Abstract
BACKGROUND AND PURPOSE
Constitutive activation of the signal transducer and activator of transcription 3 (STAT3) pathway is frequently encountered in several human cancers including multiple myeloma (MM). Thus, agents that suppress STAT3 phosphorylation have a potential for treatment of MM. In the present report, we investigated whether thymoquinone (TQ), the main component isolated from the medicinal plant Nigella sativa, modulated the STAT3 signalling pathway in MM cells.
EXPERIMENTAL APPROACH
The effect of TQ on both constitutive and IL-6-induced STAT3 activation, associated protein kinases, STAT3-regulated gene products involved in proliferation, survival and angiogenesis, cellular proliferation and apoptosis in MM cells, was investigated.
KEY RESULTS
We found that TQ inhibited both constitutive and IL-6-inducible STAT3 phosphorylation which correlated with the inhibition of c-Src and JAK2 activation. Vanadate reversed the TQ-induced down-regulation of STAT3 activation, suggesting the involvement of a protein tyrosine phosphatase. Indeed, we found that TQ can induce the expression of Src homology-2 phosphatase 2 that correlated with suppression of STAT3 activation. TQ also down-regulated the expression of STAT3-regulated gene products, such as cyclin D1, Bcl-2, Bcl-xL, survivin, Mcl-1 and vascular endothelial growth factor. Finally, TQ induced the accumulation of cells in sub-G1 phase, inhibited proliferation and induced apoptosis, as indicated by poly ADP ribose polymerase cleavage. TQ also significantly potentiated the apoptotic effects of thalidomide and bortezomib in MM cells.
CONCLUSIONS AND IMPLICATIONS
Our study has identified STAT3 signalling as a target of TQ and has thus raised its potential application in the prevention and treatment of MM and other cancers.
Keywords: STAT3, thymoquinone, multiple myeloma, proliferation
Introduction
Thymoquinone (TQ) is a major bioactive constituent of the volatile oil of black seed (Nigella sativa) which has been shown to exert anti-inflammatory, antioxidant and anti-neoplastic effects both in vitro and in vivo. For example, it has been shown to inhibit the proliferation of a wide variety of tumour cells including breast adenocarcinoma, ovarian adenocarcinoma (Shoieb et al., 2003), colorectal cancer (Gali-Muhtasib et al., 2004a), human pancreatic adenocarcinoma, neoplastic keratinocytes (Worthen et al., 1998), human osteosarcoma (Roepke et al., 2007), fibrosarcoma, lung carcinoma (Kaseb et al., 2007) and myeloblastic leukemia (El-Mahdy et al., 2005). In animal models, TQ has also been shown to have promising anti-tumour effects (Salem, 2005; Gali-Muhtasib et al., 2006). It inhibited the incidence of fibrosarcoma tumours in mice induced with 20-methylcholanthrene (Badary and Gamal El-Din, 2001), as well as forestomach tumours induced with benzo(a)pyrene (Badary et al., 1999). TQ has been shown to attenuate ifosfamide-induced Fanconi syndrome in rats and to enhance its anti-tumour activity in mice (Badary, 1999). Moreover, TQ recently has shown to augment the anti-tumour activity of gemcitabine and oxaliplatin in an orthotopic model of pancreatic cancer (Banerjee et al., 2009). How TQ exerts these activities is not completely understood, but it has been shown to down-regulate the expression of pro-inflammatory and proliferative mediators such as COX-2 (El Mezayen et al., 2006), inducible NOS (El-Mahmoudy et al., 2002), 5-lipooxygenase (El-Dakhakhny et al., 2002), tumour necrosis factor (TNF) (El-Mahmoudy et al., 2005) and cyclin D1 (Gali-Muhtasib et al., 2004b), and inhibit the activation of transcription factor NF-κB (Sethi et al., 2008), Akt and extracellular signal-regulated kinase (ERK) signalling pathways (Yi et al., 2008).
Signal transducer and activator of transcription (STAT) proteins have been shown to play an important role in tumour cell survival and proliferation (Yu et al., 2002; Yu and Jove, 2004; Aggarwal et al., 2006; Yue and Turkson, 2009). The activation of STATs involves the phosphorylation of a critical tyrosine residue by Janus kinases (JAKs) or the Src family kinases, leading to dimerization of STAT monomers, nuclear translocation and binding to specific DNA response elements in the promoters of target genes (Bowman et al., 2000; Brierley and Fish, 2005). Among the STATs, STAT3 is perhaps the most closely linked to tumourigenesis, is often constitutively activated in many human cancer cells and plays an active role at all levels of tumourigenesis (Ihle, 1996; Darnell, 2002). STAT3 is responsible for generating pro-proliferative signals and has been shown to up-regulate anti-apoptotic proteins (e.g. Bcl-xL, Bcl-2, survivin, Mcl-1) (Costantino and Barlocco, 2008; Aggarwal et al., 2009). In addition, STAT3 can control vascular endothelial growth factor (VEGF) expression, which is necessary for angiogenesis and the maintenance of tumour vasculature (Niu et al., 2002). Finally, STAT3 has been implicated in the inhibition of immune responses to tumour growth by blocking expression of pro-inflammatory factors (Wang et al., 2004).
On the basis of these critical roles of STAT3 in tumour progression and survival, we hypothesized that TQ may mediate its effects in part through the suppression of the STAT3 pathway. We found that TQ could indeed suppress both constitutive, as well as inducible STAT3 expression in multiple myeloma (MM) cells. This correlated with down-regulation of expression of cell survival, proliferative and angiogenic gene products, leading to suppression of proliferation and enhancement of apoptosis induced by thalidomide and bortezomib.
Methods
Cell lines
Human MM cell lines U266 and RPMI 8226 were a kind gift from Dr Chng Wee Joo at National University Hospital, Singapore. U266 and RPMI 8226 cells were cultured in RPMI 1640 medium containing 1× antibiotic–anti-mycotic solution with 10% fetal bovine serum (FBS). Human embryonic kidney A293 cells were a kind gift from Dr Bharat B. Aggarwal at M D Anderson Cancer Center (Houston, TX, USA). A293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 1× antibiotic–anti-mycotic solution with 10% FBS. Wild-type and STAT3 knock-out mouse fibroblasts were a kind gift from Dr Valeria Poli, University of Turin, Turin, Italy.
Western blotting
For detection of phospho-proteins, TQ-treated whole-cell extracts were lysed in lysis buffer [20 mM Tris (pH 7.4), 250 mM NaCl, 2 mM EDTA (pH 8.0), 0.1% Triton X-100, 0.01 mg·mL−1 aprotinin, 0.005 mg·mL−1 leupeptin, 0.4 mM PMSF and 4 mM NaVO4]. Lysates were then centrifuged at 15 800×g for 10 min to remove insoluble material, and resolved by a 7.5% SDS gel. After electrophoresis, the proteins were electrotransferred to a nitrocellulose membrane, blocked with 5% non-fat milk and probed with anti-STAT antibodies (1:1000) overnight at 4°C. The blot was washed, exposed to horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h and finally examined by chemiluminescence (ECL; GE Healthcare, Little Chalfont, Buckinghamshire, UK).
