Abstract
BACKGROUND AND PURPOSE
Although spontaneous Ca2+ waves in interstitial cells of Cajal (ICC)-like cells (ICC-LCs) primarily arise from endoplasmic reticulum (ER) Ca2+ release, the interactions among mitochondrial Ca2+ buffering, cellular energetics and ER Ca2+ release in determining the spatiotemporal dynamics of intracellular Ca2+ remain to be elucidated.
EXPERIMENTAL APPROACH
Spontaneous Ca2+ transients in freshly isolated ICC-LCs of the rabbit urethra were visualized using fluo-4 Ca2+ imaging, while the intracellular distribution of mitochondria was viewed with MitoTracker Red.
KEY RESULTS
Spontaneous Ca2+ waves invariably originated from the perinuclear region where clusters of mitochondria surround the nucleus. Perinuclear Ca2+ dynamics were characterized by a gradual rise in basal Ca2+ that preceded each regenerative Ca2+ transient. Caffeine evoked oscillatory Ca2+ waves originating from anywhere within ICC-LCs. Ryanodine or cyclopiazonic acid prevented Ca2+ wave generation with a rise in basal Ca2+, and subsequent caffeine evoked a single rudimentary Ca2+ transient. Inhibition of glycolysis with 2-deoxy-glucose or carbonyl cyanide 3-chlorophenylhydrazone, a mitochondrial protonophore, increased basal Ca2+ and abolished Ca2+ waves. However, caffeine still induced oscillatory Ca2+ transients. Mitochondrial Ca2+ uptake inhibition with RU360 attenuated Ca2+ wave amplitudes, while mitochondrial Ca2+ efflux inhibition with CGP37157 suppressed the initial Ca2+ rise to reduce Ca2+ wave frequency.
CONCLUSIONS AND IMPLICATIONS
Perinuclear mitochondria in ICC-LCs play a dominant role in the spatial regulation of Ca2+ wave generation and may regulate ER Ca2+ release frequency by buffering Ca2+ within microdomains between both organelles. Glycolysis inhibition reduced mitochondrial Ca2+ buffering without critically disrupting ER function. Perinuclear mitochondria may function as sensors of intracellular metabolites.
Keywords: ICC-like cell, urethra, spontaneous Ca2+ wave, perinuclear mitochondria, endoplasmic reticulum, inhibition of glycolysis
Introduction
Smooth muscle of the urethra generates spontaneous tone to maintain urinary continence (Bridgewater et al., 1993). This tone arises from smooth muscle bundles generating spontaneous phasic contractions triggered upon Ca2+ influx through L-type Ca2+ channels opened during the plateau phase of slow waves (Hashitani et al., 1996; Hashitani and Edwards, 1999; channel nomenclature follows Alexander et al., 2009). These slow waves are considered to result from the temporal summation of spontaneous transient depolarizations (STDs) that arise from the release of Ca2+ from intracellular stores and the opening of Ca2+-activated chloride channels (Hashitani and Edwards, 1999).
STDs in the urethra appear to be generated in specialized cells that have similar morphological characteristics to interstitial cells of Cajal (ICC) in the gastrointestinal (GI) tract (Sanders et al., 2006), and are clearly distinguishable from smooth muscle cells (Sergeant et al., 2000). The cells can be identified by their immunereactivity to Kit antibodies, a specific marker for ICC, and thus are referred to as ICC-like cells (ICC-LCs, Hashitani and Suzuki, 2007; Lyons et al., 2007). ICC-LCs in the urethra do not form an extensive network (Lyons et al., 2007), nor act as a synchronous electrical pacemaker of smooth muscle activity as do ICC in the GI tract (Hashitani and Suzuki, 2007). Rather, ICC-LCs are thought to electrically drive individual smooth muscle bundles by means of their localized STD discharge, which increases the overall excitability of the urethral smooth muscle.
The development of freshly isolated single ICC-LC preparations from the rabbit urethra has confirmed many of the suppositions made from previous in situ experiments. Spontaneous inward currents (STICs) and their corresponding STDs have been directly demonstrated to arise from the opening of Ca2+-activated Cl- channels that are triggered by the release of stored Ca2+ from the endoplasmic reticulum (ER) (Sergeant et al., 2000; 2001; Johnston et al., 2005). Moreover, the initiation of spontaneous activity of ICC-LCs primarily depends on the Ca2+ release through ryanodine receptor Ca2+ channels in the ER membrane. Inositol trisphosphate receptor (IP3R) Ca2+ channels are thought to play a fundamental role in the intracellular propagation of Ca2+ waves to cause a synchronous activation of the Ca2+-activated chloride channels underlying slow wave generation (Johnston et al., 2005).
More recently, the involvement of mitochondrial Ca2+ buffering in the generation of spontaneous Ca2+ waves and STICs has been suggested (Sergeant et al., 2008). Similar cooperation between ER and mitochondria in generating spontaneous electrical and Ca2+ activity has been shown for ICC in the GI tract (Ward et al., 2000), ICC-LCs in the gall bladder (Balemba et al., 2008) and in atypical smooth muscle cells, the pacemaker cells in the renal pelvis (Hashitani et al., 2009). However, what role these two distinct organelles play in the spatiotemporal dynamics of intracellular Ca2+ is not yet well understood. As ATP production by mitochondria is not critically involved in the generation of spontaneous Ca2+ waves (Ward et al., 2000; Balemba et al., 2008; Sergeant et al., 2008), even though refilling of the ER fundamentally depends on Ca2+ uptake via the sarco-ER Ca2+ ATPase (SERCA) pump, the metabolic basis for spontaneous activity in ICC or ICC-like cells has yet to be resolved.
In the present study, spontaneous Ca2+ waves in ICC-LCs freshly isolated from the rabbit urethra were visualized using fluo-4 fluorescence, and their spatial relationship with the intracellular distribution of mitochondria was established with the vital mitochondria stain, MitoTracker Red. We also examined the effects of pharmacologically manipulating Ca2+ uptake into and release from the ER and mitochondria, as well as the effects of inhibiting glycolysis on Ca2+ wave generation and propagation.
Methods
Tissue preparation
All animal care and experimental procedures have been approved by the animal experimentation ethics committee of Nagoya City University. Male rabbits, weighing 2–3 kg, were killed by exsanguination under pentobarbitone anaesthesia. The urethra and bladder were removed, and the urethra was dissected free of the bladder approximately 3 cm distal to the bilateral ureteral entries. The dorsal wall of the urethra was opened longitudinally; the mucosa and periurethral connective tissues were then dissected away. Under a dissecting microscope, the most proximal part of the urethra, approximately 1 cm wide, was isolated from the remaining parts of the tissue. During preliminary experiments, ICC-LCs were most readily isolated from the longitudinal smooth muscle layer in comparison with either the circular smooth muscle layer or lamina propria. Thus, longitudinal layers were carefully dissected away from circular smooth muscle layers, and were then processed for cell isolation.
