Abstract
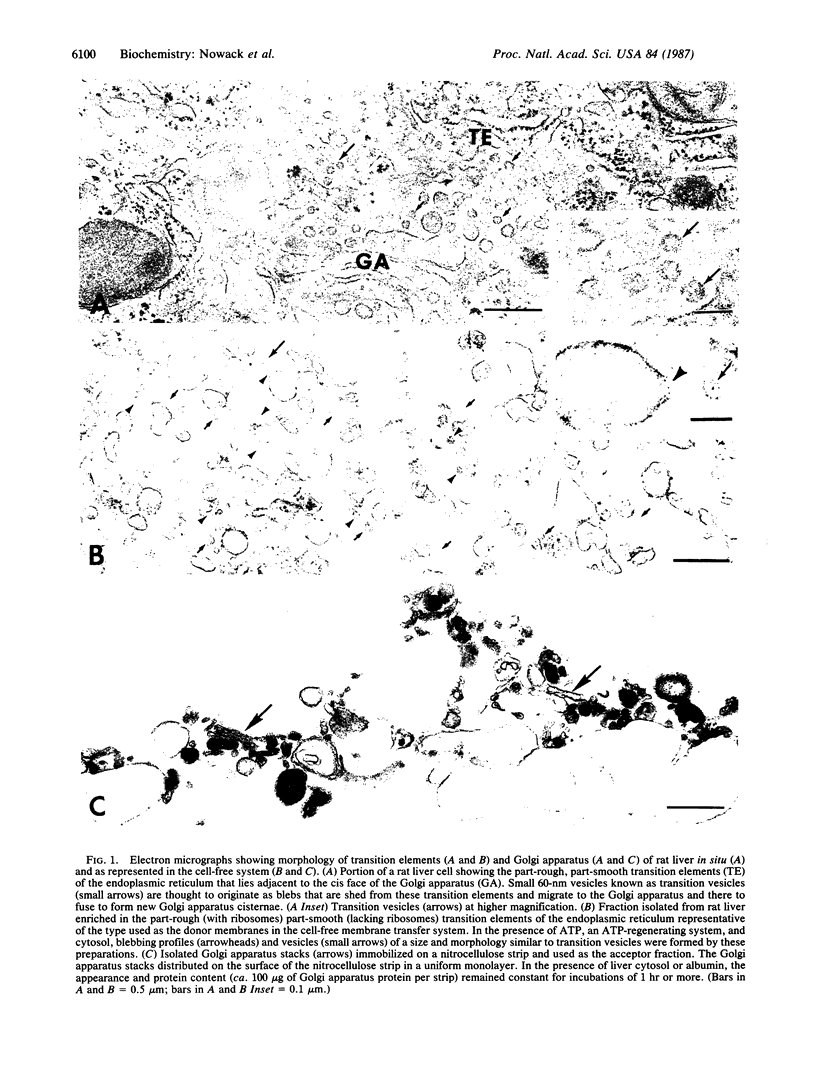
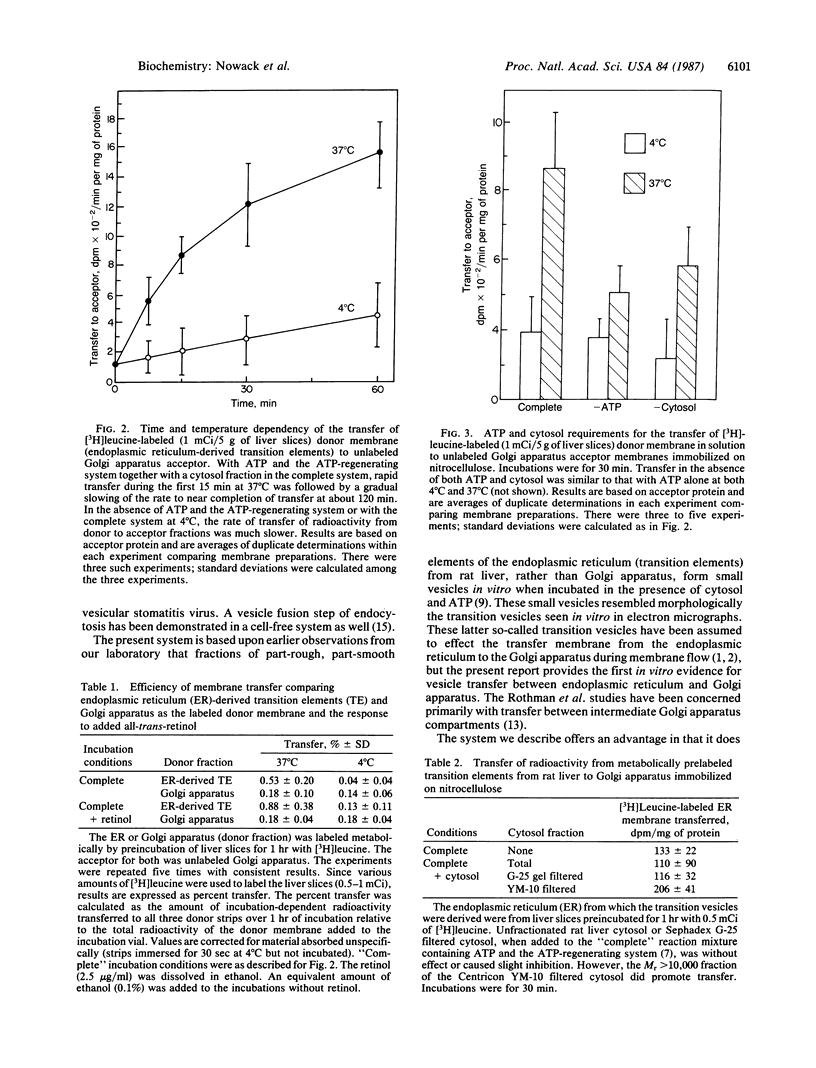
Transfer of membrane between endoplasmic reticulum and Golgi apparatus in situ is considered to occur via 60-nm transition vesicles derived from part-rough, part-smooth transition elements of the endoplasmic reticulum. A procedure is described for the isolation of a fraction enriched in these transition elements from rat liver. The isolated fraction generates small vesicles morphologically resembling transition vesicles when incubated with nucleoside triphosphate at 37 degrees C. In the cell-free system consisting of a donor fraction enriched in transition elements and an acceptor fraction consisting of intact Golgi apparatus immobilized on nitrocellulose strips, transfer in vitro of radiolabeled membranes was demonstrated. Nucleoside triphosphates were required for transfer, and transfer was facilitated by a cytosol fraction of Mr greater than 10,000. In the presence of both nucleoside triphosphate and cytosol, radiolabeled proteins were transferred in a manner dependent upon both time and temperature. Transfer appeared to be both vectorial and specific in that, with Golgi apparatus (or endoplasmic reticulum) as both donor and acceptor, only negligible time and temperature-dependent transfer was observed. The test system described is expected to facilitate further investigation of the transfer process and to provide a convenient assay to guide transition vesicle isolation and characterization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze E. M., Morré D. J., Morré