To detect STAT3-regulated proteins and poly ADP ribose polymerase (PARP), U266 cells (2 × 106 mL−1) were treated with 50 µM TQ for the indicated times. The cells were then washed and extracted by incubation for 30 min on ice in 0.05 mL buffer containing 20 mM HEPES, pH 7.4, 2 mM EDTA, 250 mM NaCl, 0.1% NP-40, 2 µg·mL−1 leupeptin, 2 µg·mL−1 aprotinin, 1 mM PMSF, 0.5 µg·mL−1 benzamidine, 1 mM dithiothreitol and 1 mM sodium vanadate. The lysate was centrifuged and the supernatant was collected. Whole-cell extract protein (30 µg) was resolved by 12% SDS–PAGE, electrotransferred onto a nitrocellulose membrane, blotted with various antibodies and then detected by chemiluminescence (ECL; Amersham).
Immunocytochemistry for STAT3 localization
TQ-treated U266 cells were placed on a glass slide by centrifugation using a Cytospin (Thermo Fisher Scientific, Asheville, NC, USA), air-dried for 1 h at room temperature and fixed with cold acetone. After a brief washing in phosphate-buffered saline (PBS), the slides were blocked with 5% normal goat serum for 1 h and then incubated with rabbit polyclonal anti-human STAT3 antibody (dilution, 1/100). After an overnight incubation, the cells were washed and then incubated with goat anti-rabbit IgG-Alexa 594 (1/100) for 1 h and counterstained for nuclei with Hoechst 33342 (50 ng·mL−1) for 5 min. Stained cells were mounted with a mounting medium (Sigma-Aldrich, St Louis, MO, USA) and analysed under a fluorescence microscope (Olympus DP 70, Tokyo, Japan).
STAT3 luciferase reporter assay
For ease of transfection, A293 cells were used for STAT3 luciferase reporter assay. A293 cells were plated in 96-well plates with 1 × 104 cells per well in DMEM containing 10% FBS. The STAT3-responsive elements linked to a luciferase reporter gene were transfected with wild-type or dominant-negative STAT3-Y705F (STAT3F). These plasmids were a kind gift from Dr Bharat B. Aggarwal at M D Anderson Cancer Center. Transfections were done according to the manufacturer's protocols using FuGENE 6 (Roche, Indianapolis, IN, USA). At 24 h post-transfection, cells were pretreated with indicated concentrations of TQ for 4 h, and then induced by IL-6 for additional 24 h before being washed and lysed in luciferase lysis buffer (Promega, Madison, WI, USA). Luciferase activity was measured with a luminometer by using a luciferase assay kit (Promega). All luciferase experiments were performed in triplicate and repeated three or more times. The data are shown as the mean and the SD.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
The anti-proliferative effect of TQ against MM cells was determined by the MTT dye uptake method as described previously (Sethi et al., 2008). Briefly, the cells (5 × 103 mL−1) were incubated in triplicate in a 96-well plate in the presence or absence of indicated concentrations of TQ in a final volume of 0.2 mL for different time intervals at 37°C. Thereafter, 20 µL MTT solution (5 mg·mL−1 in PBS) was added to each well. After a 2 h incubation at 37°C, 0.1 mL lysis buffer (20% SDS, 50% dimethylformamide) was added; incubation was continued overnight at 37°C, and then the optical density at 570 nm was measured by Tecan plate reader (Durham, NC, USA).
Flow cytometric analysis
To determine the effect of TQ on the cell cycle, U266 cells were first synchronized by serum starvation and then exposed to TQ in the presence of serum for the indicated time intervals. Thereafter, the cells were washed, fixed with 70% ethanol and incubated for 30 min at 37°C with 0.1% RNase A in PBS. The cells were then washed again, resuspended and stained in PBS containing 50 µg·mL−1 propidium iodide (PI) for 30 min at room temperature. Cell distribution across the cell cycle was analysed with CyAn ADP flow cytometer (Dako Cytomation, Carpinteria, CA, USA).
Transfection with constitutive STAT3 construct
A293 cells were plated in chamber slides in DMEM containing 10% FBS. After 24 h, the cells were transfected with constitutive STAT3 plasmid by FuGENE 6 according to the manufacturer's protocol (Roche). The cells were treated with TQ for 24 h, and viability of the cells was determined by LIVE/DEAD assay (see below). STAT3 constitutive plasmid that has been described before (Zhang et al., 2002b; Lufei et al., 2007) was a kind gift from Dr Xinmin Cao.
LIVE/DEAD Assay
Apoptosis of cells was also determined by LIVE/DEAD assay (Molecular Probes, Eugene, OR, USA) that measures intracellular esterase activity and plasma membrane integrity as described previously (Bhutani et al., 2007). Briefly, 1 × 106 cells were incubated with TQ/thalidomide/bortezomib alone or in combination for 24 h at 37°C. The cells were stained with the LIVE/DEAD reagent (5 µM ethidium homodimer, 5 µM calcein-AM) and then incubated at 37°C for 30 min. The cells were analysed under a fluorescence microscope (Olympus DP 70).
Statistical analysis
Statistical analysis was performed by one-way anova. P value less than 0.05 was considered statistically significant.
Materials
TQ, Hoechst 33342, MTT, Tris, glycine, NaCl, SDS, BSA and thalidomide were purchased from Sigma-Aldrich. TQ was dissolved in dimethylsulphoxide as a 10 mM stock solution and stored at 4°C. Further dilution was done in cell culture medium. RPMI 1640, FBS, 0.4% Trypan blue vital stain, antibiotic–anti-mycotic mixture and LIVE/DEAD assay kit were obtained from Invitrogen (Carlsbad, CA, USA). Rabbit polyclonal antibodies to STAT3 and STAT5, and antibodies against phospho-STAT3 (Tyr 705) and phospho-STAT5 (Tyr 694), phosphor-Akt, phospho-Erk, Erk2, Akt, Bcl-2, Bcl-xL, cyclin D1, SH-PTP2, survivin, Mcl-1, VEGF, procaspase-3 and PARP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to phospho-specific Src (Tyr 416), Src, phospho-specific JAK2 (Tyr 1007/1008) and JAK2 were purchased from Cell Signaling Technology (Beverly, MA, USA). Goat anti-rabbit–HRP conjugate and goat anti-mouse HRP were purchased from Sigma-Aldrich. Bacteria-derived recombinant human IL-6 was purchased from ProSpec-Tany TechnoGene Ltd (Rehovot, Israel). Bortezomib was kindly provided by Dr Chng Wee Joo at National University Hospital.