Cell isolation
Longitudinal smooth muscle portions were cut into small pieces approximately 3 × 3 mm and less than 1 mm thickness, and stored for 30 min in Ca2+ free HEPES-buffered dispersal solution (DS) [composition (in mM): Na+ 126, K+ 6, Mg2+ 1, Cl– 134, glucose 10 and HEPES 10; pH adjusted to 7.4 with NaOH]. The muscle pieces were then incubated in Ca2+-free DS containing 3 mg collagenase (Worthington, Lakewood, NJ, USA, type I), 0.2 mg protease (Sigma type XXIV), 2 mg BSA (Sigma, St. Louis, MO, USA) and 2 mg trypsin inhibitor (Sigma), for approximately 10 min at 37°C. The preparations were then placed in low Ca2+ DS ([Ca2+] = 100 µM) for 30 min at room temperature. Single cells were obtained by gentle agitation for 5–10 min with a wide-bore pipette; cells in suspension were transferred to glass bottom recording chambers and kept for 10–20 min at room temperature to allow adherence to the glass coverslip.
Intracellular Ca2+ imaging
To image the intracellular Ca2+ dynamics in freshly isolated ICC-LCs, cells were incubated in HEPES-buffered low-Ca2+ DS ([Ca2+] = 100 µM) containing 1 µM fluo-4 AM (special packaging, Dojindo, Kumamoto, Japan) for 5–10 min at room temperature. Following incubation, the cells were superfused with dye-free, warmed (36°C) HEPES-buffered PSS [composition (in mM): Na+ 137.5, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, H2PO4- 1.2, Cl- 134, glucose 10 and HEPES 10; pH adjusted to 7.4 with NaOH] at a constant flow rate (about 2 mL·min–1) for 30 min.
The recording chamber was mounted on the stage of an upright epifluorescence microscope (BX51WI, Olympus, Tokyo, Japan) equipped with an electron multiplier CCD camera (C9100, Hamamatsu Photonics, Hamamatsu, Japan) and a high-speed scanning polychromatic light source (C7773, Hamamatsu Photonics). Cells were viewed with a water immersion objective (LUMPlanFI x60, Olympus) and illuminated at 495 nm. The fluorescence emission was captured through a barrier filter above 515 nm, and images were obtained every 23–200 ms (frame interval) with an exposure time of 17.4–58.7 ms using a micro-photoluminescence measurement system (AQUACOSMOS, Hamamatsu Photonics). Relative amplitude of Ca2+ transients was expressed as the ratio (F/F0) of the fluorescence generated by an event (F) against baseline (F0).
Intracellular imaging of mitochondria, ER and nuclei
To assess the intracellular distribution of mitochondria or nuclei, ICC-LCs were incubated with either 10 nM MitoTracker Red (Invitrogen, Carlsbad, CA, USA) for 10 min at room temperature, or 100 nM Hoechst 33342 (Invitrogen) for 5 min at room temperature respectively. ICC-LCs were then superfused with dye-free, warmed (36°C) HEPES-buffered PSS ([Ca2+] = 2.5 mM) at a constant flow rate (about 2 mL·min–1) for 20 min. Images of fluorescent organelles were acquired using the same epifluorescence microscope and objectives as above. MitoTracker Red was excited at 580 nm, and emission was collected at 600 nm. Hoechst 33342 was excited at 360 nm, and emission was detected at 460 nm. Only ICC-LCs in which staining of either mitochondria or nuclei did not disrupt the generation of spontaneous Ca2+ waves were included for spatial Ca2+ dynamics analysis.
To assess the intracellular distribution of ER relative to the mitochondria distribution, ICC-LCs which had been stained with MitoTracker Red were subsequently incubated with 1 µM ER-Tracker Blue-White DPX (Invitrogen) for 15 min at room temperature. ICC-LCs were then observed immediately after incubation in the probe solution, as staining was reduced considerably on its removal (Cole et al., 2000). ER-Tracker Blue-White DPX was excited at 360 nm, and emission was detected at 560 nm.
Data analysis
The following parameters of spontaneous Ca2+ transients were measured: peak amplitude, measured as the value from the resting level to the peak of events; half-amplitude duration, measured as the time between 50% peak amplitude on the rising and falling phases; and frequency, which was defined as an average over 3 or 5 min of recording. In some cells, the conducting velocity of spontaneous Ca2+ waves, the rate of initial Ca2+ rise or the decay of Ca2+ transients was also calculated.
Measured values were expressed as mean ± SD. Statistical significance was tested using paired Student's t-test, and considered significant if P < 0.05. The number of cells examined was expressed by n, while N indicates the number of animals.
Materials
Drugs used were caffeine, cyclopiazonic acid (CPA), carbonyl cyanide 3-chlorophenylhydrazone (CCCP), 2-deoxy-glucose (2DG), nicardipine, nifedipine (from Sigma-Aldrich), CGP37157, RU360 and ryanodine (from Calbiochem, La Jolla, CA, USA). 2DG and RU360 were dissolved in distilled water, and CPA, CCCP, CGP37157 and nicardipine were dissolved in dimethyl sulphoxide (DMSO). Nifedipine was dissolved in ethanol. Caffeine was directly dissolved in PSS. Glucose-free/2DG solution was prepared by replacing glucose with 2DG. Nominally, a Ca2+-free solution was prepared by omitting CaCl2 from PSS. The final concentration of DMSO or ethanol in the PSS did not exceed 1:1000.
Results
Intracellular distribution of mitochondria and ER
ICC-LCs freshly isolated from the longitudinal smooth muscle layer of the rabbit urethra had a variety of morphologies that were distinct from the smooth muscle cells that had a smooth spindle-shaped cell body. ICC-LCs had a long, thin cell body with either several distinct angles (42.0% of n = 278 cells from N = 48 rabbits, Figure 1Aa), spiky processes (31.2%, Figure 1Ba) or a short spindle-shaped cell body with many spiky processes (26.8%, Figure 2Ca). In all ICC-LCs, nuclei were readily viewed under bright field illumination (Figure 1Ab), and their location could be confirmed by staining with Hoechst 33342 (Figure 1Ba–c). Upon staining with MitoTracker Red, mitochondria were observed to be preferentially clustered around the nucleus (Figure 1Ab,c and Bb,c), while other short filamentous mitochondria were scattered along the long axis of the cell body in a daisy chain arrangement (Figure 1Ad).
