008). Many of the ERK1/2 activating paths that have been identified operating in glioblastoma are shown inFigure 1, with references in the legend but are not further discussed in text.
The drugs
Each drug has advantages and disadvantages. All three drugs are full antagonists, are fairly well tolerated with side effects that are readily reversible on discontinuation. Side effect reversibility might not hold for psychiatric patients taking these over decades but does hold true for the proposed use here as glioblastoma treatment adjunct.
Table 1 lists basic pharmacological parameters of the three drugs (Opler and Feinberg, 1991; Tueth and Cheong, 1993; Möller, 2005; Smith et al., 2006). Common side effects are given below. All these drugs have a risk for the rare neuroleptic malignant syndrome. All three drugs have good safety margins when taken in overdose with suicidal intent. That safety margin allows clinical psychiatric practice not to use blood levels for titration. These drugs are dosed up or down by desired effect versus tolerability of side effects.
Table 1.
All three drugs are eliminated by renal excretion
| Drug | Ki (Nm) | T1/2 | Metabolism | Common blood levels in psychiatric patients (ng·mL−1) |
|---|---|---|---|---|
| Pimozide | 0.5 | 2 days | 3A4, 1A2 | 1–5 |
| Risperidone | 1.3 | 10–20 h | 2D6 | 10–120 |
| Paliperidone | 1.3 | 1 day | none | 10–120 |
Ki refers to drug affinity at serotonin receptor 7, 5-HT7. T1/2 is the circulating half-life. 3A4, 1A2 and 2D6 refer to hepatic P450 enzyme system responsible for primary catabolism of the drug.
Although not potently so, all three drugs do have antinausea effects that can be helpful during standard chemotherapy. All side effects listed below are fully reversible on discontinuation or lowering of dose.
The three suggested 5-HT7 antagonists are thought to exert their antipsychotic effects by a combination of antagonism at the dopamine-2 and the serotonin 2A receptors (Opler and Feinberg, 1991, Tueth and Cheong, 1993; Möller, 2005; Smith et al., 2006). Metabolic disturbances such as weight gain, increased cholesterol and diminished glucose tolerance or frank diabetes tend to be seen after long-term use in psychiatric patients and are thought to result from antagonism at these receptors as well. Antagonism at alpha-adrenoceptors is mild and usually not clinically significant (Owens, 1994).
Pimozide advantages (Opler and Feinberg, 1991; Tueth and Cheong, 1993)
Pimozide has the highest affinity at 5-HT7 of any marketed drug about which we have data. It is also the oldest. It is available as a generic drug, therefore potentially the cheapest.
Pimozide disadvantages
QTc prolongation is common but is not usually problematic in clinical practice but can rarely become so. Because of the risk of torsade de points and other arrhythmias increases with increased QTc, close monitoring of the QTc would be required if pimozide was used. Patients would best be shifted to an alternative 5-HT7 inhibitor if prolongation exceeded 15% or 60 ms. Pimozide's inhibition at 5-HT7 is reversible so potentially less potent at blocking 5-HT7 signalling. Akathesia and parkinsonian signs and symptoms are common at higher doses. Prolactin elevation can be expected. Paucity of thought can occur.
Risperidone advantages (Möller, 2005)
Risperidone is now available as generic drug. Its interaction with 5-HT7 is unusual in that inhibition of a given receptor is permanent for the life of that particular 5-HT7 receptor (Smith et al., 2006). Risperidone might covalently bind to 5-HT7.
Risperidone disadvantages
Prolactin elevations are probably greatest with risperidone. Akathesia and parkinsonian signs and symptoms are common. Galactorrhea and paucity of thought can occur.
Paliperidone ( = 9-OH-risperidone, Invega®, Janssen Pharmaceuticals Inc., Titusville, NJ, USA)
Advantages (Marino and Caballero, 2008)
Clinical experience in psychiatric patients indicates that paliperidone might be the easiest of these drugs for patients to tolerate. Paliperidone inhibition of a given receptor is permanent for the life of that particular 5-HT7 receptor. This drug is the least likely to cause akathesia or parkinsonian problems. Oros® delivery system offers smoother blood levels.
