Abstract
BACKGROUND AND PURPOSE
Cannabinoid CB1 receptor antagonists reduce food intake and body weight, but clinical use in humans is limited by effects on the CNS. We have evaluated a novel cannabinoid antagonist (AM6545) designed to have limited CNS penetration, to see if it would inhibit food intake in rodents, without aversive effects.
EXPERIMENTAL APPROACH
Cannabinoid receptor binding studies, cAMP assays, brain penetration studies and gastrointestinal motility studies were carried out to assess the activity profile of AM6545. The potential for AM6545 to induce malaise in rats and the actions of AM6545 on food intake and body weight were also investigated.
KEY RESULTS
AM6545 binds to CB1 receptors with a Ki of 1.7 nM and CB2 receptors with a Ki of 523 nM. AM6545 is a neutral antagonist, having no effect on cAMP levels in transfected cells and was less centrally penetrant than AM4113, a comparable CB1 receptor antagonist. AM6545 reversed the effects of WIN55212-2 in an assay of colonic motility. In contrast to AM251, AM6545 did not produce conditioned gaping or conditioned taste avoidance in rats. In rats and mice, AM6545 dose-dependently reduced food intake and induced a sustained reduction in body weight. The effect on food intake was maintained in rats with a complete subdiaphragmatic vagotomy. AM6545 inhibited food intake in CB1 receptor gene-deficient mice, but not in CB1/CB2 receptor double knockout mice.
CONCLUSIONS AND IMPLICATIONS
Peripherally active, cannabinoid receptor antagonists with limited brain penetration may be useful agents for the treatment of obesity and its complications.
Keywords: AM6545, CB1 receptor antagonist, food intake, conditioned gaping, conditioned taste avoidance
Introduction
Agonist activation of cannabinoid 1 (CB1) receptors (nomenclature follows Alexander et al., 2009) by cannabinoids stimulates food intake in humans and rodents (Jarbe and DiPatrizio, 2005), and conversely antagonists/inverse agonists and neutral antagonists inhibit food intake (Salamone et al., 2007). With obesity-related medical problems becoming an increasing burden on health-care systems in the Western world, there has been a great deal of research into the cannabinoid system as a therapeutic target in obesity. The CB1 receptor antagonist/inverse agonist SR141716 (rimonabant) (Colombo et al., 1998; Vickers et al., 2003) and the pharmacologically and structurally similar CB1 receptor antagonist/inverse agonist, AM251 (Hildebrandt et al., 2003; McLaughlin et al., 2003; Chambers et al., 2004; 2006;), have well characterized anorexigenic actions in rodents. Unfortunately, CB1 receptor antagonist/inverse agonists also have the potential to induce centrally mediated effects and, owing to these side effects, rimonabant as an anti-obesity agent has been removed from the European market and did not receive approval from the Food and Drug Administration in the United States (Bifulco and Pisanti, 2009). Despite this, studies with rimonabant support the overall finding that antagonist/inverse agonist actions at CB1 receptors in humans can be used as a successful weight-loss therapy.
It has been shown that the peripheral endocannabinoid system is influenced by both feeding status and obesity. The levels of CB1 receptor mRNA and protein expression in vagal afferent neurons that innervate the gut of food-deprived lean rats are greatly increased compared with the levels during the fed state (Burdyga et al., 2004). Similarly, during periods of fasting, levels of the endocannabinoid, anandamide, are elevated in the rat duodenum (Gómez et al., 2002). Furthermore, in obese rodents, an increase in mRNA for CB1 receptors is observed in the stomach (Di Marzo et al., 2008) and in the nodose ganglia (Paulino et al., 2009). Endocannabinoid levels in the duodenum, pancreas and liver are similarly elevated in obese rodents (Izzo et al., 2009). These reports suggest that the peripheral endocannabinoid system is implicated in the regulation of energy balance.
It has been suggested that peripheral, as opposed to central, CB1 receptors mediate the inhibition of food intake observed with CB1 receptor antagonism/inverse agonism (Gómez et al., 2002). Rimonabant, when injected intracerebroventricularly (i.c.v.) did not inhibit food intake in rats while similar and lower doses injected intraperitoneally (i.p.) induced hypophagia (Gómez et al., 2002). Furthermore, this study demonstrated that rats treated with the neurotoxin capsaicin, which destroys primary afferent fibres innervating the gastrointestinal (GI) tract, did not display hypophagia to rimonabant (Gómez et al., 2002). However, the delivery of rimonabant directly into the CNS by another group was shown to inhibit food intake and body weight (Nogueiras et al., 2008), while a study by Madsen et al. (2009) found that neither subdiaphragmatic vagotomy nor subdiaphragmatic vagal de-afferentation prevented the effects of rimonabant on food intake and body weight. Therefore, while negative psychological effects of CB1 receptor antagonists are no doubt mediated through actions in the brain, the role of peripheral CB1 receptors on the control of food intake and body weight remains controversial. LoVerme et al., (2009) have recently synthesized a mixed CB1 receptor neutral antagonist/CB2 receptor agonist with limited access to the CNS; URB447. This compound was shown to inhibit food intake and body weight gain in mice (LoVerme et al., 2009), but whether this was due to CB1 receptor antagonism or CB2 receptor stimulation was not determined.
In the present study, we describe a novel cannabinoid CB1 receptor specific neutral antagonist with limited CNS penetration: AM6545 (Makriyannis et al., 2010; Tam et al., 2010). We tested the hypothesis that AM6545 would inhibit food intake in rodents in the absence of adverse effects. We show that AM6545 is a neutral CB1 antagonist with low CNS penetration. It inhibited food intake and body weight gain in rodents and at these doses it did not appear to have aversive side effects.
Methods
Animals
All animal care and experimental procedures were in accordance with the guidelines of the Canadian Council on Animal Care and approved by the University of Calgary Animal Care Committee or University of Guelph Institutional Animal Care Committee. Male Sprague Dawley (SD) rats with or without a complete subdiaphragmatic truncal vagotomy and male and female C57BL/6 mice were obtained from Charles River (Montreal, Quebec, Canada). Two breeding pairs of heterozygous CB1+/−C57BL/6N mice were obtained from B. Lutz (University Medical Center Mainz) and bred in our facility to obtain CB1−/−C57BL/6N mice (Marsicano et al., 2002). Animals used in these studies were backcrossed to C57BL/6N for six generations and were used at the same age (female, 9–14 weeks) and maintained under the same conditions as the wild-type mice. All CB1−/− mice were genotyped using established protocols and were confirmed as homozygous gene deficient animals (CB1−/−C57BL/6N) prior to inclusion in the study. CB1−/−/CB2−/− double knockout mice, bred on a C57BL/6J background, were obtained from A. Zimmer (University of Bonn) and bred in our facility. All animals were housed, under a 12 h light-dark cycle (lights off 19:00, unless otherwise stated), in plastic sawdust floor cages and allowed free access to tap water and standard laboratory chow, unless otherwise stated.