Results
The present study was undertaken to determine the effect of TQ on STAT3 signalling pathway. We investigated the effect of TQ on both constitutive and IL-6-inducible STAT3 activation in MM cells. We also evaluated the effect of TQ on various mediators of cellular proliferation, cell survival and apoptosis. The structure of TQ is shown in Figure 1A. The dose and duration of TQ used to modulate STAT3 activation did not affect cell viability, indicating that down-regulation of STAT3 was not due to cell killing (data not shown).
Figure 1.
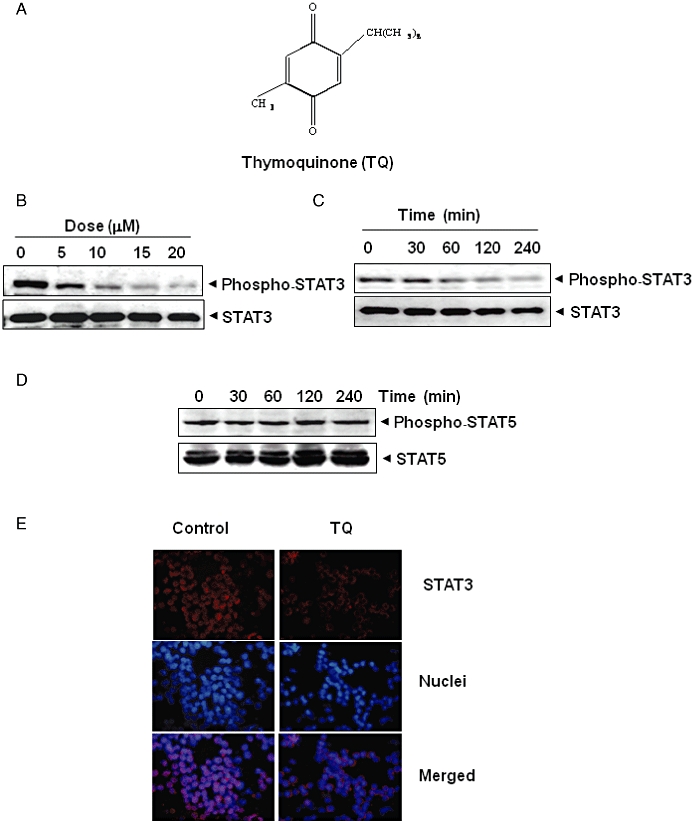
TQ inhibits constitutively active STAT3 in U266 cells. (A) The structure of TQ. (B) TQ suppressed phospho-STAT3 levels in a dose-dependent manner. U266 cells (2 × 106 mL−1) were treated with the indicated concentrations of TQ for 4 h, after which whole-cell extracts were prepared, and 30 µg of protein was resolved by 7.5% SDS–PAGE gel, electrotransferred onto nitrocellulose membranes and probed for phospho-STAT3. (C) TQ suppresses phospho-STAT3 levels in a time-dependent manner. U266 cells (2 × 106 mL−1) were treated with 15 µM TQ for the indicated times, after which Western blotting was performed as described for (B). The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (D) TQ has no effect on phospho-STAT5 and STAT5 protein expression. U266 cells (2 × 106 mL−1) were treated with 15 µM TQ for the indicated times. Whole-cell extracts were prepared, fractionated on SDS–PAGE and examined by Western blotting using antibodies against phospho-STAT5 and STAT5. (E) TQ causes inhibition of translocation of STAT3 to the nucleus. U266 cells (1 × 105 mL−1) were incubated with or without 15 µM TQ for 4 h, and then analysed for the intracellular distribution of STAT3 by immunocytochemistry. The same slides were counterstained for nuclei with Hoechst 33342 (50 ng·mL−1) for 5 min.
TQ inhibits constitutive STAT3 phosphorylation in MM cells
Whether TQ can modulate the constitutive STAT3 activation in MM cells was investigated. U266 cells were incubated with different concentrations of TQ for 4 h; whole-cell extracts were prepared and examined for phosphorylated STAT3 by Western blot analysis using antibodies which recognize STAT3 phosphorylated at tyrosine 705. As shown in Figure 1B, TQ inhibited the constitutive activation of STAT3 in U266 cells in a dose-dependent manner, with maximum inhibition occurring at around 15 µM. TQ had no effect on the expression of STAT3 protein (Figure 1B, lower panel). As shown in Figure 1C, the inhibition was time dependent, with maximum inhibition occurring at around 4 h, again with no effect on the expression of STAT3 protein (Figure 1C, lower panel).
Effect of TQ on STAT3 phosphorylation is specific
Whether TQ affects the activation of other STAT proteins in U266 cells was also investigated. Under the conditions where TQ completely inhibited STAT3 phosphorylation, it altered neither the levels of constitutively phosphorylated STAT5 nor the expression of STAT5 proteins (Figure 1D).
TQ depletes nuclear pool of STAT3 in MM cells
Because nuclear translocation is central to the function of transcription factors and because it is not certain whether phosphorylation is mandatory for nuclear transport of STAT3 and its oncogenic functions (Bowman et al., 2000; Brierley and Fish, 2005), we determined whether TQ suppresses nuclear translocation of STAT3. Figure 1E clearly demonstrates that TQ inhibited the translocation of STAT3 to the nucleus in U266 cells.
TQ inhibits inducible STAT3 phosphorylation in MM cells
Because IL-6 induces STAT3 phosphorylation (Kawano et al., 1988; Klein et al., 1995), we determined whether TQ could inhibit IL-6-induced STAT3 phosphorylation. RPMI 8226 cells that lack constitutively active STAT3 (Bharti et al., 2003) were treated with different concentrations of IL-6 and then examined for phosphorylated STAT3. IL-6 induced phosphorylation of STAT3 in a dose-dependent manner, with maximum activation observed at 10–50 ng·mL−1 (Figure 2A). IL-6 also induced phosphorylation of STAT3 in a time-dependent manner (Figure 2B). In RPMI 8226 cells incubated with TQ for different times, IL-6-induced STAT3 phosphorylation was suppressed in a time-dependent manner. Exposure of cells to TQ for 4–6 h was sufficient to completely suppress IL-6-induced STAT3 phosphorylation (Figure 2C).
Figure 2.