Paliperidone disadvantages
Is still proprietary and by far the most expensive. As the newest we have least experience with it.
Each drug seems to have its advantage. Nothing will replace empirical testing. Although pimozide has higher affinity to 5-HT7, risperidone and paliperidone even with slightly lower binding affinity might be more effective in inhibiting 5-HT7 because of the unique irreversible nature of their interaction with 5-HT7 (Smith et al., 2006).
Parenthetical notes on the special nature of il-6
Data showing autocrine IL-6 mediated growth promotion of glioblastoma was first shown in 1997 and 1998 (Candi et al., 1997; Goswami et al., 1998). By different techniques using glioblastoma biopsy tissue, primary ex vivo culture, and in vitro glioblastoma cell lines, IL-6 continues to be shown to be an active growth promoting signalling system (Liu et al., 2010).
Interleukin-6 is a 26 kDa multifunctional cytokine synthesized by many different cell types, of relevance here prominently so by monocyte lineage cells, glia and the cells and vasculature of glioblastoma as outlined and referenced below. The important role of IL-6 in glioblastoma growth was reviewed in 2006 with suggested drugs then to lower IL-6 signalling (Kast and Altschuler, 2006).
Evidence continues to accrue since then attesting to IL-6's role in glioblastoma's growth and determining some of glioblastoma's attributes. Lieb et al. (2005) documented that agonists at 5-HT7 stimulated IL-6 synthesis and release from human glioblastoma cell line U373MG and in partial confirmation of the predictions of this paper; pimozide was shown to block this in vitro 5-HT7-induced IL-6 increase (Lieb et al., 2005).
In vitro exposure of microglia cell line MC-3 to serotonin also results in increased synthesis and secretion of IL-6 via agonism at 5-HT7 (Mahéet al., 2004). Fresh, primary resected human glioblastoma tissue has a disproportionatly high number of IL-6 receptors (Kudo et al., 2009). Antibody to IL-6 receptors inhibited in vitro growth of U87MG glioblastoma cell line (Kudo et al., 2009).
Glioblastoma patients' circulating monocytes are more active in IL-6 synthesis than are normal monocytes (Samaras et al., 2007; 2009;). IL-6 driven up-regulation of vascular endothelial growth factor, VEGF, goes through a signal transducer and activator of transcription-3, STAT-3, mediated step (Loeffler et al., 2005) leading to VEGF promoter's stimulation (Choi et al., 2002; Brantley and Benveniste, 2008). In the glioblastoma cell line U-251 STAT-3 activation was shown to be secondary to autocrine IL-6 signalling (Rahaman et al., 2002). STAT-3 is so uniformly up-regulated in glioblastoma tissue that it has been called a growth factor signalling hub (Brantley and Benveniste, 2008).
Resected glioblastoma tissue is strongly positive for IL-6 by immunohistochemistry (Samaras et al., 2007; 2009;) and elisa on tissue homogenates (Choi et al., 2002). Correspondingly high amplification of IL-6 gene was noted in half of surgical glioblastoma specimens (Tchirkov et al., 2001; Samaras et al., 2009), and that half has significantly shorter survival than did the non-amplified half (Tchirkov et al., 2001; Samaras et al., 2009). IL-6 gene amplification and protein overexpression was independently found also in half of cases examined (Sasaki et al., 2001).
Independent concordant immunohistochemical documentation of increased IL-6 protein was seen in about half of all cases (Chang et al., 2005) with average survival in that group of 7 months compared with 16 months in IL-6 immunohistochemical negative group (Chang et al., 2005).