Cannabinoid receptor binding assay
AM6545 was tested for its affinity for the cannabinoid receptors using membrane preparations made from rat brain (CB1) or HEK293 cells expressing either mouse CB2 (mCB2) or human CB2 (hCB2) and [3H]CP-55,940, as previously described (Morse et al., 1995; Lan et al., 1999; McLaughlin et al., 2006).
cAMP assay
The binding of an inverse agonist to cannabinoid receptors raises levels of intracellular cyclic AMP (cAMP) whereas a neutral antagonist has no effect on cAMP levels (Janero and Makriyannis, 2009). We looked at the effect of AM6545 on forskolin-induced cAMP levels to determine whether it is a neutral antagonist. The effects of an inverse agonist, AM251, were also investigated as a positive control. Intracellular cAMP levels were measured with a competitive binding assay using intact HEK293 cells expressing hCB1 or hCB2 as previously described (McLaughlin et al., 2006). After lysing the cells and centrifugation, a cAMP assay kit (Sigma-Aldrich, St. Louis, MO, USA) was used to determine cAMP released in the resulting supernatant.
Brain penetration assay
Rats (280–310 g) were killed (sodium pentobarbital, 80–100 mg·kg−1, i.p.) 1, 3 and 5 h after receiving an i.p. injection of vehicle (4% DMSO, 1% Tween 80 in physiological saline; n = 2), AM6545 (10 mg·kg−1; n = 4) or AM4113 (10 mg·kg−1; n = 4). In further experiments, female C57BL/6 mice (18.5–21.0 g) were administered an i.p. injection of vehicle (n = 2) or AM6545 (5 mg·kg−1; n = 4) and were killed 1, 3 and 17 h post-injection. From both rats and mice, blood was collected and centrifuged for plasma, and brains were removed. All samples were flash-frozen in liquid nitrogen and stored at −80°C. Tissues (plasma or brain) were extracted following published procedures (Folch et al., 1957) and analysed in SRM mode after APCI+ ionization using a Thermo-Finnigan Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron, San Jose, CA, USA) with an Agilent 1100 HPLC (Agilent Technologies, Wilmington, DE, USA) front-end. Compounds were eluted from the Phenomenex Gemini C18 column (Phenomenex, Torrance, CA, USA) (2 × 50 mm, 5 µ) with a C18 guard column using a gradient elution consisting of 0.1% formic acid in both methanol (A) and water (B). The detection limits in this assay for AM6545 were: rat plasma, 9 ng·mL−1; mouse plasma, 2.6 ng·mL−1 and for AM4113, in rat plasma, 1.5 ng·mL−1. For brain samples, the corresponding lower limits were AM6545 (rat) 7.5 ng·g−1 (mouse) 5.4 ng·g−1 and AM4113 (rat) 5.3 ng·g−1
Colonic propulsion assay
Cannabinoid agonists slow GI transit and thus the actions of AM6545 on WIN55212-2-induced slowing of colonic propulsion was used to confirm the functional blockade of peripheral CB1 receptors by AM6545 (Pinto et al., 2002). Male C57BL/6 mice (19–26 g) were lightly anesthetized with isoflurane (4% in air) before a 2.5 mm spherical glass bead was inserted 2 cm intrarectally. The time to the expulsion of the bead was recorded. AM6545 (10 mg·kg−1, n = 5–10 or 20 mg·kg−1, n = 6–7) or vehicle (4% DMSO, 1% Tween 80 in physiological saline, n = 9–18) was injected i.p. 60 min prior to the administration of WIN55212-2 (1 mg·kg−1, n = 7–16), loperamide (1 mg·kg−1, n = 5–9) or vehicle (n = 7–18). Twenty minutes later, colonic propulsion was measured. Doses of WIN55212-2 (Pinto et al., 2002) and loperamide (Yamada and Onoda, 1993) were based on previous work.
Conditioned gaping
All rats were implanted with intra-oral cannulae, according to the procedure previously described (Rock et al., 2009). For 3 days following surgery, the rats were weighed and their cannulae flushed daily with chlorhexidine. Three days after the intraoral cannulation surgery, the rats received an adaptation trial; they were individually placed in the Plexiglas taste reactivity (TR) chamber (22.5 × 26 × 20 cm) with the cannula attached to an infusion pump (Harvard Apparatus, South Natick, MA, USA) for fluid delivery. Water was infused into the intra-oral cannulae over a period of 2 min at the rate of 1 mL·min−1.
Rats received the conditioning trial 24 h after the adaptation trial. They were individually placed in the TR chamber and were intra-orally infused with 0.1% saccharin for 2 min at a rate of 1 mL·min−1. During the infusion, their orofacial and somatic responses were videotaped from a mirror at a 45° angle below the TR chamber. Immediately following the saccharin infusion, the rats were injected (8 mL·kg−1) with vehicle (2-HPBCD, n = 7), AM251 (n = 7) or AM6545 (n = 7). We have previously demonstrated that a dose of 8 mg·kg−1, of the inverse agonist, AM251, produces conditioned gaping reactions (McLaughlin et al., 2005). Classically, conditioned responses, such as conditioned gaping, are most readily formed when the conditioned stimulus (saccharin) precedes the unconditioned stimulus (potential nausea induced by the antagonist). Therefore, the temporal arrangement ensured the optimal opportunity to detect the nauseating property of the antagonist. Had the drug been administered prior to the saccharin, then the arrangement would have been one of backward conditioning which is much less likely to result in an association between taste and nausea.
The animals were given a second adaptation trial with a 2 min intra-oral infusion of water 48 h following the conditioning trial, prior to TR testing. The drug free TR testing occurred 72 h following the conditioning trial. The rats were placed in the TR chamber and infused with 0.1% saccharin over a period of 2 min (1 mL·min−1) while their orofacial reactions were videorecorded [Sony DCR-HC48 (Noldus, Inc., Leesburg, VA, USA), with firewire feed to a PC] from the mirror beneath the chamber. The videos were later scored by an observer unaware of the experimental conditions using ‘The Observer’ (Noldus, Inc.) software for the aversive behaviour of gaping (number of large openings of the mouth and jaw, with lower incisors exposed). Additionally, the number of 2 s bouts of tongue protrusions (forward and lateral protrusions of the tongue from the mouth) and mouth movements (movement of the mandible with a closed mouth) were summated to provide a positive hedonic reaction score.