Figure 1.
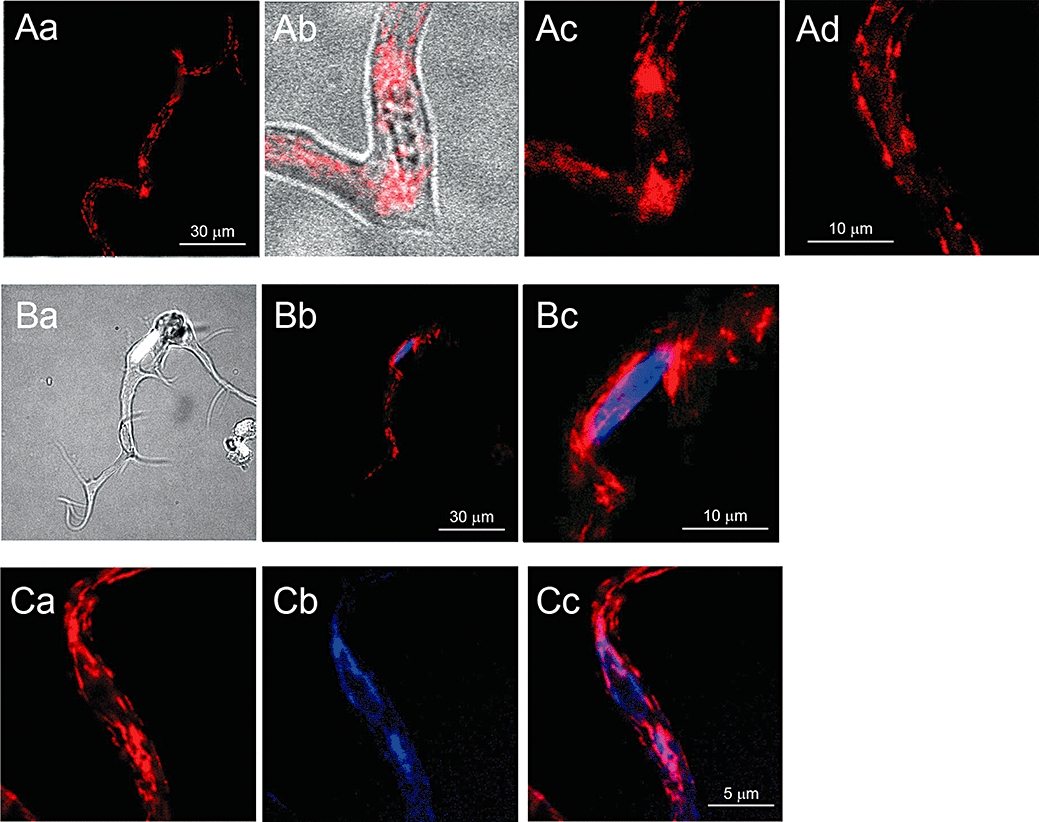
Intracellular distribution of mitochondria and ER in urethral ICC-like cells. Fluorescent images of an ICC-LC loaded with MitoTracker-Red (Aa). Perinuclear clusters of mitochondria are evident on both sides of the nucleus in an enlarged overlay image of bright field and MitoTracker-Red (Ab). Enlarged images indicate perinuclear clusters of mitochondria (Ac), while filamentous mitochondria are distributed in the cell periphery (Ad). An overlay image of another ICC-LC with a bright field view and Hoechst 33342 staining (Ba), and a corresponding overlay fluorescent image with Hoechst 33342 and MitoTracker-Red staining (Bb). An enlarged overlay image of perinuclear region illustrating that clusters of mitochondria surround the nucleus (Bc). In a different ICC-LC, in which perinuclear clusters of mitochondria were stained with MitoTracker-Red (Ca), the ER, stained with ER-Tracker, was also distributed around the nucleus (Cb). An overlay image showing a close apposition of perinuclear mitochondria with ER (Cc).
Figure 2.
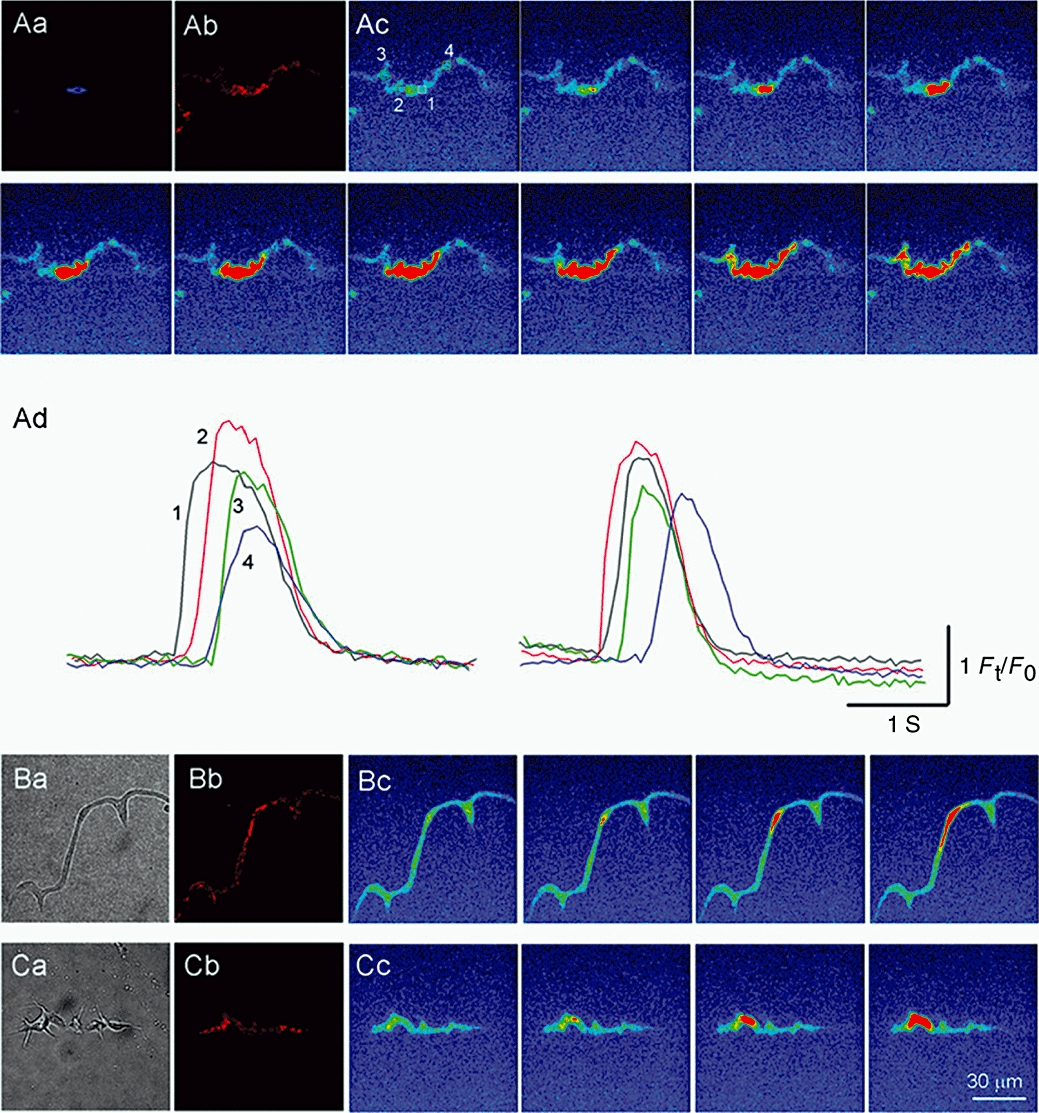
Spatial relationship between perinuclear mitochondria and spontaneous Ca2+ waves in urethral ICC-LCs. Fluorescent images of an ICC-LC loaded with Hoechst Blue (Aa) and MitoTracker-Red (Ab). Sequential fluo-4 fluorescent images of a spontaneous Ca2+ wave (Ac) with frame interval of 100 ms. Temporal sequence of Ca2+ transients captured from four areas in ICC-LC (Ad). Spontaneous Ca2+ waves originated from either side of perinuclear mitochondria and spread to the cell periphery. Bright field (Ba) and corresponding MitoTracker-Red fluorescent (Bb) images of another ICC-LC. Sequential fluo-4 fluorescent images showing a Ca2+ wave originating from perinuclear mitochondria (Bc). Bright field (Ca) and corresponding MitoTracker-Red (Cb) images of a different ICC-LC. Sequential fluo-4 fluorescent images showing a Ca2+ wave originating from perinuclear mitochondria (Cc).
The intracellular distribution of ER was also examined by staining ICC-LCs with ER-Tracker Blue-White DPX. In ICC-LCs that expressed perinuclear mitochondrial staining (Figure 1Ca), ER was also clustered around the nucleus (Figure 1Cb), demonstrating the co-localization of mitochondria and ER in perinuclear regions of ICC-LCs (Figure 1C,c, n = 23, N = 8).
Spatial dynamics of Ca2+ waves
All experiments were carried out in the presence of nifedipine (1 µM) or nicardipine (1 µM) to exclude a possible contribution of Ca2+ influx through L-type Ca2+ channels to the intracellular Ca2+ dynamics in ICC-LCs.