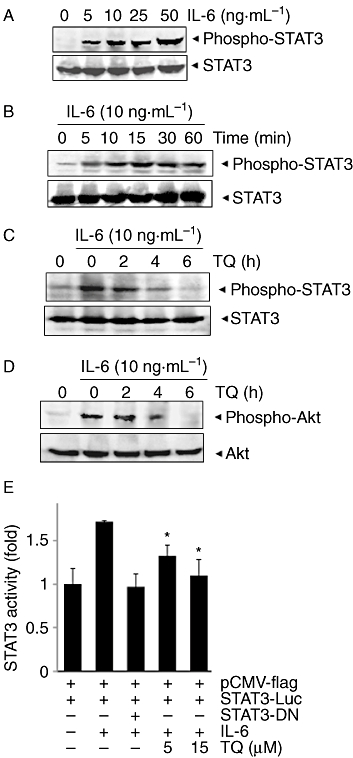
TQ down-regulates IL-6-induced phospho-STAT3. (A) RPMI 8266 cells (2 × 106 mL−1) were treated with indicated concentrations of IL-6 for 15 min, whole-cell extracts were prepared and phospho-STAT3 was detected by Western blot. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (B) RPMI 8266 cells (2 × 106 mL−1) were treated with IL-6 (10 ng·mL−1) for the indicated times, whole-cell extracts were prepared and phospho-STAT3 was detected by Western blot. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (C) RPMI 8266 cells (2 × 106 mL−1) were treated with 15 µM TQ for the indicated times, and then stimulated with IL-6 (10 ng·mL−1) for 15 min. Whole-cell extracts were then prepared and analysed for phospho-STAT3 by Western blotting. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. The results shown are representative of three independent experiments. (D) RPMI 8266 cells (2 × 106 mL−1) were treated with 15 µM TQ for the indicated times, and then stimulated with IL-6 (10 ng·mL−1) for 15 min. Whole-cell extracts were then prepared and analysed for phospho-Akt by Western blotting. The same blots were stripped and reprobed with Akt antibody to verify equal protein loading. The results shown are representative of three independent experiments. (E) A293 cells (5 × 105 mL−1) were transfected with STAT3-luciferase (STAT3-Luc) plasmid, incubated for 24 h and treated with 5 and 15 µM TQ for 4 h and then stimulated with IL-6 (10 ng·mL−1) for 24 h. Whole-cell extracts were then prepared and analysed for luciferase activity. The results shown are representative of three independent experiments. *P < 0.05.
TQ inhibits IL-6-inducible Akt phosphorylation in MM cells
Activated Akt has been shown to play a critical role in the mechanism of action of IL-6 (Tu et al., 2000). Hence, we also examined whether TQ could modulate IL-6-induced Akt activation. Treatment of RPMI 8226 cells with IL-6 induced phosphorylation of Akt, and pretreatment with TQ suppressed the activation in a time-dependent manner (Figure 2D). Under these conditions, TQ had no effect on the expression of Akt protein.
TQ suppresses IL-6-induced STAT3-dependent reporter gene expression
Our results showed that TQ inhibited the phosphorylation and nuclear translocation of STAT3. We next determined whether TQ affects STAT3-dependent gene transcription. When cells transiently transfected with the pSTAT3-Luc construct were stimulated with IL-6, STAT3-mediated luciferase gene expression significantly increased. Dominant-negative STAT3 blocked this increase, indicating specificity. When the cells were pretreated with TQ, IL-6-induced STAT3 activity was inhibited in a dose-dependent manner (Figure 2E).
TQ suppresses constitutive activation of c-Src
STAT3 has also been reported to be activated by soluble tyrosine kinases of the Src kinase families (Schreiner et al., 2002). Hence, we determined whether TQ affected the constitutive activation of Src kinase in U266 cells. We found that TQ suppressed the constitutive phosphorylation of c-Src kinases (Figure 3A). The levels of non-phosphorylated Src kinases remained unchanged under the same conditions.
Figure 3.
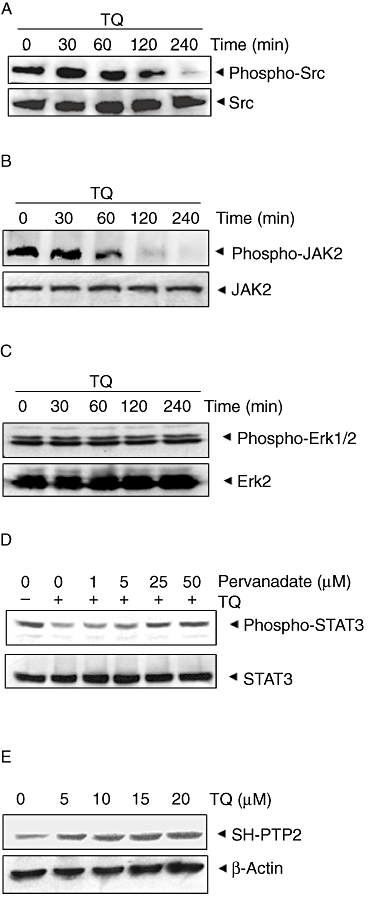
(A) TQ suppresses phospho-Src levels in a time-dependent manner. U266 cells (2 × 106 mL−1) were treated with 15 µM TQ, after which whole-cell extracts were prepared and 30 µg aliquots of those extracts were resolved by 10% SDS–PAGE, electrotransferred onto nitrocellulose membranes and probed for phospho-Src antibody. The same blots were stripped and reprobed with Src antibody to verify equal protein loading. (B) TQ suppresses phospho-JAK2 levels in a time-dependent manner. U266 cells (2 × 106 mL−1) were treated with 15 µM TQ for indicated time intervals, after which whole-cell extracts were prepared and 30 µg portions of those extracts were resolved by 10% SDS–PAGE, electrotransferred onto nitrocellulose membranes and probed for JAK2 antibody. The same blots were stripped and reprobed with JAK2 antibody to verify equal protein loading. (C) U266 cells (2 × 106 mL−1) were treated with 15 µM TQ for indicated time intervals, after which whole-cell extracts were prepared and 30 µg portions of those extracts were resolved by 10% SDS–PAGE, electrotransferred onto nitrocellulose membranes and probed for phospho-ERK1/2 antibody. The same blots were stripped and reprobed with ERK2 antibody to verify equal protein loading. (D) Pervanadate reverses the phospho-STAT3 inhibitory effect of TQ. U266 cells (2 × 106 mL−1) were treated with the indicated concentrations of pervanadate and 15 µM TQ for 4 h, after which whole-cell extracts were prepared and 30 µg portions of those extracts were resolved by 7.5% SDS–PAGE gel, electrotransferred onto nitrocellulose membranes and probed for phospho-STAT3 and STAT3. (E) TQ induces the expression of SH-PTP2 protein in a dose-dependent manner in U266 cells. U266 cells (2 × 106 mL−1) were treated with indicated concentrations of TQ for 4 h, after which whole-cell extracts were prepared and 30 µg portions of those extracts were resolved by 10% SDS–PAGE, electrotransferred onto nitrocellulose membranes and probed for SH-PTP2 antibody. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
TQ suppresses constitutive activation of JAK2
STAT3 has been reported to be activated by soluble tyrosine kinases of the Janus family (JAKs) (Ihle, 1996), so we determined whether TQ affects constitutive activation of JAK2 in U266 cells. We found that TQ suppressed the constitutive phosphorylation of JAK2 (Figure 3B). The levels of non-phosphorylated JAK2 remained unchanged under the same conditions (Figure 3B, bottom panel).