Conditioned taste avoidance
Different flavors of Ensure Plus™ Liquid diet (53.3% carbohydrate, 29% fat, 16.7% protein, 1.41 kcal·g−1) (Abbot Laboratories, Abbott Park, IL, USA) were used to assess whether rats would avoid a flavour associated with the effect of AM6545. Naive male SD rats (250–275 g) were restricted to 15 g of rodent chow per day in order to promote food intake during training and testing trials. On the first day of the experiment, rats were given access to vanilla-flavoured Ensure Plus in order to become familiar with the diet. The vanilla flavour was deliberately chosen to be different to those that would be used in the conditioning study. On the second day, rats were given access to either chocolate- or strawberry-flavoured Ensure Plus followed 45 min later by an i.p. vehicle injection. On the third day, rats were given access to the opposite flavour from the day before followed 45 min later by AM6545 (10 mg·kg−1, n = 7). The dose of AM6545 was based on that used in the conditioned gaping studies. An additional group of rats were treated the same way with saline vehicle and LiCl (10 mL·kg−1 of 0.15 M, n = 6) to provide a positive control. On the fourth day, each rat was given access to each flavour for 45 min. Evidence of conditioned taste avoidance was observed by the preference for one flavour over another, and expressed as food intake of a flavour (paired to a treatment) as a percentage of total food consumed.
Short-term food intake in rats
Rats were individually housed and fed chocolate-flavoured Ensure Plus Liquid diet. Rats were habituated to testing and handling procedures for 3 days prior to the start of the study. Food and water were presented in drip-free inverted glass bottles that attached to the outside of the cage. Food was available for 18 h each day starting at 16:00 h (12 h light-dark cycle; lights off 16:00 h). Prior to the first day of treatment, rats were assigned to vehicle (1 mL·kg−1) (mean body weight ± s.e.mean; 252 ± 5 g, n = 6), 5 mg·kg−1 AM6545 (253 ± 6 g; n = 6) or 10 mg·kg−1 AM6545 (252 ± 3 g, n = 6) treatment groups. Drugs were administered i.p. starting at 15:45. Food intake was measured at 1, 3 and 5 h. Doses were used based on the conditioned gaping and conditioned taste avoidance studies.
Chronic feeding study in rats
Daily food intake and body weight were monitored in rats treated with vehicle (256 ± 6 g, n = 4) or 10 mg·kg−1 AM6545 (255 ± 6 g, n = 4) to determine if the peripherally restricted antagonist would affect body weight over 7 days. Rats were maintained on vanilla-flavoured Ensure Plus Liquid diet as described previously. Injections were given i.p. each day starting at 15:45. Food intake and body weight were measured each day. The dose of AM6545 was chosen based on the results from the short-term food intake study and conditioned taste avoidance assay.
Short-term food intake in vagotomized rats
Upon arrival from Charles River, vagotomized rats were immediately placed on vanilla-flavoured Ensure Plus Liquid diet and allowed to recover for 10 days. Food was available each day from 16:00 to 09:00. Food intake and body weight were monitored daily. The day before the experiment, rats were assigned to either vehicle (281.2 ± 13 g; n = 5) or 10 mg·kg−1 AM6545 (281.8 ± 15 g; n = 5) treatment groups. The dose was chosen based on results from the early feeding study and conditioned taste avoidance assay. On the day of the first experiment, rats were injected i.p. at 15:30. Food intake was measured at 1, 3, and 5 h post-injection.
Short-term food intake in CB1 and CB1/CB2 receptor knockout mice
Female CB1−/− (Marsicano et al., 2002) and littermate control mice, CB1−/−/CB2−/− (Jarai et al., 1999) and C57BL/6J control mice were individually housed 4 days prior to the experiment and placed on a medium fat diet (51.4% carbohydrate, 31.8% fat, 16.8% protein; 4.41 kcal·g−1; Diet # D12266B, Research Diets, New Brunswick, NJ, USA). Access to food was restricted to the hours of 16:30 to 09:30 with free access to tap water at all times. The day before testing the effect of 5 mg·kg−1 AM6545 on food intake, mice were assigned to either vehicle (wild-type, 23.4 ± 2.0 g; n = 9; CB1−/−, 21.9 ± 0.9 g; n = 8; C57BL/6, 27.5 ± 0.8 g; n = 5; CB1−/−/CB2−/−, 21.6 ± 1.1 g; n = 5) or AM6545 (wild-type, 23.9 ± 3.9 g; n = 9; CB1−/−, 22.28 ± 1.6 g; n = 9; C57BL/6, 27.7 ± 0.8 g; n = 6; CB1−/−/CB2−/−, 21.9 ± 2.0 g; n = 6) treatment groups. Similarly, the day before testing the effect of 20 mg·kg−1 AM6545 on food intake, mice were assigned to either vehicle (wild-type, 23.0 ± 1.9 g; n = 8; CB1−/−, 22.2 ± 0.9 g; n = 10) or AM6545 (wild-type, 23.3 ± 1.5 g; n = 11; CB1−/−, 21.6 ± 1.7 g; n = 12) treatment groups. Drugs were administered i.p. starting at 16:00. Food intake was measured at 1, 2, 3 and 17 h. Doses were chosen based on the colonic propulsion study in mice and feeding studies in rats.
Data analysis
Results from the competition binding assays were analysed using non-linear regression to determine the IC50 values for the ligand; Ki values were calculated from the IC50 (Cheng and Prusoff, 1973) (Prism by GraphPad Software, Inc., La Jolla, CA, USA). Each experiment was performed in triplicate and Ki values determined from at least two independent experiments. The results from the cAMP assay were expressed as percent inhibition of forskolin-stimulated cAMP accumulation and EC50 curves were generated with the use of GraphPad Prism software. All other data were expressed as the mean ± s.e.mean. Data were analysed (GraphPad Prism software) using t-tests, one-way anova or Kruskal–Wallis test followed by Bonferroni's or Dunn's multiple comparisons post hoc test or by two-way mixed design anova with time as the repeated measure followed by Bonferroni's post hoc test, as appropriate.
Materials
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (AM251), N-piperidin-1-yl-2,4-dichlorophenyl-1H-pyrazole-3-carboxamide analogue (AM4113) and 5-(4-(4-cyanobut-l-ynyl)phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(l,l-ioxothiomorpholino)-lH-pyrazole-3-carboxamide (AM6545) were synthesized at the Center for Drug Discovery, Northeastern University. The cannabinoid receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN55212-2 mesylate) was purchased from Tocris (Ellisville, MI, USA) and the µ-opioid receptor agonist, loperamide hydrochloride, was purchased from Sigma-Aldrich. Sodium pentobarbital was supplied by Ceva Sante Animale, Libourne, France and isoflurane by Halocarbon Products Corporation, NJ, USA. Lithium chloride (LiCl) was purchased from Sigma-Aldrich, made up to a concentration of 0.15 M in sterile water and injected i.p., at 10 mL kg-1 rat. All other drugs were dissolved in a vehicle of 4% DMSO, 1% Tween 80 in physiological saline unless otherwise stated. Drugs used in the conditioned gaping studies were dissolved in 45% 2-hydroxypropyl-β-cyclodextrin (2-HPBCD) with sterile water to 1 mg·mL−1. Injections were administered i.p. to mice at 100 µL·10 g−1 body weight and to rats at 100 µL·100 g−1 body weight.