D. M., Kartenbeck J., Franke W. W. Distribution of clathrin and spiny-coated vesicles on membranes within mature Golgi apparatus elements of mouse liver. Eur J Cell Biol. 1982 Aug;28(1):130–138. [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmental organization of the Golgi stack. Cell. 1985 Aug;42(1):13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Fries E., Rothman J. E. Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3870–3874. doi: 10.1073/pnas.77.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Cheetham R. D., Nyquist S. E. A simplified procedure for isolation of golgi apparatus from rat liver. Prep Biochem. 1972;2(1):61–69. doi: 10.1080/00327487208061453. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Kartenbeck J., Franke W. W. Membrane flow and intercoversions among endomembranes. Biochim Biophys Acta. 1979 Apr 23;559(1):71–52. doi: 10.1016/0304-4157(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Orci L., Glick B. S., Rothman J. E. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986 Jul 18;46(2):171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Pâquet M. R., Pfeffer S. R., Burczak J. D., Glick B. S., Rothman J. E. Components responsible for transport between successive Golgi cisternae are highly conserved in evolution. J Biol Chem. 1986 Apr 5;261(10):4367–4370. [PubMed] [Google Scholar]
- ZEIGEL R. F., DALTON A. J. Speculations based on the morphology of the Golgi systems in several types of proteinsecreting cells. J Cell Biol. 1962 Oct;15:45–54. doi: 10.1083/jcb.15.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]