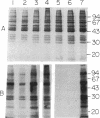
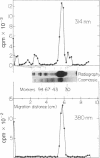
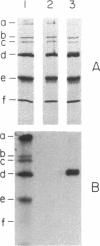
Abstract
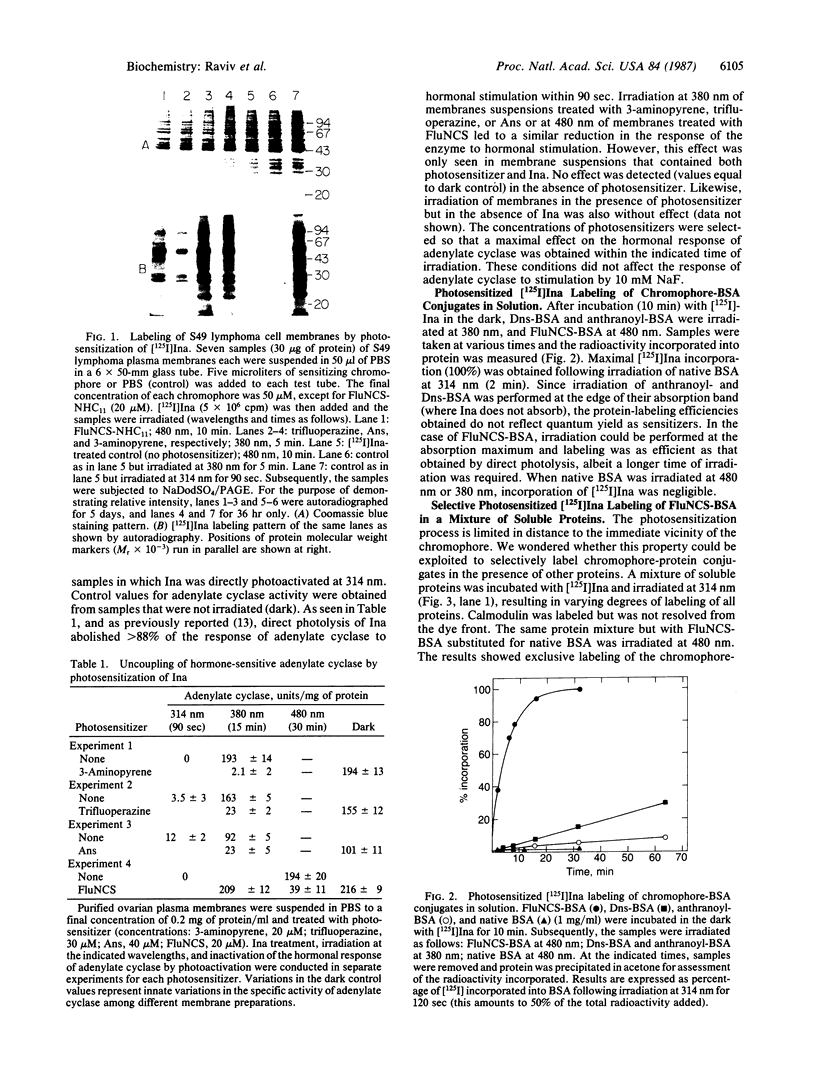
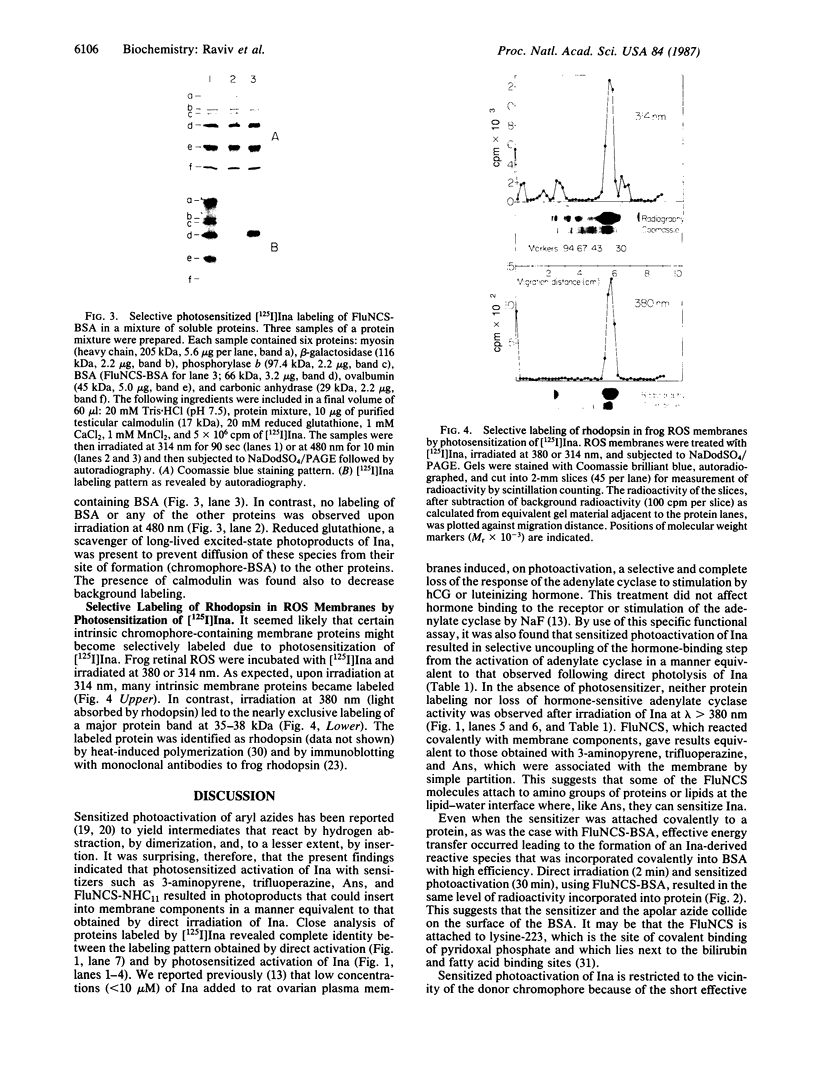
The apolar azide of 5-iodonaphthalene-1-azide (Ina) partitions into the lipid bilayer of biological membranes. Upon photolysis at 314 nm, it is rapidly converted into the reactive nitrene, which efficiently attaches covalently to lipid-embedded domains of proteins and, to a lesser extent, to membrane phospholipids. Above 370 nm, Ina absorption is negligible and photolysis at these wavelengths does not occur. However, on addition of the photosensitizing molecule 3-aminopyrene, trifluoperazine, or 8-anilinonaphthalene-1-sulfonate, followed by irradiation at 380 nm, efficient conversion of Ina to reactive species was observed, as measured by [125I]Ina-labeling of membrane proteins and inactivation of the hormonal response of adenylate cyclase. Irradiation at 480 nm in the presence of a fluorescein derivative of n-undecylamine also resulted in a pattern of [125I]Ina-labeled membrane proteins and hormone uncoupling indistinguishable from that obtained following direct photolysis at 314 nm. Photosensitization of the azide molecules is confined to the vicinity of the photosensitizer chromophore. This allowed selective labeling of chromophore-bearing proteins in solution or in membranes. Bovine serum albumin-fluorescein conjugate, in the presence of nonderivatized soluble proteins, was exclusively labeled by [125I]Ina when irradiated at 480 nm, but random labeling occurred on photolysis at 314 nm. Likewise, rhodopsin in rod outer segment membranes from frog retina was exclusively labeled by [125I]Ina upon photosensitization at 380 nm. Random labeling again occurred on direct irradiation at 314 nm. The results suggest that selective labeling in complex biological systems may be achieved by photosensitized activation of azides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 1. Phenylnitrene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 2. Phenylcarbene and adamantylidene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2420–2423. doi: 10.1021/bi00605a026. [DOI] [PubMed] [Google Scholar]
- Bayley H., Knowles J. R. Photogenerated reagents for membranes: selective labeling of intrinsic membrane proteins in the human erythrocyte membrane. Biochemistry. 1980 Aug 19;19(17):3883–3892. doi: 10.1021/bi00558a001. [DOI] [PubMed] [Google Scholar]