Results
The novel compound AM6545 was synthesized (Makriyannis et al., 2010) as a neutral antagonist of the cannabinoid receptors, with selectivity for CB1 over CB2 receptors, and with limited brain penetration. Pharmacological and functional assays were used to verify these properties.
Cannabinoid receptor binding assay
Binding assays were performed to determine the affinity of AM6545 for the cannabinoid receptors. AM6545 bound with high affinity to CB1 receptors exhibiting 302-fold selectivity for CB1 versus mCB2 receptors and 38-fold selectivity for CB1 versus hCB2 receptors (Table 1).
Table 1.
Binding data for AM6545
| Assay | AM6545 |
|---|---|
| CB1 binding Ki | 1.73 ± 0.92 |
| 95% confidence | (0.94, 3.20) |
| r2-value | 0.942 |
| mCB2 binding Ki | 523 ± 143 |
| 95% confidence | (288, 1,050) |
| r2-value | 0.929 |
| hCB2 binding Ki | 65.2 ± 123 |
| 95% confidence | (43.0, 99.0) |
| r2-value | 0.963 |
Values for Ki are in nM ± standard deviation of four (CB1 receptors), two (mCB2 receptors) or three (hCB2 receptors) assays done in triplicate (shown with 95% confidence intervals and r2-value).
cAMP assay
A cAMP assay was used to demonstrate that AM6545 acts as a neutral antagonist (as opposed to an inverse agonist) of the CB1 receptors. When carried out in HEK293 cell cultures expressing hCB1, AM6545, at concentrations up to 3 µM, produced no change in forskolin-stimulated cAMP levels, while the inverse agonist AM251 further stimulated cAMP production (Figure 1).
Figure 1.
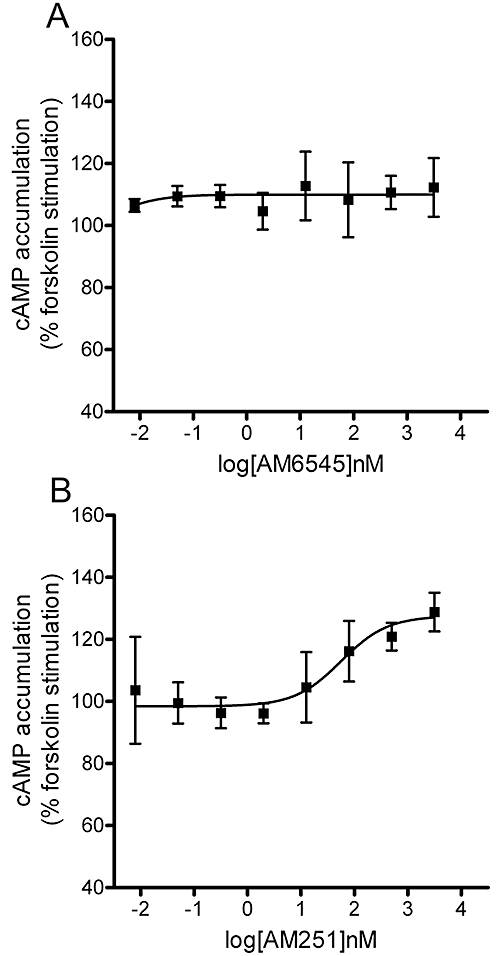
Representative cAMP assay using hCB1-HEK293 cells for AM6545 (A) and AM251 (B). n = 3.
Brain penetration assay
A unique property of the novel CB1 receptor neutral antagonist, AM6545, is its limited penetration of the CNS. To verify this, the levels in the brain and plasma of AM6545 and AM4113 (a centrally active compound) were measured after the systemic administration of each compound. Neither AM6545 nor AM4113 were detected in the samples taken from rats receiving a vehicle injection. AM6545 was not detected in brain or plasma taken from mice following vehicle administration. The brain : plasma ratios demonstrate that AM4113 has a much greater brain penetration than AM6545 (Table 2). Similarly, low brain : plasma ratios of AM6545 were measured in mouse tissues (Table 2).
Table 2.
AM6545 or AM4113 levels in the brain and plasma of rats or mice at specified time points after the i.p. administration of the antagonist
| Species | Time point (h) | Treatment (mg·kg−1) | Brain [antagonist] (ng·g−1) | Plasma [antagonist] (ng·mL−1) | Brain : plasma ratio |
|---|---|---|---|---|---|
| Rat | 1 | 10 AM6545 | 29.7 ± 9.8 | 178.1 ± 99.4 | 0.18 ± 0.11 |
| 10 AM4113 | 63.6 ± 6.2 | 49.3 ± 5.9 | 1.30 ± 0.18 | ||
| 3 | 10 AM6545 | 58.8 ± 4.8 | 267.9 ± 91.9 | 0.23 ± 0.06 | |
| 10 AM4113 | 123.4 ± 15.6 | 36.2 ± 8.5 | 4.73 ± 2.62 | ||
| 5 | 10 AM6545 | 41.9 ± 26.3 | 123.5 ± 114.0 | 0.41 ± 0.12 | |
| 10 AM4113 | 79.5 ± 27.3 | 22.7 ± 14.1 | 3.56 ± 0.61 | ||
| Mouse | 1 | 5 AM6545 | 20.7 ± 5.5 | 197.0 ± 0.1 | 0.13 ± 0.08 |
| 3 | 5 AM6545 | 39.4 ± 11.4 | 195.0 ± 0.2 | 0.10 ± 0.01 | |
| 17 | 5 AM6545 | 11.3 ± 1.4 | 153.4 ± 0.2 | 0.20 ± 0.06 |
Data represent the mean ± s.e.mean, n = 3–4.
Colonic propulsion assay
Experiments were carried out to confirm the functional blockade of peripheral CB1 receptors by AM6545. The cannabinoid receptor agonist WIN55212-2, when systemically administered, has been shown to slow GI transit through the activation of peripheral CB1 receptors (Izzo et al., 2000). The ability of AM6545 to reverse WIN55212-2-induced actions on the GI tract was investigated. WIN55212-2 (1 mg·kg−1) slowed colonic propulsion (P < 0.001; Figure 2) and this effect was reversed in a dose-dependent manner by AM6545 (Figure 2). To further confirm the specificity of AM6545, it was tested against the action of loperamide, a µ–opioid receptor agonist. Loperamide (1 mg·kg−1) slowed colonic propulsion (P < 0.001) to a similar magnitude as WIN55212-2 and this effect was not modified by AM6545 (Figure 2). AM6545 (10 or 20 mg·kg−1) alone did not modify colonic propulsion (P > 0.05; Figure 2).
Figure 2.