Two hundred sixteen ICC-LCs isolated from 48 animals exhibited spontaneous Ca2+ waves that invariably originated from the perinuclear region. In Figure 2, three representative ICC-LCs firing spontaneous Ca2+ waves have also been stained to illustrate their spatial distribution of mitochondria. In an ICC-LC in which mitochondria mostly surrounded the nucleus (Figure 2Aa,b), spontaneous Ca2+ waves originated from either side of the perinuclear region (Figure 2Ac; Supporting Information Video Clip S1). Not only did these Ca2+ transients travel to the periphery on the same side of the nucleus from which they originated, they also readily travelled across the nucleus and spread towards the periphery of the opposite side (Figure 2Ad). In ICC-LCs that had either long, thin cell bodies (Figure 2B) or had short spindle cell bodies with many spiky branches (Figure 2C), mitochondria were clustered around the perinuclear regions, as well as scattered throughout the periphery (Figure 2B). In both cell types, spontaneous Ca2+ waves originated from the perinuclear regions and propagated to their cell periphery. Thus, regardless of cell morphology or the location of the nucleus, spontaneous Ca2+ waves invariably originated from the perinuclear regions, suggesting that perinuclear mitochondria may play an important role in determining the spatiotemporal dynamics of Ca2+ waves in ICC-LCs.
Properties of perinuclear and peripheral Ca2+ transients
The triggering of spontaneous perinuclear Ca2+ waves in some cells was characterized by an initial gradual rise in Ca2+ level that preceded individual Ca2+ transients (52.5% of n = 216 cells, Figure 3A). In 13.9% of cells, perinuclear Ca2+ waves were preceded by a number of Ca2+ transients which gradually grew in amplitude, all of which were triggered from a relatively stable (flat) baseline (Figure 3C). The remaining 37% of cells exhibited a combination of these two discharge patterns such that increasing amplitude Ca2+ transients were recorded on a rising baseline (Figure 3B) before the main Ca2+ wave discharge. In peripheral regions of ICC-LCs, small amplitude non-propagating Ca2+ transients were often recorded (Figure 3D). These small non-propagating Ca2+ transients invariably failed to fire during the period following the passing of a propagating Ca2+ waves (Figure 3D). Alternatively, the cell periphery could be abruptly ‘awakened’ from quiescence by Ca2+ waves that propagated from the perinuclear regions.
Figure 3.