TQ does not affect constitutive activation of ERK
Apart from tyrosine phosphorylation, STAT3 also undergoes phosphorylation at serine residues. IL-6 can activate the mitogen-activated protein kinase (RAS/MAPK) pathway for STAT3 activation (Chung et al., 1997b). We therefore investigated whether TQ affects constitutive expression of p-ERK kinase in U266 cells. We found that TQ did not affect the constitutive activation of ERK kinase in U266 cells (Figure 3C). The levels of non-phosphorylated ERK2 also remained unchanged under the same conditions (Figure 3C, bottom panel).
Tyrosine phosphatases are involved in TQ-induced inhibition of STAT3 activation
Because protein tyrosine phosphatases (PTPases) have also been implicated in STAT3 activation (Han et al., 2006), we next determined whether TQ-induced inhibition of STAT3 tyrosine phosphorylation could be due to activation of a PTPase. Treatment of U266 cells with the broad-acting tyrosine phosphatase inhibitor, sodium pervanadate (Carballo et al., 1999), prevented the TQ-induced inhibition of STAT3 activation (Figure 3D). This suggests that tyrosine phosphatases are involved in TQ-induced inhibition of STAT3 activation.
TQ induces the expression of SH-PTP2 in MM cells
The PTP Src homology 2 phosphatase 2 (SH-PTP2) is a member of a small family of Src homology 2 (SH2) domain-containing PTPs, and has been reported to play an important role in the negative regulation of Jak/STAT signalling (Servidei et al., 1998; You et al., 1999; Ohtani et al., 2000). We therefore examined whether TQ can modulate expression of SH-PTP2 in U266 cells. Cells were incubated with different concentrations of TQ for 4 h; whole-cell extracts were prepared and examined for SH-PTP2 protein by Western blot analysis. As shown in Figure 3E, TQ induced the expression of SH-PTP2 protein in U266 cells in a dose-dependent manner, with maximum expression at 15–20 µM. This stimulation of SH-PTP2 expression by TQ correlated with down-regulation of constitutive STAT3 activation in U266 cells (Figure 1B).
TQ down-regulates the expression of cyclin D1, Bcl-2, Bcl-xL, surcivin, Mcl-1 and VEGF
STAT3 activation has been shown to regulate the expression of various gene products involved in cell survival, proliferation and angiogenesis (Aggarwal et al., 2006; Costantino and Barlocco, 2008). We found that expression of the cell cycle regulator cyclin D1; the anti-apoptotic proteins Bcl-2, Bcl-xL, survivin and Mcl-1; and the angiogenic gene product VEGF were modulated by TQ treatment. Their expression decreased in a time-dependent manner, with maximum suppression observed at around 36–48 h (Figure 4).
Figure 4.
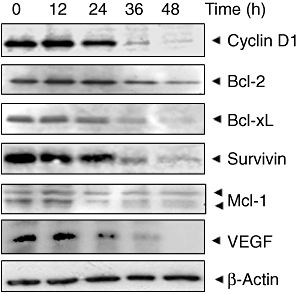
TQ suppresses STAT3-regulated gene products involved in proliferation, survival and angiogenesis. U266 cells (2 × 106 mL−1) were treated with 15 µM TQ for indicated time intervals, after which whole-cell extracts were prepared and 30 µg portions of those extracts were resolved by 10% SDS–PAGE; membrane sliced according to molecular weight; and probed against cyclin D1, Bcl-2, Bcl-XL, survivin, Mcl-1 and VEGF antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
TQ is a potent inhibitor of the proliferation of MM cells
Because TQ down-regulated the expression of cyclin D1, the gene critical for cell proliferation, we next investigated whether TQ affects the proliferation of MM cells by using the MTT method. We found that TQ inhibited the proliferation of both U266 and RPMI-8226 cells in a dose- and time-dependent manner (Figure 5A).
Figure 5.
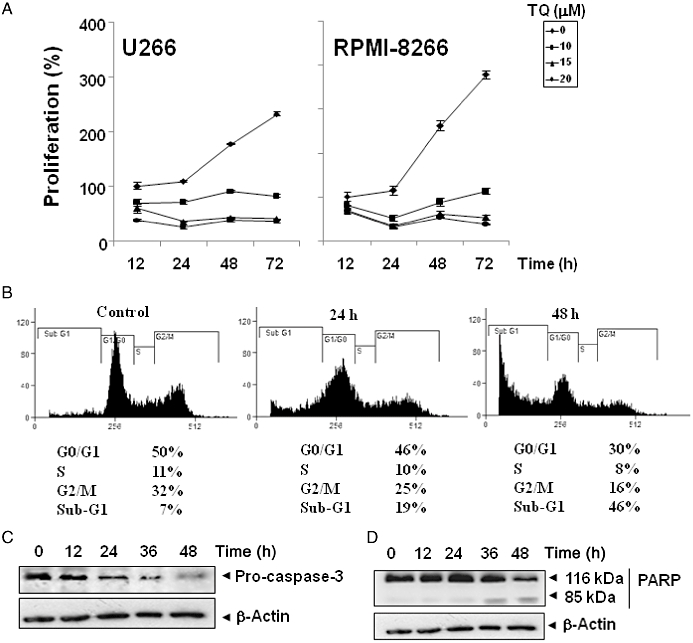
TQ suppresses proliferation, causes accumulation of cells in sub-G1 phase and activates caspase-3. (A) U266 and RPMI 8226 cells (5 × 103 mL−1) were plated in triplicate; treated with indicated concentrations of TQ; and then subjected to MTT assay after 12, 24, 48 and 72 h to analyse proliferation of cells. Standard deviations between the triplicates are indicated. (B) U266 cells (2 × 106 mL−1) were synchronized by incubation overnight in the absence of serum, and then treated with 15 µM TQ in the presence of serum for the indicated times, after which the cells were washed, fixed, stained with PI and analysed for DNA content by flow cytometry. (C) U266 cells were treated with 15 µM TQ for the indicated times; whole-cell extracts were prepared, separated by SDS–PAGE and subjected to Western blotting against pro-caspase-3 antibody. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. (D) U266 cells were treated with 15 µM TQ for the indicated times, and whole-cell extracts were prepared, separated by SDS–PAGE and subjected to Western blot against PARP antibody. The same blot was stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of three independent experiments.