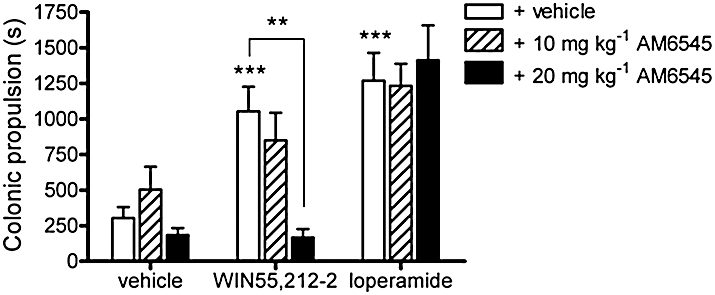
The effect of vehicle (4% DMSO, 1% Tween 80 in physiological saline), 10 mg·kg−1 or 20 mg·kg−1 AM6545 administered 1 h prior to vehicle, WIN55212-2 (1 mg·kg−1) or loperamide (1 mg·kg−1) on colonic propulsion in mice. Bars represent the mean ± s.e.mean, n = 6–7. **P < 0.01 and ***P < 0.001 denote a significant difference between groups analysed using one-way anova followed by Bonferroni's multiple comparisons post hoc test.
Conditioned gaping and conditioned taste avoidance
In order to confirm whether AM6545 induces signs of nausea, we examined the potential for AM6545 to generate conditioned gaping and conditioned taste avoidance in rats. There was a significant difference between treatments in the number of conditioned gaping reactions, F(2,16) = 14.43, P = 0.003, and hedonic reactions, F(2,16) = 5.41, P = 0.016 (Figure 3A,B). Post hoc analysis revealed that AM251 (8 mg·kg−1; P < 0.05), but not AM6545 (8 mg·kg−1; P > 0.05) produced aversive conditioned gaping reactions (Figure 3A) and suppressed positive conditioned ingestive reactions (Figure 3B) relative to vehicle. Furthermore, while the positive control LiCl produced conditioned taste avoidance (P < 0.001; Figure 3C), AM6545 (10 mg·kg−1; P > 0.05) did not (Figure 3D).
Figure 3.
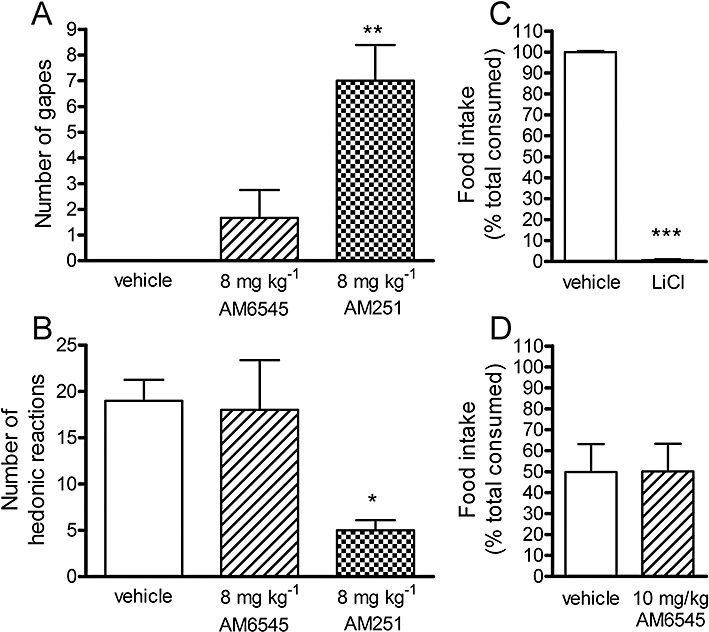
The effect of vehicle (2-HPBCD), AM6545 (8 mg·kg−1) or AM251 (8 mg·kg−1) on subsequent conditioned gaping (A) and positive hedonic reactions (B) elicited by 0.1% saccharin solution. The effect of AM6545 (10 mg·kg−1) on the consumption of flavoured food previously paired to vehicle, lithium chloride (LiCl, C) or 10 mg·kg−1 AM6545 (D) in naive rats. Bars represent the mean ± s.e.mean, n = 6–7. *P < 0.05, **P < 0.01 and ***P < 0.001 denote a significant difference from the vehicle control analysed using either one-way anova followed by Dunnett's post hoc test (A and B) or using paired t-test (C and D).
Short-term food intake in rats
Investigations were carried out to examine whether AM6545 could inhibit food intake in rats. Two-way repeated measures anova showed there was a significant treatment effect [F(2,32) = 5.83, P = 0.0126]. Post hoc analysis revealed that AM6545 (5 mg·kg−1) had no effect on food intake in rats at 1, 3 and 5 h post-injection (Figure 4A) and that food intake in rats was significantly (P < 0.001) inhibited 3 h after the administration of a higher dose of AM6545 (10 mg·kg−1) and, while the trend for inhibition was still evident, the action was not significant (P > 0.05) at 5 h (Figure 4A).
Figure 4.
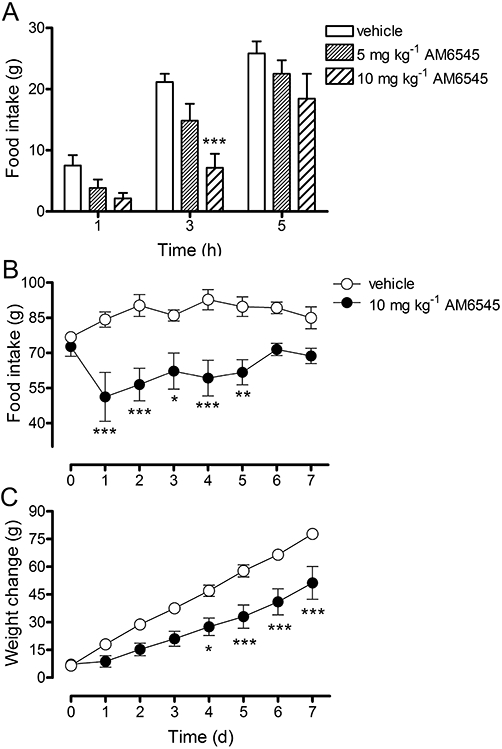
The effect of vehicle (4% DMSO, 1% Tween 80 in physiological saline) or AM6545 (5 or 10 mg·kg−1) on short-term food intake in rats (A). Bars represent the mean ± s.e.mean, n = 6–7. ***P < 0.001 denotes a significant difference to vehicle control analysed using one -way anova followed by Bonferroni's post hoc test. The effect of vehicle (4% DMSO, 1% Tween 80 in physiological saline) or AM6545 (10 mg·kg−1) on food intake (B) and weight change (C) in rats. Data points represent the mean ± s.e.mean, n = 4. *P < 0.05, **P < 0.01 and ***P < 0.001 denote a significant difference from vehicle control analysed using two-way mixed design anova with time as the repeated measure followed by Bonferroni's post hoc test.