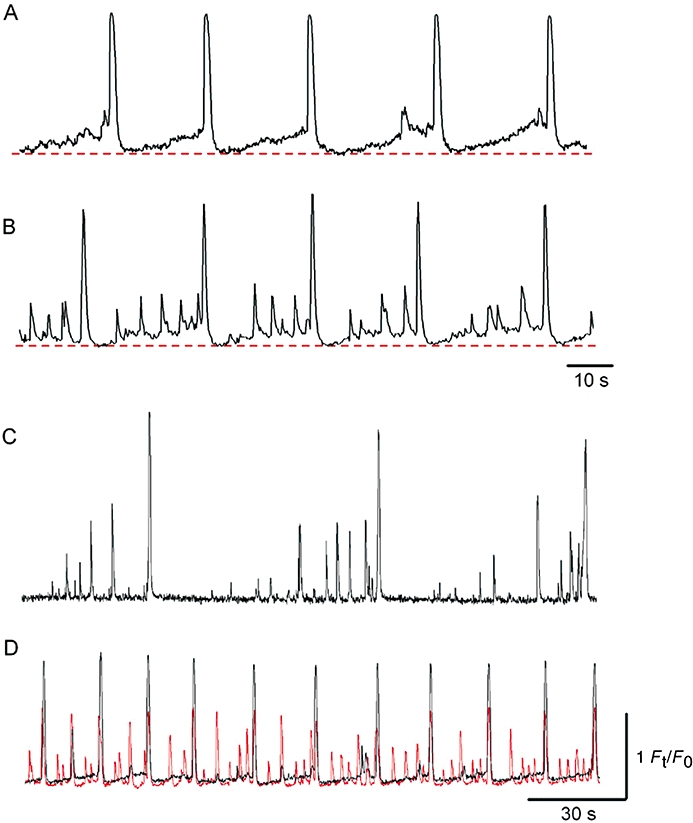
Properties of perinuclear Ca2+ transients in ICC-LCs. In an ICC-LC, perinuclear Ca2+ transients were characterized by an initial gradual rise in basal Ca2+ that preceded each regenerative Ca2+ transient (A). In another ICC-LC, a gradual rise in basal Ca2+ that was superimposed by ongoing Ca2+ fluctuations preceded each Ca2+ transient (B). The time-scale for (B) also refers to (A). In a different ICC-LC, each Ca2+ transient was followed by a quiescent period and subsequent increasing amplitude small Ca2+ fluctuations that preceded the next Ca2+ transient (C). A simultaneous recording of perinuclear and peripheral Ca2+ transients in an ICC-LC (D). In the cell periphery (red), small Ca2+ fluctuations were generated, while regenerative Ca2+ transients were triggered by Ca2+ waves propagated from perinuclear regions. The time-scale for (D) also refers to (C); the fluorescence scale for (D) refers to all traces.
Spontaneous Ca2+ waves were generated in perinuclear regions at a frequency of 2.6 ± 1.2 min−1, and propagated to the cell periphery at a velocity of 68.8 ± 25.2 µm·ms–1 (n = 51, N = 23). Perinuclear Ca2+ transients had an amplitude of 1.94 ± 0.75 Ft/F0 and a half-width of 1.1 ± 0.38 s (n = 51, N = 23). Perinuclear Ca2+ transients had a faster falling phase in comparison to peripheral Ca2+ transients: the decay time constant (τ) for perinuclear Ca2+ transients was 410 ± 128 ms and significantly smaller than the τ value for peripheral Ca2+ transients (550 ± 174 ms; P < 0.05, n = 45, N = 20).
Effects of caffeine on Ca2+ dynamics
Caffeine (10 mM) evoked Ca2+ waves that gradually reduced in amplitude and prevented the further generation of spontaneous Ca2+ waves (Figure 4, n = 7, N = 6), suggesting that continuous activation of ryanodine receptors results in a functional depletion of Ca2+ in the ER. The initial perinuclear Ca2+ transient induced by caffeine had an amplitude of 2.3 ± 0.51 Ft/F0, which was 108.5 ± 5.6% of the amplitude of their corresponding control spontaneous Ca2+ transients. In ICC-LCs that remained quiescent (n = 3, N = 3) or generated only small Ca2+ fluctuations (n = 2, N = 2), caffeine evoked oscillatory Ca2+ waves. It should be noted that caffeine-induced Ca2+ waves originated from sites anywhere along the length of each ICC-LC, and thus perinuclear regions did not act as a dominant site of the origin of caffeine-induced Ca2+ waves (Figure 4).
Figure 4.
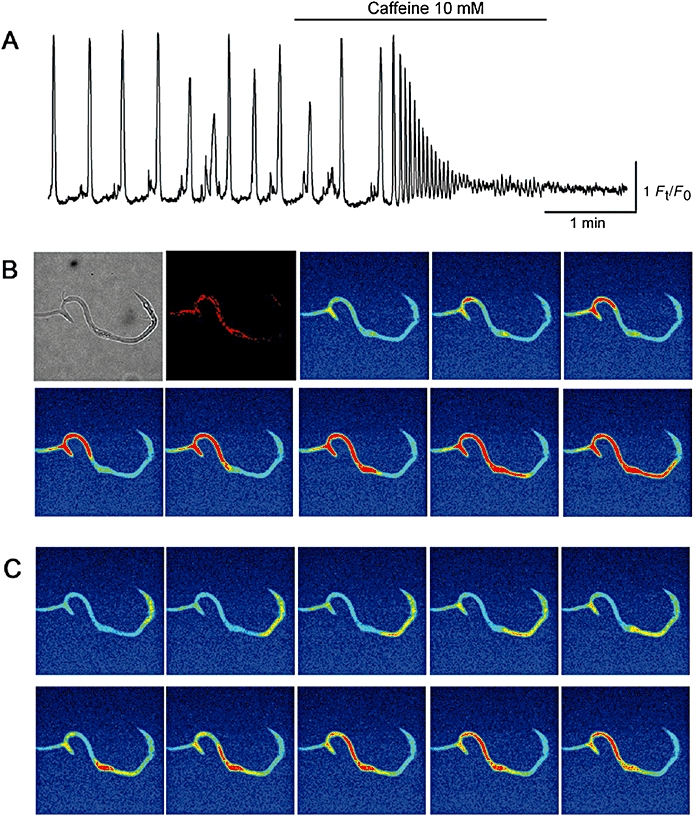
Effects of caffeine on Ca2+ dynamics in ICC-LCs. In an ICC-LC that generated spontaneous Ca2+ waves, caffeine (10 mM) evoked oscillatory Ca2+ transients (A). The amplitude of caffeine-induced Ca2+ transients gradually reduced until the generation of spontaneous Ca2+ waves ceased. In another ICC-LC which expressed MitoTracker Red fluorescence in its perinuclear regions, caffeine triggered Ca2+ waves from distinct peripheral sites that travelled across the nucleus towards the other side of the cell periphery (B, C).
Role of ER Ca2+ handlings and extracellular Ca2+ in Ca2+ wave generation
The role of Ca2+ handling by ER in the temporal dynamics of perinuclear Ca2+ transients was examined by manipulating ER Ca2+ sequestration and release, using CPA and ryanodine respectively. The ER Ca2+ content remaining after exposure to either CPA or ryanodine was estimated by exposing cells to caffeine (10 mM).
CPA (10 µM) increased the basal Ca2+ level by 0.36 ± 0.11 Ft/F0 and prevented the further generation of spontaneous Ca2+ waves (Figure 5Aa, n = 10, N = 9). Subsequent exposure to caffeine (10 mM) caused a single, rudimentary Ca2+ transient that had an absolute amplitude of 0.21 ± 0.08 Ft/F0. The peak value of Ca2+ recorded upon exposure to caffeine was 25.1 ± 5.3% of the untreated spontaneous Ca2+ transients. These results are summarized in Figure 5Ab, and suggest that CPA prevention of Ca2+ waves results from the depletion of ER Ca2+ content, and that continuous Ca2+ uptake by SERCA is fundamental in maintaining both ER levels of releasable Ca2+ and a low basal cytosolic Ca2+.