The direct relationship between intracellular cAMP and IL-6 synthesis has been extensively reviewed (Kast, 2000; 2005; 2007;). The relationship has been documented specifically in glioblastoma cells (Lin et al., 2009). Because serotonin stimulates the Gs-coupled 5-HT7 receptor, stimulation of IL-6 by 5-HT7 agonists was to be expected (Kast, 2007). There are many paths to stimulate adenylate cyclase. Perhaps all of them must be blocked for effective control of IL-6.
Earlier efforts to find paths to lower IL-6 in glioblastoma focused on reducing the component of increased IL-6 in glioblastoma contributed by histaminergic agonism at H1 receptors (Kast and Altschuler, 2006; Kast, 2007) using potent H1 receptor blockers (technically they are inverse agonists) currently in wide clinical use – doxopin, mirtazapine or olanzapine (Kast and Altschuler, 2006; Kast, 2007).
Conclusion
That targeting of specific growth factors utilized by a specific cancer in a specific patient will improve outcomes is a current line of thinking (Kast and Altschuler, 2006; Kudo et al., 2009) to which this paper contributes.
We do not know the dosing that will shut down glioblastoma 5-HT7 signalling enough to be of clinical use. Patients with psychosis treated with risperidone at the lower end of its dosing range, 3.5 mg per day, showed 50% to 70% dopamine receptor(2) occupancy and close to 100% 5-HT2A occupancy (Reimold et al., 2007). Although inhibition of 5-HT7 function by paliperidone, pimozide or risperidone is unlikely to have profound growth retarding or prognosis improving effects given the large number and redundant intersecting nature of growth promoting systems active in glioblastoma, the benign and reversible nature of expected side effects warrant a clinical trial if rodent study confirms activity in growth slowing by 5-HT7 inhibition.
Glossary
Abbreviations
- 5-HT
serotonin
- 5-HT7
the serotonin receptor 7
- cAMP
cyclic ademosine monophosphate
- ERK1/2
extracellular regulated kinase 1/2
- H1
histamine receptor 1
- IL-6
interleukin-6
- STAT-3
signal transducer and activator of transcription-3
- VEGF
vascular endothelial growth factor
Conflict of interest
The author has no conflict of interest in any matter related to this work.
Supporting Information
Supporting Information: Teaching Materials; Fig 1 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. see specifically S11 through S13 for comprehensive database and references to 5-HT7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, et al. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112:2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari A, Larocca LM, Deaglio S, Finisguerra V, Boe A, Raggi C, et al. Transferrin receptor 2 is frequently and highly expressed in glioblastomas. Transl Oncol. 2010;3:123–134. doi: 10.1593/tlo.09274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Knight RA, Spinedi A, Guerrieri P, Melino G. A possible growth factor role of IL-6 in neuroectodermal tumours. J Neurooncol. 1997;31:115–122. doi: 10.1023/a:1005706019048. [DOI] [PubMed] [Google Scholar]
- Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12:930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Choi C, Gillespie GY, Van Wagoner NJ, Benveniste EN. Fas engagement increases expression of IL-6 in human glioma cells. J Neurooncol. 2002;56:13–19. doi: 10.1023/a:1014467626314. [DOI] [PubMed] [Google Scholar]
- Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- Gessi S, Sacchetto V, Fogli E, Merighi S, Varani K, Baraldi PG, et al. Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A(3) adenosine receptors. Biochem Pharmacol. 2010;79:1483–1495. doi: 10.1016/j.bcp.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Goswami S, Gupta A, Sharma SK. IL-6-mediated autocrine growth promotion in human glioblastoma multiforme cell line U87MG. J Neurochem. 1998;71:1837–1845. doi: 10.1046/j.1471-4159.1998.71051837.x. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. Identification of 5-hydroxytryptamine receptors positively coupled to adenylyl cyclase in rat cultured astrocytes. Br J Pharmacol. 1997;120:509–515. doi: 10.1038/sj.bjp.0700921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juillerat-Jeanneret L, Celerier J, Chapuis Bernasconi C, Nguyen G, Wostl W, Maerki HP, et al. Renin and angiotensinogen expression and functions in growth and apoptosis of human glioblastoma. Br J Cancer. 2004;90:1059–1068. doi: 10.1038/sj.bjc.6601646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast RE. Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int J Immunopharmacol. 2000;22:1001–1006. doi: 10.1016/s0192-0561(00)00046-1. [DOI] [PubMed] [Google Scholar]
- Kast RE. Evidence of a mechanism by which etanercept increased TNF-alpha in multiple myeloma: new insights into the biology of TNF-alpha giving new treatment opportunities – the role of bupropion. Leuk Res. 2005;29:1459–1463. doi: 10.1016/j.leukres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Kast RE. Melanoma inhibition by cyclooxygenase inhibitors: role of interleukin-6 suppression, a putative mechanism of action, and clinical implications. Med Oncol. 2007;24:1–6. doi: 10.1007/BF02685897. [DOI] [PubMed] [Google Scholar]
- Kast RE. Why cerebellar glioblastoma is rare and how that indicates adjunctive use of the FDA approved antiemetic aprepitant might retard cerebral glioblastoma growth: a new hypothesis answering an old question. Clin Transl. Oncology. 2009;11:408–410. doi: 10.1007/s12094-009-0379-x. [DOI] [PubMed] [Google Scholar]
- Kast RE. Glioblastoma: synergy of growth promotion between CCL5 and NK-1R can be thwarted by blocking CCL5 with miraviroc, an FDA approved anti-HIV drug and blocking NK-1R with aprepitant, an FDA approved anti-nausea drug. J Clin Pharm Ther. 2010 doi: 10.1111/j.1365-2710.2009.01148.x. DOI: 10.1111/j.1365-2710.2009.01148.x [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kast RE, Altschuler EL. Current drugs available now for interleukin-6 suppression as treatment adjunct in gllioblastoma: anakinra, aprepitant, mirtazapine and olanzapine. Int J Cancer Res. 2006;2:303–314. [Google Scholar]
- Kim JY, Kim YJ, Lee S, Park JH. The critical role of ERK in death resistance and invasiveness of hypoxia selected glioblastoma cells. BMC Cancer. 2009;9:27. doi: 10.1186/1471-2407-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Jono H, Shinriki S, Yano S, Nakamura H, Makino K, et al. Antitumor effect of humanized anti-interleukin-6 receptor antibody (tocilizumab) on glioma cell proliferation. J Neurosurg. 2009;111:219–225. doi: 10.3171/2008.12.JNS081284. [DOI] [PubMed] [Google Scholar]
- Lieb K, Biersack L, Waschbisch A, Orlikowski S, Akundi RS, Candelario-Jalil E, et al. Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J Neurochem. 2005;93:549–559. doi: 10.1111/j.1471-4159.2005.03079.x. [DOI] [PubMed] [Google Scholar]
- Lin HY, Sun M, Tang HY, Lin C, Luidens MK, Mousa SA, et al. L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol. 2009;296:C980–C991. doi: 10.1152/ajpcell.00305.2008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Li G, Li R, Shen J, He Q, Deng L, et al. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol. 2010 doi: 10.1007/s11060-010-0158-0. Epub ahead of print] PubMed PMID: 20361349. [DOI] [PubMed] [Google Scholar]
- Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- Loew S, Schmidt U, Unterberg A, Halatsch ME. The epidermal growth factor receptor as a therapeutic target in glioblastoma multiforme and other malignant neoplasms. Anticancer Agents Med Chem. 2009;9:703–715. doi: 10.2174/187152009788680019. [DOI] [PubMed] [Google Scholar]