Chronic feeding study with AM6545
Following the observation that AM6545 could inhibit short-term food intake after an acute injection, investigations were carried out into the long-term effect on food intake and body weight that chronic administration produced. There was a significant (treatment-time) interaction, F(7,42) = 3.25, P = 0.0075, of daily AM6545 treatment on food intake (Figure 4B). Daily administration of AM6545 (10 mg·kg−1) inhibited food intake in rats, F(1,42) = 21.98, P = 0.003, with significant (P < 0.05) differences between vehicle- and AM6545-treated animals observed at days 1 through 5 (Figure 4B). There was also a significant (treatment-time) interaction, F(7,42) = 6.88, P < 0.0001, on body weight change induced by daily AM6545 (Figure 4C). The rats treated daily with AM6545 (10 mg·kg−1) exhibited a reduction in weight change, F(1,42) = 12.04, P = 0.013, with significant (P < 0.05) differences between vehicle- and AM6545-treated animals seen at days 4 to 7 (Figure 4C).
Short-term food intake in vagotomized rats
Experiments were carried out in vagotomized rats to investigate the role of the vagus nerve in the action of AM6545 on food intake. A two-way repeated measures anova revealed a significant (treatment-time) interaction, F(2,16) = 7.19, P = 0.006. AM6545 (10 mg·kg−1) significantly inhibited short-term food intake in vagotomized rats, F(1,16) = 53.01, P < 0.0001, with post hoc tests showing there was a significant difference from vehicle-treated rats at all time points (Figure 5).
Figure 5.
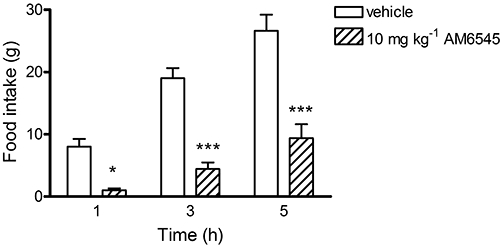
The effect of vehicle (4% DMSO, 1% Tween 80 in physiological saline) or AM6545 (10 mg·kg−1) on food intake in vagotomized rats. Bars represent the mean ± s.e.mean, n = 5. *P < 0.05 and ***P < 0.001 denote a significant difference from vehicle control analysed using two-way mixed design anova with time as the repeated measure followed by Bonferroni's post hoc test.
Short-term food intake in CB1 and CB1/CB2 receptor knockout mice
CB1 receptor knockout and CB1/CB2 receptor knockout mice were used in an attempt to specify the mechanism of action of AM6545 on food intake. AM6545 (20 mg·kg−1) inhibited food intake in wild-type mice. Two-way repeated measures anova showed a significant (treatment-time) interaction, F(3,48) = 11.86, P < 0.0001. Also, there was a significant treatment effect, F(1,48) = 29.14, P < 0.0001. Bonferroni's post hoc analysis showed there was a significant inhibition (P < 0.001) in food intake induced by AM6545 (1.79 ± 0.39 g) at 17 h as compared with vehicle-treated mice (3.90 ± 0.29 g). At the earlier time points of 1, 2 and 3 h, there was no difference in food intake (P > 0.05) between vehicle-treated mice (1 h, 0.25 ± 0.08 g; 2 h, 0.48 ± 0.10 g; 3 h, 0.70 ± 0.08 g) and 20 mg·kg−1 AM6545-treated mice (1 h, 0.07 ± 0.03 g; 2 h, 0.11 ± 0.04 g; 3 h, 0.12 ± 0.04 g). AM6545 (20 mg·kg−1) also inhibited food intake in CB1 knockout mice with a significant (treatment-time) interaction, F(3,63) = 17.15, P < 0.0001, and significant treatment effect, F(1,63) = 30.84, P < 0.0001. Food intake was reduced at 17 h (P < 0.001) in mice receiving 20 mg·kg−1 AM6545 (1.65 ± 0.21 g) compared with vehicle-treated mice (3.12 ± 0.24 g). At earlier time points, AM6545 (20 mg·kg−1) did not modify food intake (P > 0.05) in CB1 knockout mice (1 h, 0.08 ± 0.02 g; 2 h, 0.09 ± 0.02 g; 3 h, 0.11 ± 0.03 g) compared with vehicle-treated mice (1 h, 0.16 ± 0.03 g; 2 h, 0.29 ± 0.03 g; 3 h, 0.50 ± 0.06 g). AM6545 at the lower dose of 5 mg·kg−1 inhibited 17 h food intake in control mice (P = 0.0004) and in CB1 knockout mice (P = 0.009), but had no effect in CB1/CB2 double knockout mice (P = 0.08) compared with vehicle (Figure 6).
Figure 6.
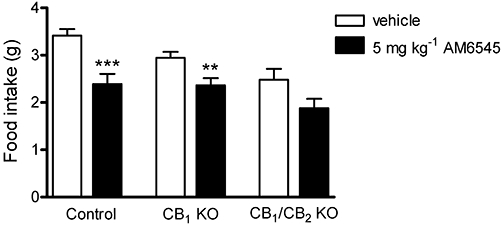
The effect of vehicle (4% DMSO, 1% Tween 80 in physiological saline) or AM6545 (5 mg·kg−1) on food intake in control (wild-type or C57BL/6J), CB1 receptor knockout (CB1 KO) or CB1/CB2 receptor double knockout (CB1/CB2 KO) mice. Bars represent the mean ± s.e.mean, n = 5–14. **P < 0.01 and ***P < 0.001 denote a significant difference from vehicle control analysed using t-test.
Discussion
We report the actions of a novel cannabinoid CB1 receptor antagonist, AM6545, on food intake and body weight in rats and mice. This compound is a neutral antagonist of CB1 receptors and shows limited penetration into the CNS. Furthermore, we demonstrated the ability of AM6545 to inhibit short-term food intake, and to reduce body weight gain, in rats without the potential for inducing aversive reactions. As AM6545 has limited brain penetrance, these findings suggest that the hypophagic actions of CB1 receptor antagonists are not dependent on actions in the CNS, making AM6545 of potential therapeutic value for the treatment of obesity and its complications.
Brain penetration studies showed that at doses, route of administration and time points which were relevant to the other investigations in the present study, the levels of AM6545 in the brain were much lower than the centrally active compound, AM4113, suggesting limited penetration of the brain by this compound. AM6545 showed selectivity for CB1 receptors over CB2. To determine the CB1 selective, functional activity of AM6545, we examined its ability to block a well-established cannabinoid-mediated action in the GI tract. The action of the cannabinoid CB1/CB2 receptor agonist WIN55212-2 on the motility of the GI tract is well documented, with this compound slowing gastric emptying (Fichna et al., 2009), upper GI transit (Carai et al., 2006), colonic propulsion (Pinto et al., 2002) and whole GI transit (Carai et al., 2006) in mice. Furthermore, the action of the cannabinoid-induced inhibition of physiological GI transit has been shown to be mediated by peripherally located CB1 receptors (Izzo et al., 2000). AM6545 blocked the inhibitory action of WIN55212-2 on colonic propulsion in a dose-dependent manner while having no effect on the inhibitory action of a µ-opioid receptor agonist, confirming its functionality as a peripherally active, specific CB1 receptor antagonist. Cannabinoid receptor antagonists such as AM251 (present study) and rimonabant (Landsman et al., 1997) are in fact inverse agonists, blocking constitutive activity of the receptor, while others, such as AM4113 (Chambers et al., 2007; Sink et al., 2008), are neutral antagonists which block the receptor binding site preventing agonist stimulation. A forskolin-stimulated cAMP assay confirmed that while AM251 acts as an inverse agonist, further stimulating the production of cAMP, AM6545 behaved as a neutral antagonist. Together, these data confirm the CB1 receptor specific, neutral antagonist characteristics of AM6545.