Figure 5.
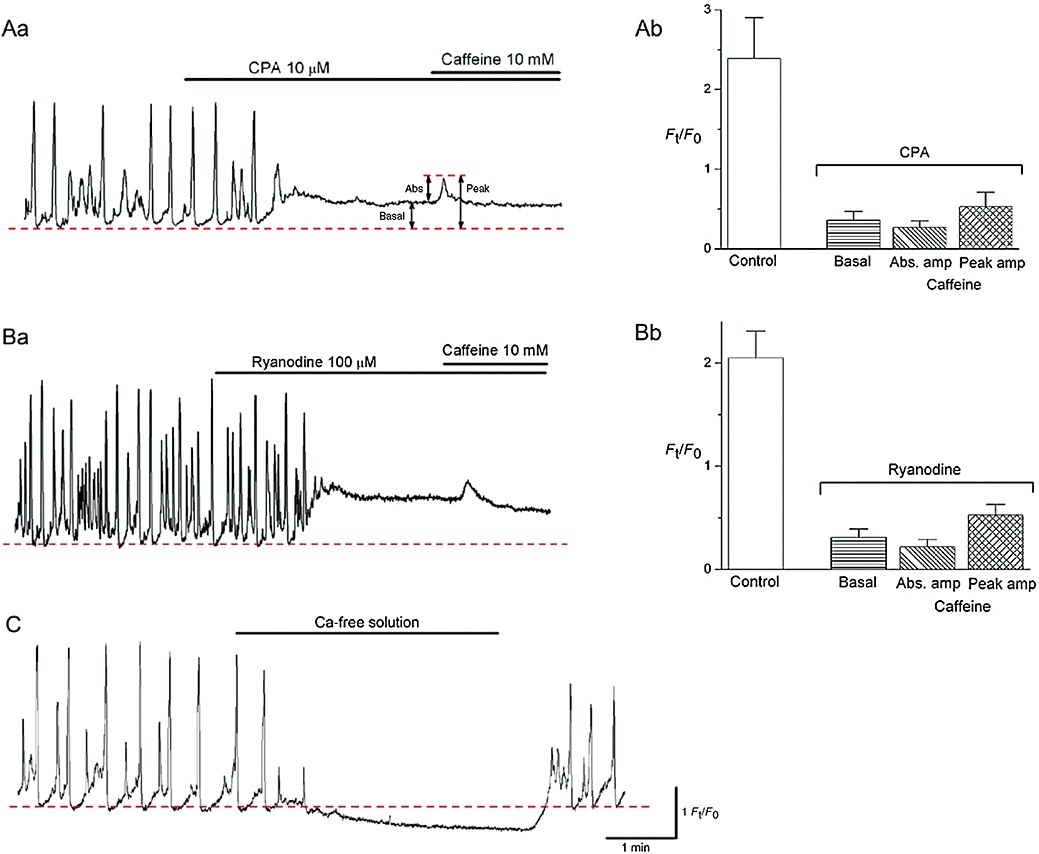
Effects of CPA, ryanodine and Ca2+-free solution on perinuclear Ca2+ transients in ICC-LCs. CPA (10 µM) prevented the generation of spontaneous Ca2+ transients with a rise in the basal Ca2+ (Aa). Subsequent application of caffeine evoked a single rudimentary Ca2+ transient. The results obtained from 10 ICC-LCs are summarized in (Ab). In another spontaneously active ICC-LC, ryanodine (100 µM) abolished spontaneous Ca2+ transients with an increase in basal Ca2+ (Ba). The subsequent addition of caffeine caused only a single small Ca2+ transient. The results obtained from six ICC-LCs were summarized in (Bb). In (Ab) and (Bb), control indicates the amplitude of spontaneous Ca2+ transients, and basal, Abs.amp and peak amp indicate the corresponding values shown in (Aa). In a different ICC-LC, switching from PSS to nominally Ca2+-free solution caused a reduction in basal Ca2+ and cessation of Ca2+ transients that recovered upon the re-admission of extracellular Ca2+ (C). The scale bars for (C) also refer to Aa and Ba.
Ryanodine (100 µM, n = 8, N = 6) abolished spontaneous Ca2+ waves with a rise in the basal Ca2+ level of 0.26 ± 0.11 Ft/F0 (Figure 5Ba). The subsequent application of caffeine evoked only a single small Ca2+ transient that had an amplitude of 0.2 ± 0.06 Ft/F0 and a peak value that was 26.4 ± 6.7% of control spontaneous Ca2+ transients. These results are summarized in Figure 5Bb, and suggest that the ryanodine-induced rises in basal Ca2+ level may be due to the activation of ryanodine receptors that results in a critical reduction of releasable Ca2+ from the ER.
Although perinuclear Ca2+ transient generation relied on ER Ca2+ handing, nominally Ca2+-free PSS readily prevented the generation of Ca2+ transients associated with a fall in the basal Ca2+ level of 0.31 ± 0.10 Ft/F0 (n = 5, N = 4; Figure 5C). Upon returning to normal PSS, both spontaneous Ca2+ transients and basal Ca2+ levels were immediately restored.
Role of mitochondrial Ca2+ handlings in Ca2+ wave generations
The role of mitochondrial Ca2+ uptake in the temporal dynamics of spontaneous perinuclear Ca2+ waves was examined by disrupting the mitochondrial inner membrane potential (ΔΨm), using the protonophore CCCP, which results in an inhibition of Ca2+ influx. The Ca2+ content of the ER under these conditions was again estimated by exposing cells to caffeine.
CCCP (1 µM) increased basal Ca2+ levels by 0.40 ± 0.12 Ft/F0 and prevented the generation of spontaneous Ca2+ waves (Figure 6Aa). Subsequent exposure to caffeine (10 mM) induced oscillatory Ca2+ transients with a mean amplitude of 1.68 ± 0.12 Ft/F0; the peak value of the caffeine-induced Ca2+ transient was not significantly different (102 ± 5%, P > 0.05, n = 8, N = 6) from the control spontaneous Ca2+ transients. These results are summarized in Figure 6Ab and suggest that disruption of the mitochondrial inner membrane potential was not associated with a critical suppression of SERCA or ER Ca2+ storage. More likely, abolition of Ca2+ waves induced by CCCP resulted from a reduction of Ca2+ influx into mitochondria subsequent to the collapse of ΔΨm.
Figure 6.