- Bercovici T., Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978 Apr 18;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Bridges C. D., Fong S. L. Use of lectins to investigate photoreceptor membranes. Methods Enzymol. 1982;81:65–77. doi: 10.1016/s0076-6879(82)81014-8. [DOI] [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Findlay J. B. Modification of ovine opsin with the photosensitive hydrophobic probe 1-azido-4-[125I]iodobenzene. Labelling of the chromophore-attachment domain. Biochem J. 1986 Mar 1;234(2):413–420. doi: 10.1042/bj2340413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler C., Bercovici T. Use of lipophilic photoactivatable reagents to identify the lipid-embedded domains of membrane proteins. Ann N Y Acad Sci. 1980;346:199–211. doi: 10.1111/j.1749-6632.1980.tb22100.x. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Chakrabarti P., Khorana H. G. Incorporation of fatty acids containing photosensitive groups into phospholipids of Escherichia coli. Proc Natl Acad Sci U S A. 1976 Jan;73(1):86–90. doi: 10.1073/pnas.73.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P. L., Karlish S. J., Gitler C. Evidence for the organization of the transmembrane segments of (Na,K)-ATPase based on labeling lipid-embedded and surface domains of the alpha-subunit. J Biol Chem. 1982 Jul 10;257(13):7435–7442. [PubMed] [Google Scholar]
- Klip A., Darszon A., Montal M. Labelling of rhodopsin moieties confined to the membrane lipid bilayer. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1350–1358. doi: 10.1016/s0006-291x(76)80163-5. [DOI] [PubMed] [Google Scholar]
- Klip A., Gitler C. Photoactive covalent labeling of membrane components from within the lipid core. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1155–1162. doi: 10.1016/0006-291x(74)90433-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapidot Y., Rappoport S., Wolman Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J Lipid Res. 1967 Mar;8(2):142–145. [PubMed] [Google Scholar]
- McDowell J. H., Kühn H. Light-induced phosphorylation of rhodopsin in cattle photoreceptor membranes: substrate activation and inactivation. Biochemistry. 1977 Sep 6;16(18):4054–4060. doi: 10.1021/bi00637a018. [DOI] [PubMed] [Google Scholar]
- Mintz Y., Amir Y., Amsterdam A., Lindner H. R., Salomon Y. Properties of LH-sensitive adenylate cyclase in purified plasma membranes from rat ovary. Mol Cell Endocrinol. 1978 Sep;11(3):265–283. doi: 10.1016/0303-7207(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Raviv Y., Bercovici T., Gitler C., Salomon Y. Selective photoinduced uncoupling of the response of adenylate cyclase to gonadotropins by 5-iodonaphthyl 1-azide. Biochemistry. 1984 Jan 31;23(3):503–508. doi: 10.1021/bi00298a016. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Sidman C. L., Bercovici T., Gitler C. Membrane insertion of lymphocyte surface molecules. Mol Immunol. 1980 Dec;17(12):1575–1583. doi: 10.1016/0161-5890(80)90183-2. [DOI] [PubMed] [Google Scholar]
- Witt P. L., Hamm H. E., Bownds M. D. Preparation and characterization of monoclonal antibodies to several frog rod outer segment proteins. J Gen Physiol. 1984 Aug;84(2):251–263. doi: 10.1085/jgp.84.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]