Antagonist/inverse agonist actions at CB1 receptors are widely acknowledged to reduce food intake in both lean and obese laboratory animals and in obese humans. However, CB1 receptor antagonist/inverse agonists can induce such side effects as nausea. Rimonabant was shown to induce nausea in humans during clinical trials, with 8.7% of patients reporting the side effect compared with 3.6% of patients who received placebo (Rosenstock et al., 2008). Investigating the potential of a compound to induce nausea in laboratory rodents is made more difficult by the subjective nature of the sensation; however, models do exist. One such model is the conditioned taste avoidance test. Here, a flavoured food or liquid is paired with a compound and subsequent avoidance of this flavour can reflect the potential of the compound to be noxious and induce illness. Rimonabant (10 mg·kg−1) (De Vry et al., 2004) and AM251 (8 mg·kg−1) (McLaughlin et al., 2005) have been shown to induce conditioned taste avoidance in rats, suggesting they possess the potential to induce nausea. In the present study, AM6545 did not produce conditioned taste avoidance when paired with a flavour of Ensure Liquid diet, whereas in separate experiments, rats avoided consumption of the flavour of diet that was paired to the reference compound, LiCl. However, one drawback to this model is that conditioned taste avoidance is also observed with compounds which possess rewarding properties such as amphetamine (Berger, 1972). To further confirm that AM6545 does not have the potential to induce nausea, it was also tested in a more selective model of nausea, the taste reactivity test in rats. When noxious compounds are paired with an intra-oral infusion of saccharin solution, rats display gaping of the mouth and a reduction in the number of hedonic behaviours; these effects are only observed in response to compounds which produce emesis in animals capable of vomiting and not to rewarding drugs (Parker, 2003; Parker et al., 2008). The CB1 receptor antagonist/inverse agonist AM251 induced gaping in the taste reactivity test in rats (McLaughlin et al., 2005); however, the neutral antagonist AM4113 did not demonstrate a potential to induce nausea in this paradigm (Sink et al., 2008). We also did not observe gaping in response to AM6545 in the present study, suggesting that this peripherally restricted, neutral CB1 antagonist does not induce nausea in two rat models.
In rats fed a chow diet, acute systemic administration of rimonabant inhibits short-term food intake (Arnone et al., 1997; Ward et al., 2009) and operant food intake (McLaughlin et al., 2003; De Vry et al., 2004). Acute systemic administration of AM251 also inhibits short-term food intake in rats (McLaughlin et al., 2003; 2005;) and mice (Riedel et al., 2009), and in rats, this effect is still observed 5 days post-injection (Chambers et al., 2004). We report that acute systemic administration of AM6545 dose-dependently inhibited food intake in the short term, with significant reductions observed 3 h after an injection of a 10 mg·kg−1 dose in rats. A reduction in food intake by AM6545 in rats has also been demonstrated by another group (Randall et al., 2010), further confirming the effect. In an attempt to further characterize the mechanism of action of AM6545 on short-term food intake, we investigated its action in CB1 knockout and CB1/CB2 receptors double knockout mice and found that while food intake was reduced in the absence of CB1 receptors, there was no effect of AM6545 on feeding in the absence of both CB1 and CB2 receptors. It has been shown that CB2 receptor ligands can inhibit food intake in C57BL/6 mice (Onaivi et al., 2008), suggesting that the hypophagic action of AM6545 may involve the CB2 receptor. Furthermore, AM6545 showed no affinity for an additional 61 targets investigated in the NovaScreen ‘side effect’ profile assay (Caliper LifeSciences, Hanover, MD, USA; data not shown), further suggesting the specificity of the compound.
To further characterize the effect of AM6545 on feeding, we investigated the effect of a 7 day chronic treatment schedule on body weight change and food intake. After 4 days of daily injections of AM6545, body weight gain was inhibited and this effect persisted until the end of the study despite the fact that there was not a significant inhibition in food intake after day 5. This trend was also observed in lean rats administered rimonabant once daily for 14 days (Colombo et al., 1998). Tolerance to the effect on food intake was observed at treatment day 5 while the inhibitory action on body weight gain continued for 7 days after rimonabant treatment ended (Colombo et al., 1998). However, tolerance did not develop to the inhibitory action of AM251 (Chambers et al., 2006) and AM4113 (Chambers et al., 2007) on food intake in chronic studies where the compound was administered daily for 15 and 5 days, respectively. The current findings suggest that the inhibitory actions of AM6545 on body weight gain may not be solely due to an inhibition of food intake. Further investigations are required to determine the effect of AM6545 on energy expenditure.
As we have shown that AM6545 inhibits food intake and has limited brain penetration, it was of interest to investigate the role of the vagus nerve in the mediation of the hypophagic action of AM6545. Short-term food intake was inhibited by AM6545 in rats with a complete subdiaphragmatic truncal vagotomy implying that an intact vagus nerve is not essential in the mediation of this effect. The site of action of AM6545 in these studies remains to be determined. The circumventricular organs (CVOs) that lack a blood-brain-barrier are one potential site mediating the effects of AM6545. The area postrema is believed to be an important CVO site in the regulation of feeding (Cottrell and Ferguson, 2004). While it is not known whether cannabinoid CB1 receptors are expressed in other CVOs, it has been shown that they are present in the area postrema (Partosoedarso et al., 2003; Van Sickle et al., 2003). The present study also revealed the interesting finding that AM6545-induced hypophagia in vagotomized rats was enhanced compared with rats with an intact vagus nerve. Indeed, the effect of AM6545 on food intake in naïve rats was only seen 3 h post-injection whereas in vagotomized rats AM6545 inhibited food intake sooner (at 1 h post-injection) and the effect was observed for as long as 5 h post-injection. Gómez et al. (2002) reported that the inhibitory action of rimonabant on food intake in rodents was abolished by capsaicin-induced deafferentation, suggesting that the effect was mediated in the periphery. Receptor targets for peripherally released orexigenic and anorexigenic compounds are found in the nodose ganglia and these receptors are translocated to either central or peripheral terminals (Dockray, 2009). It is known that gene and protein expression levels of these receptors in the nodose ganglia are altered in response to differing feeding states (Dockray, 2009). This suggests that the hypophagic action of AM6545 may be suppressed by the action of endogenous compounds acting via the vagus nerve.