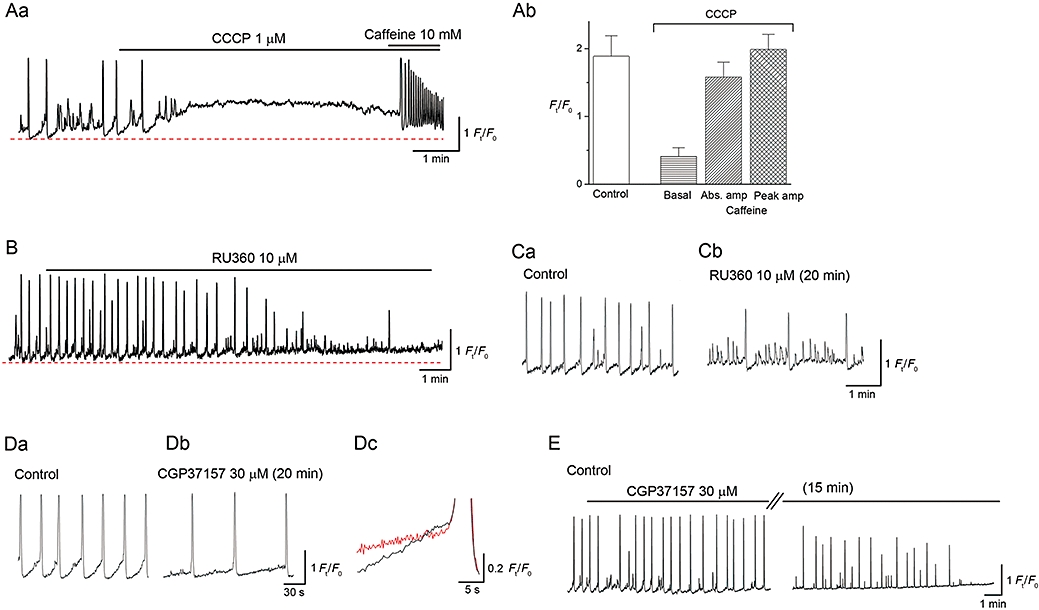
Effects of CCCP, RU360 and CGP37157 on perinuclear Ca2+ transients in ICC-LCs. In an ICC-LC developing spontaneous Ca2+ transients, CCCP (1 µM) prevented the generation of Ca2+ transients with a rise in the basal Ca2+ (A). Subsequent caffeine exposure evoked oscillatory Ca2+ transients. The results obtained from eight ICC-LCs are summarized in (B). In another spontaneously active ICC-LC, RU360 (10 µM) abolished spontaneous Ca2+ transients with an increase in basal Ca2+ (C). In a different ICC-LC, RU360 reduced the frequency of spontaneous Ca2+ transients and induced small Ca2+ fluctuations (Ca,b). CGP37157 (30 µM) reduced the frequency of spontaneous Ca2+ transients (Da,b) by suppressing the initial gradual rise in Ca2+ (Dc). In a different ICC-LC, CGP37157 eventually abolished spontaneous Ca2+ transients with an increase in basal Ca2+ (E).
The role of mitochondrial Ca2+ influx in spontaneous Ca2+ dynamics was more specifically investigated using RU360, an inhibitor of the mitochondrial Ca2+ uniporter. In six (N = 4) out of nine ICC-LCs (N = 6), RU360 (10 µM for 5–10 min) increased the basal Ca2+ level and prevented the generation of spontaneous Ca2+ waves, although some small, non-propagating Ca2+ fluctuations remained (Figure 6B). In the three other (N = 3) ICC-LCs, RU360 reduced the frequency of spontaneous Ca2+ waves and increased the occurrence of small Ca2+ fluctuations, but did not prevent Ca2+ wave generation even after 30 min of exposure (Figure 6C).
In seven ICC-LC (N = 6), CGP37157, an inhibitor of the mitochondrial Na+ : Ca2+ exchanger, reduced the frequency of spontaneous Ca2+ waves by suppressing the initial gradual rise in basal Ca2+ level that preceded Ca2+ transients, but did not abolish Ca2+ waves even after 30 min (Figure 6D). The slope of the initial Ca2+ rises was 0.022 ± 0.005 Ft/F0 s−1 in control, 0.009 ± 0.004 Ft/F0 s−1 in CGP37157 (P < 0.05, n = 7). In three other cells (N = 2), CGP37157 (30 µM) reduced the frequency of spontaneous Ca2+ waves and eventually prevented Ca2+ wave generation after 15–25 min in a manner associated with an increase in basal Ca2+ level (Figure 6E).
Role of glycolysis in the generation of Ca2+ waves
Because reducing the electrical driving force for ATP synthesis with CCCP did not critically reduce the Ca2+ content of the ER (Figure 5), mitochondrial ATP production may not be fundamental for a functioning SERCA, at least over the short period of our experiments. This view is supported by the recent finding that oligomycin, a blocker of mitochondrial ATP synthetase, did not affect spontaneous Ca2+ waves in urethral ICC-LCs (Sergeant et al., 2008).
To investigate the role of glycolysis in both ATP production and mitochondrial substrates supply, the effects of 2DG on ICC-LC Ca2+ waves were examined in 15 ICC-LCs (N = 12). 2DG (10 mM) prevented the generation of spontaneous Ca2+ waves within 5–20 min, which was associated with a rise in basal Ca2+ level. These effects were readily reversible upon returning to normal glucose containing PSS (Figure 7A). However, in nine ICC-LCs (N = 9), the subsequent addition of caffeine (10 mM) in the continuing presence of 2DG still induced oscillatory Ca2+ transients, suggesting that inhibition of glycolysis did not critically reduce the ER Ca2+ content. The rise in basal Ca2+ in 2DG suggests that reducing the supply of mitochondrial substrates is vital to maintaining ΔΨm, which, in turn, is essential in keeping the cytosolic Ca2+ concentration at low levels.
Figure 7.