- Lopez-Gines C, Gil-Benso R, Benito R, Mata M, Pereda J, Sastre J, et al. The activation of ERK1/2 MAP kinases in glioblastoma pathobiology and its relationship with EGFR amplification. Neuropathology. 2008;28:507–515. doi: 10.1111/j.1440-1789.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- Mahé C, Bernhard M, Bobirnac I, Keser C, Loetscher E, Feuerbach D, et al. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br J Pharmacol. 2004;143:404–410. doi: 10.1038/sj.bjp.0705936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino J, Caballero J. Paliperidone extended-release for the treatment of schizophrenia. Pharmacotherapy. 2008;28:1283–1298. doi: 10.1592/phco.28.10.1283. [DOI] [PubMed] [Google Scholar]
- Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, et al. Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. J Neuroimmunol. 2008;199:35–45. doi: 10.1016/j.jneuroim.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Möller HJ. Risperidone: a review. Expert Opin Pharmacother. 2005;6:803–818. doi: 10.1517/14656566.6.5.803. [DOI] [PubMed] [Google Scholar]
- Opler LA, Feinberg SS. The role of pimozide in clinical psychiatry: a review. J Clin Psychiatry. 1991;52:221–233. [PubMed] [Google Scholar]
- Owens DG. Extrapyramidal side effects and tolerability of risperidone: a review. J Clin Psychiatry. 1994;55:29–35. [PubMed] [Google Scholar]
- Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G. 5-HT7 receptor ligands: recent developments and potential therapeutic applications. Mini Rev Med Chem. 2007;7:945–960. doi: 10.2174/138955707781662663. [DOI] [PubMed] [Google Scholar]
- Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, et al. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- Reimold M, Solbach C, Noda S, Schaefer JE, Bartels M, Beneke M, et al. Occupancy of dopamine D(1), D (2) and serotonin(2A) receptors in schizophrenic patients treated with flupentixol in comparison with risperidone and haloperidol. Psychopharmacology (Berl) 2007;190:241–249. doi: 10.1007/s00213-006-0611-0. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, et al. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Samaras V, Piperi C, Korkolopoulou P, Zisakis A, Levidou G, Themistocleous MS, et al. Application of the ELISPOT method for comparative analysis of IL-6 and IL-10 secretion in peripheral blood of patients with astroglial tumors. Mol Cell Biochem. 2007;304:343–351. doi: 10.1007/s11010-007-9517-3. [DOI] [PubMed] [Google Scholar]
- Samaras V, Piperi C, Levidou G, Zisakis A, Kavantzas N, Themistocleous MS, et al. Analysis of interleukin (IL)-8 expression in human astrocytomas: associations with IL-6, COX-2, VEGF, and microvessel morphometry. Hum Immunol. 2009;70:391–397. doi: 10.1016/j.humimm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ishiuchi S, Kanda T, Hasegawa M, Nakazato Y. Analysis of interleukin-6 gene expression in primary human gliomas, glioblastoma xenografts, and glioblastoma cell lines. Brain Tumor Pathol. 2001;18:13–21. doi: 10.1007/BF02478920. [DOI] [PubMed] [Google Scholar]
- Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol. 2006;70:1264–1270. doi: 10.1124/mol.106.024612. [DOI] [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007;21:98–111. doi: 10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Böck BC, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27:6646–6656. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- Tchirkov A, Rolhion C, Bertrand S, Doré JF, Dubost JJ, Verrelle P. IL-6 gene amplification and expression in human glioblastomas. Br J Cancer. 2001;85:518–522. doi: 10.1054/bjoc.2001.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Hagan JJ. 5-HT7 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- Tueth MJ, Cheong JA. Clinical uses of pimozide. South Med J. 1993;86:344–349. doi: 10.1097/00007611-199303000-00019. [DOI] [PubMed] [Google Scholar]
- Xu L, Fukumura D, Jain RK. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J Biol Chem. 2002;277:11368–11374. doi: 10.1074/jbc.M108347200. [DOI] [PubMed] [Google Scholar]
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M. Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res. 2009;29:119–123. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