TQ causes the accumulation of the cells in the sub-G1 phase of the cell cycle
Because D-type cyclins are required for the progression of cells from the G1 phase of the cell cycle to the S phase (Matsushime et al., 1991), and rapid decline in levels of cyclin D1 was observed in TQ-treated cells, we determined the effect of TQ on cell cycle phase distribution. We found that TQ caused increased accumulation of cell population in the sub-G1 phase. After 24 and 48 h, 19 and 46% of the cell population, respectively, had accumulated in sub-G1 phase, which is indicative of apoptosis (Figure 5B).
TQ activates caspase-3 and causes PARP cleavage
Whether suppression of constitutively active STAT3 in U266 cells by TQ leads to apoptosis was also investigated. In U266 cells treated with TQ, there was a time-dependent activation of pro-caspase-3 (Figure 5C). Activation of downstream pro-caspase-3 led to the cleavage of a 116 kDa PARP protein into an 85 kDa fragment (Figure 5D). These results clearly suggest that TQ induces caspase-3-dependent apoptosis in U266 cells.
Over-expression of constitutively active STAT3 rescues TQ-induced apoptosis
We assessed whether the over-expression of constitutive active STAT3 can rescue TQ-induced apoptosis. A293 cells were transfected with constitutively active STAT3 plasmid, incubated for 24 h, and the cells were thereafter treated with TQ for 24 h and examined for apoptosis by esterase staining assay. The results show that the forced expression of STAT3 significantly reduces the TQ-induced apoptosis (Figure 6A).
Figure 6.
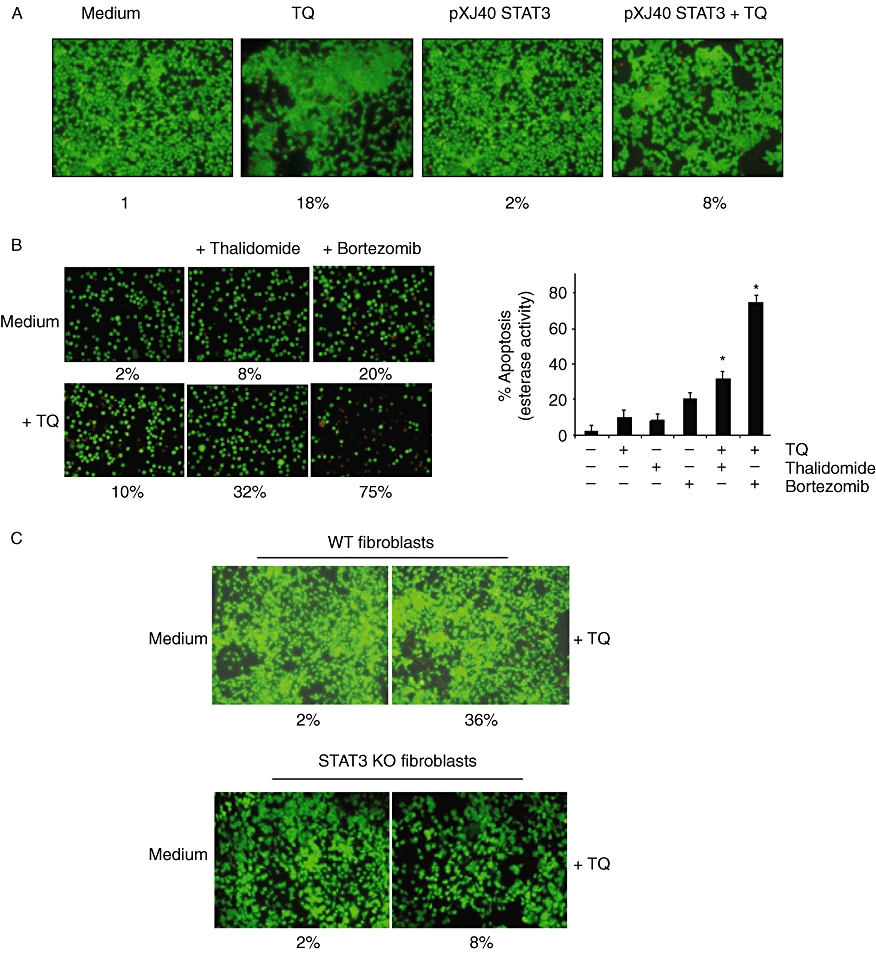
(A) Over-expression of constitutive STAT3 rescues A293 cells from TQ-induced apoptosis. First, A293 cells were transfected with constitutive STAT3 plasmid. After 24 h of transfection, the cells were treated with 15 µM TQ for 24 h, and then the apoptosis was determined by LIVE/DEAD assay and 20 random fields were counted. (B) TQ potentiates the apoptotic effect of thalidomide and bortezomib. U266 cells (1 × 106 mL−1) were treated with 5 µM TQ and 10 µg·mL−1 thalidomide or 20 nM bortezomib alone or in combination for 24 h at 37°C. The cells were stained with a LIVE/DEAD assay reagent for 30 min and then analysed under a fluorescence microscope. The % apoptosis has been plotted, and results shown are representative of three independent experiments. *P < 0.05. (C) Deletion of STAT3 inhibited the apoptotic effect of TQ. Wild-type and STAT3 deleted fibroblasts were treated with 15 µM TQ for 48 h and analysed for the percentage of apoptosis by LIVE/DEAD assay. The cells were stained with a LIVE/DEAD assay reagent for 30 min and then analysed under a fluorescence microscope.
TQ potentiates the apoptotic effect of thalidomide and bortezomib in MM cells
Thalidomide, an inhibitor of TNF expression, and bortezomib, an inhibitor of the proteasome, have been approved for the treatment of MM patients (Cavo, 2006; Glasmacher et al., 2006). Whether TQ can potentiate the effect of these drugs was examined. For this, U266 cells were treated with sub-optimal dose of TQ together with either thalidomide or bortezomib, and then examined for apoptosis by LIVE/DEAD assay, which determines plasma membrane stability, using esterase staining. As shown in Figure 6B, TQ significantly enhanced the apoptotic effects of thalidomide from 8 to 32%, and of bortezomib from 20 to 75%.
STAT3 deletion reduces TQ-induced apoptosis
We next determined the apoptotic effect of TQ on STAT3 gene deleted mouse embryonic fibroblasts that lack activation of STAT3. Apoptotic effects of TQ were measured through esterase staining (LIVE/DEAD) assay. Results shown in Figure 6C indicate that TQ-induced apoptosis was effectively abolished in the STAT3 gene deleted (8%) as compared to (36%) in wild-type fibroblasts. These results suggest that induction of apoptosis is mediated through the suppression of STAT3 by TQ.