Pavon et al., (2006) reported that a neutral CB1 receptor antagonist LH-21 with limited penetration to the CNS inhibited food intake and body weight gain in obese rats. However, it has since been reported that LH-21 is an inverse agonist at CB1 receptors and that the brain to plasma ratio following a single intravenous injection of LH-21 is 1:1, demonstrating that it is highly brain penetrant (Chen et al., 2008). It was also reported that CB1 receptor antagonists with little potential to cross the blood-brain-barrier reduced body weight of obese mice (McElroy et al., 2008). Recently, LoVerme et al. (2009) have described the effects of a peripherally restricted mixed CB1 receptor antagonist/CB2 receptor agonist, URB447, on food intake. They showed that URB447 inhibited short-term food intake in lean mice and inhibited food intake and reduced body weight in genetically obese ob/ob mice during a chronic treatment period (LoVerme et al., 2009). The authors attribute these findings to the antagonism of peripheral CB1 receptors rather than the agonist stimulation of CB2 receptors. However, we have shown in the present study that while AM6545 has a much greater affinity for CB1 over CB2 receptors, and that AM6545 blocks CB1 receptor-mediated inhibition of colonic propulsion, its effects on food intake may involve actions at CB2 receptors.
In conclusion, the present study has revealed that hypophagia mediated by cannabinoid receptors is not solely dependent on central activation. AM6545 is a neutral cannabinoid receptor antagonist with limited penetration into the CNS that is also devoid of aversive effects. These valuable characteristics indicate that peripherally restricted neutral cannabinoid receptor antagonists could be developed for the treatment of obesity and its complications, potentially without the psychiatric side effects observed with centrally active CB1 receptor antagonists.
Acknowledgments
We thank Winnie Ho for the genotyping of the cannabinoid receptor deficient mice, Catherine M. Keenan for her assistance with the colonic propulsion studies and Shivangi Joshi and Yan Peng for performing the binding and cAMP assays. These studies were supported by a grant from the Canadian Institutes of Health Research (to KAS). KAS is an Alberta Heritage Foundation for Medical Research Medical Scientist and the Crohn's and Colitis Foundation of Canada Chair in Inflammatory Bowel Disease Research. The studies at the University of Guelph were supported by a research grant from the Natural Sciences and Engineering Council of Canada to LAP and those carried out at Northeastern University were supported by grants DA7215, DA023142 and DA009158 to AM.
Glossary
Abbreviations
- CB1
cannabinoid 1 receptor
- CB2
cannabinoid 2 receptor
- GI
gastrointestinal
- i.c.v.
intracerebroventricular
Conflicts of interest
Two authors (AM and VKV) are co-applicants for a patent application which covers the synthesis and characterization of AM6545 (Makriyannis A, Vemuri VK, Thotapally R, Olszewska T. International Patent Application No: PCT/US09/01054) to be published in 2010. There are no other conflicts to declare.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Berger BD. Conditioning of food aversions by injections of psychoactive drugs. J Comp Physiol Psychol. 1972;81:21–26. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Pisanti S. End of the line for cannabinoid receptor 1 as an anti-obesity target? An opinion. Nat Rev Drug Discov. 2009;8:594. doi: 10.1038/nrd2775-c1. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajappa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA. AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol. 2006;147:109–116. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Lao JZ, Huang RR, Xiao JC, Clements MJ, et al. Pharmacological evaluation of LH-21, a newly discovered molecule that binds to cannabinoid CB1 receptor. Eur J Pharmacol. 2008;584:338–342. doi: 10.1016/j.ejphar.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–L117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97:531–536. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Fichna J, Schicho R, Andrews CN, Bashashati M, Klompus M, McKay DM, et al. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating kappa-opioid and cannabinoid receptors. Neurogastroenterol Motil. 2009;21:1326–e128. doi: 10.1111/j.1365-2982.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Pinto L, Borrelli F, Capasso R, Mascolo N, Capasso F. Central and peripheral cannabinoid modulation of gastrointestinal transit in physiological states or during the diarrhoea induced by croton oil. Br J Pharmacol. 2000;129:1627–1632. doi: 10.1038/sj.bjp.0703265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, et al. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:43–65. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, DiPatrizio NV. Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol. 2005;16:373–380. doi: 10.1097/00008877-200509000-00009. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, et al. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy J, Sieracki K, Chorvat R. Non-brain-penetrant CB1 receptor antagonists as a novel treatment of obesity and related metabolic disorders. Obesity. 2008;16:S47. [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, et al. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Madsen AN, Jelsing J, van de Wall EH, Vrang N, Larsen PJ, Schwartz GJ. Rimonabant induced anorexia in rodents is not mediated by vagal or sympathetic gut afferents. Neurosci Lett. 2009;449:20–23. doi: 10.1016/j.neulet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Makriyannis A, Vemuri VK, Thotapally R, Olszewska T. International Patent Application. 2010. No: PCT/US09/01054.
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Morse KL, Fournier DJ, Li X, Grzybowska J, Makriyannis A. A novel electrophilic high affinity irreversible probe for the cannabinoid receptor. Life Sci. 1995;56:1957–1962. doi: 10.1016/0024-3205(95)00176-7. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci. 2008;1139:426–433. doi: 10.1196/annals.1432.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol. 2003;550:149–158. doi: 10.1113/jphysiol.2003.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino G, Barbier de la SC, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon FJ, Bilbao A, Hernandez-Folgado L, Cippitelli A, Jagerovic N, Abellan G, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole – LH 21. Neuropharmacology. 2006;51:358–366. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Ferreris Torres E, Hosmer S, Nunes EJ, et al. 2010 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2010. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Fadda P, Killop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Benzaquen J, Limebeer CL, Parker LA. Potential of the rat model of conditioned gaping to detect nausea produced by rolipram, a phosphodiesterase-4 (PDE4) inhibitor. Pharmacol Biochem Behav. 2009;91:537–541. doi: 10.1016/j.pbb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Hollander P, Chevalier S, Iranmanesh A. SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care. 2008;31:2169–2176. doi: 10.2337/dc08-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010 doi: 10.1172/JCI42551. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Rawls SM, Whiteside GT, Walker EA. Age-dependent effects of the cannabinoid CB1 antagonist SR141716A on food intake, body weight change, and pruritus in rats. Psychopharmacology (Berl) 2009;206:155–165. doi: 10.1007/s00213-009-1592-6. [DOI] [PubMed] [Google Scholar]
- Yamada K, Onoda Y. Comparison of the effects of T-1815, yohimbine and naloxone on mouse colonic propulsion. J Smooth Muscle Res. 1993;29:47–53. doi: 10.1540/jsmr.29.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