Discussion and conclusion
The aim of this study was to determine whether TQ exerts its anti-cancer effects through inhibition of the STAT3 signalling pathway in MM cells. We found that this dietary agent suppressed constitutive and IL-6-inducible STAT3 activation in human MM cells in parallel with the inhibition of c-Src and JAK2 activation. TQ also down-regulated the expression of STAT3-regulated gene products, including cyclin D1, Bcl-2, Bcl-xL, survivin, Mcl-1 and VEGF. It also induced the inhibition of proliferation, accumulation of cells in sub-G1 phase and apoptosis, and finally it significantly potentiated the apoptotic effects of thalidomide and bortezomib in MM cells.
We report for the first time that TQ could suppress both constitutive and inducible STAT3 activation in MM cells, and that these effects were specific to STAT3, as TQ had no effect on STAT5 phosphorylation. In comparison as reported previously, exposure of cells to 100 µM AG490 (a rationally designed inhibitor of JAK2) for 8 h was required to suppress STAT3 activation (Bharti et al., 2003). The effects of TQ on STAT3 phosphorylation correlated with the suppression of upstream protein tyrosine kinases JAK2 and c-Src. Previous studies indicated that Src and JAK kinase activities cooperate to mediate constitutive activation of STAT3 (Campbell et al., 1997; Garcia et al., 2001). Our findings suggest that TQ may block cooperation of Src and JAK2 involved in tyrosyl phosphorylation of STAT3. TQ did not affect the expression of phospho-ERK, and it is also unlikely that this kinase is directly involved, as it is serine kinase. How TQ inhibits IL-6-induced STAT3 activation is not clear. The roles of JAK2, MAPK and Akt have been implicated in IL-6-induced STAT3 activation (Ihle, 1996; Tu et al., 2000). We found that IL-6-induced Akt activation was also suppressed by TQ. We further observed that TQ suppressed nuclear translocation and IL-6-induced reporter activity of STAT3. This finding suggests that TQ could manifest its effect on STAT3 activation through multiple mechanisms.
STAT3 phosphorylation plays a critical role in proliferation and survival of tumour cells (Aggarwal et al., 2006; Yue and Turkson, 2009). Several types of cancer, including head and neck cancers (Song and Grandis, 2000), hepatocellular carcinoma (Yoshikawa et al., 2001), lymphomas and leukemia (Zhang et al., 2002a), also have constitutively active STAT3. The suppression of constitutively active STAT3 in MM cells raises the possibility that this novel STAT3 inhibitor might also inhibit constitutively activated STAT3 in other types of cancer cells. Previously, we have reported that TQ can also suppress NF-κB activation (Sethi et al., 2008). Whether suppression of STAT3 activation by TQ is linked to inhibition of NF-κB activation is not clear. However, a recent report indicated that STAT3 prolongs NF-κB nuclear retention through acetyltransferase p300-mediated RelA acetylation, thereby interfering with NF-κB nuclear export (Lee et al., 2009). Thus, it is possible that suppression of STAT-3 activation may mediate inhibition of NF-κB activation by TQ.
We also found evidence that the TQ-induced inhibition of STAT3 activation involves a PTPase. Numerous PTPs have been implicated in STAT3 signalling including SHP1, SH-PTP2, TC-PTP, PTEN, PTP-1D, CD45, PTP-ε, low molecular weight (LMW) and PTP (Chiarugi et al., 1998; Kim and Baumann, 1999; Woetmann et al., 1999; Tanuma et al., 2000; Tenev et al., 2000; Gunaje and Bhat, 2001; Irie-Sasaki et al., 2001; Sun and Steinberg, 2002; Yamamoto et al., 2002). SH-PTP2 is implicated in negative regulation of JAK/STAT signalling pathways (Servidei et al., 1998; You et al., 1999; Ohtani et al., 2000) and has been shown to mediate the protective effect of IL-6 against dexamethasone-induced apoptosis in MM cells (Chauhan et al., 2000). Indeed, we report for the first time that TQ stimulated the expression of SH-PTP2 protein in U266 cells, which correlated with down-regulation of constitutive STAT3 phosphorylation in these cells. However, several putative inhibitors for the JAK/STAT pathways have been identified by functional or molecular screening of cDNA libraries (Chung et al., 1997a; Endo et al., 1997; Starr et al., 1997). Over-expression of another SH2 domain-containing protein known as suppressor of cytokine signalling (SOCS1) in murine monocytic leukemic M1 cell line suppressed IL-6-induced macrophage differentiation and phosphorylation of Stat3 (Starr et al., 1997). Another group identified a family of proteins with a putative zinc-binding motif, called protein inhibitor of activated STAT (PIAS) (Chung et al., 1997a). It was observed that PIAS3 specifically interacts with activated STAT3, thereby inhibiting its DNA-binding activity and induction of gene expression. Although further investigation is required for elucidation of the molecular mechanism for functions of these inhibitors, it is conceivable that JAK/STAT pathways might be regulated at multiple levels.
We also observed that TQ suppressed the expression of several STAT3-regulated genes, including proliferative (cyclin D1) and anti-apoptotic gene products (Bcl-2, Bcl-xL, survivin and Mcl-1), and angiogenic gene product (VEGF) in MM cells. Mcl-1 is highly expressed in haematopoietic cells (Epling-Burnette et al., 2001), and Niu et al. (2002) reported that inhibition of STAT3 by an Src inhibitor results in down-regulation of expression of the Mcl-1 gene in melanoma cells. In addition, activation of STAT3 signalling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells (Gritsko et al., 2006). Bcl-2 and Bcl-xL can also block cell death induced by a variety of chemotherapeutic agents, in parallel with an increase in chemoresistance (Simonian et al., 1997; Tu et al., 1998). Thus, the down-regulation of the expression of Bcl-2, Bcl-xL, survivin and Mcl-1 is likely to be linked with TQ's ability to induce apoptosis in MM cells as evident by cleavage of PARP and increased accumulation of cells in the sub-G1 phase. The down-modulation of VEGF expression is in line with our recent report in which TQ inhibited VEGF-induced angiogenesis in vivo in a Matrigel plug assay (Yi et al., 2008).
Thalidomide (a TNF blocker) and bortezomib (a proteasome inhibitor) are approved for the treatment of MM by the Food and Drug Administration (Cavo, 2006; Glasmacher et al., 2006). We also found that TQ can potentiate the apoptotic effects of thalidomide and bortezomib in MM cells. Furthermore, we observed that apoptotic effects of TQ were abolished in STAT3 deleted cells, and over-expression of STAT3 rescued the cells from the apoptotic effects of TQ, thus strengthening our hypothesis that anti-proliferative and pro-apoptotic effects of TQ were mediated through inhibition of the STAT3 signalling pathway. Our results clearly show that TQ inhibits IL-6 signalling quite effectively. Thus, it is possible that the role of TQ in experimental allergic encephalomyelitis (Mohamed et al., 2003), rheumatoid arthritis (Tekeoglu et al., 2007), experimental colitis (Mahgoub, 2003), allergic asthma (El Gazzar et al., 2006b), allergic lung inflammation (El Gazzar et al., 2006a) and immunomodulatory effects (Salem, 2005) are all due to suppression of IL-6 signalling as reported here.
TQ has been shown to target multiple pathways of tumourigenesis, including proliferation, apoptosis, angiogenesis, invasion and tumour-induced immunosuppression in various tumour cells and in vivo cancer models (Sethi et al., 2008). However, no reports exist in the literature elaborating the affect of TQ on STAT3/JAK2 signalling cascade in MM cells. Our results indicate for the first time that TQ inhibits both inducible and constitutive STAT3 activation, which makes it a potentially effective suppressor of tumour cell survival, proliferation and angiogenesis. Further in vivo studies may provide important leads for using TQ as treatment of cancers harbouring active STAT3 and other inflammatory diseases.
Acknowledgments
This work was supported by a grant from the Department of Research and Technology (R-184-000-157-133) to G.S.
Glossary
Abbreviations
- FBS
fetal bovine serum
- JAK
Janus-like kinase
- MAPK
mitogen-activated protein kinase
- MM
multiple myeloma
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- PTPase
protein tyrosine phosphatase
- STAT3
signal transducer and activator of transcription 3
Conflict of interest
The contributing authors have no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999;67:135–142. doi: 10.1016/s0378-8741(98)00242-6. [DOI] [PubMed] [Google Scholar]
- Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435–440. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–5583. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Brierley MM, Fish EN. Stats: multifaceted regulators of transcription. J Interferon Cytokine Res. 2005;25:733–744. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- Campbell GS, Yu CL, Jove R, Carter-Su C. Constitutive activation of JAK1 in Src-transformed cells. J Biol Chem. 1997;272:2591–2594. doi: 10.1074/jbc.272.5.2591. [DOI] [PubMed] [Google Scholar]
- Carballo M, Conde M, El Bekay R, Martin-Nieto J, Camacho MJ, Monteseirin J, et al. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- Cavo M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia. 2006;20:1341–1352. doi: 10.1038/sj.leu.2404278. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P, Marra F, Raugei G, Fiaschi T, Camici G, et al. The Src and signal transducers and activators of transcription pathways as specific targets for low molecular weight phosphotyrosine-protein phosphatase in platelet-derived growth factor signaling. J Biol Chem. 1998;273:6776–6785. doi: 10.1074/jbc.273.12.6776. [DOI] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997a;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997b;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino L, Barlocco D. STAT 3 as a target for cancer drug discovery. Curr Med Chem. 2008;15:834–843. doi: 10.2174/092986708783955464. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- El-Dakhakhny M, Madi NJ, Lembert N, Ammon HP. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81:161–164. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006a;6:1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, El Mezayen R, Nicolls MR, Marecki JC, Dreskin SC. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim Biophys Acta. 2006b;1760:1088–1095. doi: 10.1016/j.bbagen.2006.03.006. [DOI] [PubMed] [Google Scholar]
- El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, et al. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2:1603–1611. doi: 10.1016/s1567-5769(02)00139-x. [DOI] [PubMed] [Google Scholar]
- El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, Nikami H, Takewaki T. Macrophage-derived cytokine and nitric oxide profiles in type I and type II diabetes mellitus: effect of thymoquinone. Acta Diabetol. 2005;42:23–30. doi: 10.1007/s00592-005-0170-6. [DOI] [PubMed] [Google Scholar]
- El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett. 2006;106:72–81. doi: 10.1016/j.imlet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004a;25:857–866. [PubMed] [Google Scholar]
- Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004b;15:389–399. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, von Lilienfeld-Toal M, et al. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584–593. doi: 10.1111/j.1365-2141.2005.05914.x. [DOI] [PubMed] [Google Scholar]
- Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- Gunaje JJ, Bhat GJ. Involvement of tyrosine phosphatase PTP1D in the inhibition of interleukin-6-induced Stat3 signaling by alpha-thrombin. Biochem Biophys Res Commun. 2001;288:252–257. doi: 10.1006/bbrc.2001.5759. [DOI] [PubMed] [Google Scholar]
- Han Y, Amin H, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signalling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK-positive anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, et al. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–7788. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kim H, Baumann H. Dual signalling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999;19:5326–5338. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872. [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufei C, Koh TH, Uchida T, Cao X. Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene. 2007;26:7656–7664. doi: 10.1038/sj.onc.1210567. [DOI] [PubMed] [Google Scholar]
- Mahgoub AA. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143:133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, et al. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum. 2003;39:440–445. [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680–45687. doi: 10.1074/jbc.M204255200. [DOI] [PubMed] [Google Scholar]
- Servidei T, Aoki Y, Lewis SE, Symes A, Fink JS, Reeves SA. Coordinate regulation of STAT signaling and c-fos expression by the tyrosine phosphatase SHP-2. J Biol Chem. 1998;273:6233–6241. doi: 10.1074/jbc.273.11.6233. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–113. [PubMed] [Google Scholar]
- Simonian PL, Grillot DA, Nunez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Sun S, Steinberg BM. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J Gen Virol. 2002;83(Pt 7):1651–1658. doi: 10.1099/0022-1317-83-7-1651. [DOI] [PubMed] [Google Scholar]
- Tanuma N, Nakamura K, Shima H, Kikuchi K. Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells. J Biol Chem. 2000;275:28216–28221. doi: 10.1074/jbc.M003661200. [DOI] [PubMed] [Google Scholar]
- Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bohmer SA, Kaufmann R, Frese S, Bittorf T, Beckers T, et al. Perinuclear localization of the protein-tyrosine phosphatase SHP-1 and inhibition of epidermal growth factor-stimulated STAT1/3 activation in A431 cells. Eur J Cell Biol. 2000;79:261–271. doi: 10.1078/S0171-9335(04)70029-1. [DOI] [PubMed] [Google Scholar]
- Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, et al. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–6770. [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Woetmann A, Nielsen M, Christensen ST, Brockdorff J, Kaltoft K, Engel AM, et al. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18(3A):1527–1532. [PubMed] [Google Scholar]
- Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N, et al. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun. 2002;297:811–817. doi: 10.1016/s0006-291x(02)02291-x. [DOI] [PubMed] [Google Scholar]
- Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29–35. doi: 10.1038/ng0501-29. [DOI] [PubMed] [Google Scholar]
- You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367(Pt 1):97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N, et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002a;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- Zhang T, Seow KT, Ong CT, Cao X. Interdomain interaction of Stat3 regulates its Src homology 2 domain-mediated receptor binding activity. J Biol Chem. 2002b;277:17556–17563. doi: 10.1074/jbc.M105525200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