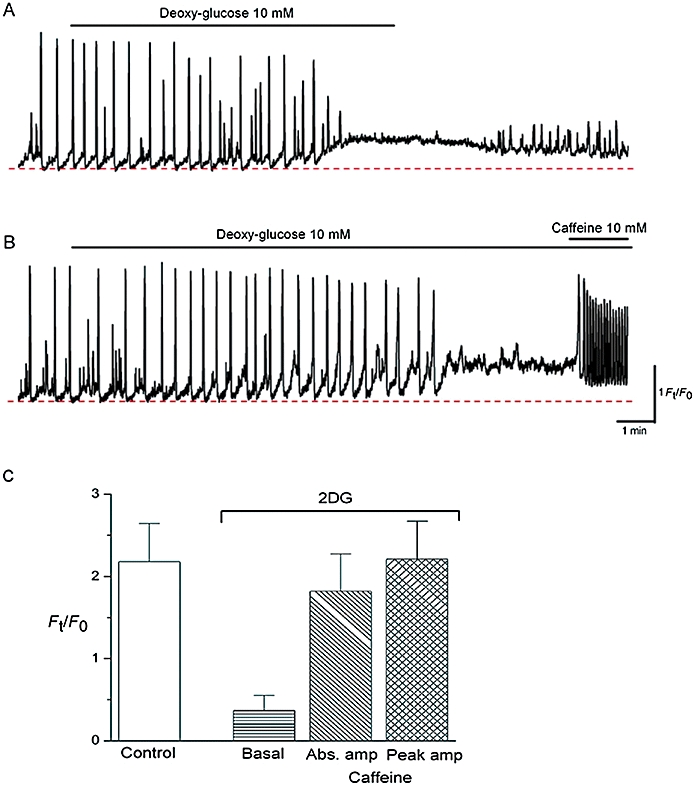
Effects of 2DG on perinuclear Ca2+ transients in ICC-LCs. In an ICC-LC that displayed spontaneous Ca2+ transients, 2DG increased the basal Ca2+ which suppressed the generation of spontaneous Ca2+ transients (A). Upon switching from 2DG/glucose-free PSS back to normal PSS, Ca2+ gradually returned to the original level and small Ca2+ fluctuations re-appeared. In another ICC-LC, 2DG increased the basal Ca2+ which eventually abolished Ca2+ wave generation (B). Upon the cessation of Ca2+ transients, a subsequent exposure to caffeine evoked oscillatory Ca2+ transients. Effects of 2DG and caffeine obtained from nine ICC-LCs are summarized in (C).
Discussion and conclusions
In ICC-LCs freshly isolated from longitudinal smooth muscle layer of the rabbit urethra, clusters of mitochondria surround the nucleus, while filamentous mitochondria were scattered throughout the cell periphery. Spontaneous Ca2+ waves invariably originated from the perinuclear regions where these mitochondrial clusters were situated, and propagated to the cell periphery, suggesting that perinuclear mitochondria may play a fundamental role in determining spatial dynamics of Ca2+ in ICC-LCs. Spontaneous Ca2+ waves in ICC-LCs are associated with the generation of STDs (Sergeant et al., 2000) that transmit to adjacent smooth muscle cells to depolarize their membranes (Hashitani and Suzuki, 2007). Besides the generation of electrical activity, intracellular Ca2+ signalling is also crucially involved in many other cell functions, including gene transcription, protein synthesis and cell proliferation, and thus spatio-temporal Ca2+ dynamics have to be finely controlled (Bootman et al., 2001). The initiation of spontaneous Ca2+ waves in perinuclear regions may indicate a cross-talk between the nucleus and mitochondria.
Mitochondrial Ca2+ uptake is also critical in regulating mitochondrial oxidative metabolism, that is, ATP synthesis, because several mitochondrial dehydrogenases are sensitive to mitochondrial Ca2+ concentrations (Robb-Gaspers et al., 1998; Jouaville et al., 1999). In parotid acinar cells, in which a majority of mitochondria are located around the nucleus, agonist-evoked Ca2+ waves are delayed as they propagate through the nucleus, and are accompanied by a substantial increase in NADH autofluorescence (Bruce et al., 2004). It has been suggested that perinuclear mitochondria may have a potential role in shaping nuclear Ca2+ signals, but more importantly, drive mitochondrial metabolism to generate ATP close to the nucleus (Bruce et al., 2004). A similar tight energetic transport between the perinuclear mitochondria and the nucleus in urethra ICC-LCs may explain the lack of inhibition of SERCA after pharmacological disruption of mitochondrial function in the present experiments.
The Ca2+ release from ER through ryanodine receptors has been considered to be the primary step of Ca2+ dynamics in ICC-LCs, while IP3-dependent Ca2+ release may be required in the propagation or coordination of Ca2+ waves (Johnston et al., 2005). The importance of mitochondrial Ca2+ buffering in regulating temporal Ca2+ dynamics in ICC-LCs is now becoming apparent as a reduction of mitochondrial Ca2+ uptake results in the cessation of spontaneous Ca2+ waves, while the acceleration of Ca2+ uniporter increases Ca2+ wave frequency (Sergeant et al., 2008). Because mitochondria Ca2+ buffering appears to be important in regulating the temporal dynamics of Ca2+ in ICC-LCs, the far higher density of mitochondria in perinuclear regions in comparison with the cell periphery may indicate a larger Ca2+ buffering capacity in restricted areas surrounding the nucleus. This view may be supported by the smaller decay time constant for perinuclear Ca2+ transients than that for peripheral Ca2+ transients, as well as the recording of small Ca2+ fluctuations more frequently in the cell periphery. In our experiments, blockade of the Ca2+ uniporter with bath applied RU 360 took longer to exert its inhibitory action on Ca2+ waves, and did not consistently abolish their generation, in comparison to when cells were dialysed with RU360 (Sergeant et al., 2008). Nevertheless, non-propagating Ca2+ fluctuations in the perinuclear region were increased in cells treated with RU360 and re-appeared more quickly than the appearance of spontaneous Ca2+ waves, upon the wash-out of 2DG with normal glucose-containing PSS. Therefore, perinuclear mitochondria may buffer Ca2+ concentrations in microdomains, and play a role in restricting Ca2+ release from the ER and allowing sufficient ER Ca2+ sequestration, necessary for the generation of regenerative Ca2+ waves. In cardiac cells, inhibition of mitochondrial Ca2+ uptake results in an increase in the frequency and duration of Ca2+ sparks (Pacher et al., 2002). Because of the low affinity of the mitochondrial Ca2+ uniporter, Ca2+ uptake preferentially operates in Ca2+-loaded microdomains in close proximity to ER Ca2+ release channels. The possibility of such microdomains in urethral ICC-LCs is suggested by our demonstration that the ER in ICC-LCs stained with ER-Tracker displayed a similar perinuclear distribution as mitochondria. Such close contacts and functional interactions between mitochondria and ER/SR have been reported in other cell types (Rizzuto et al., 1998; Szalai et al., 2000). Although we have not investigated the intracellular distribution of ryanodine receptors, perinuclear clusters of ryanodine receptors have been reported in ICC-LCs of the rabbit portal vein (Harhun et al., 2006). In the cell periphery where the mitochondria : ER ratio may be lower than perinuclear regions so that microdomains are not so well established, Ca2+ release and uptake from ryanodine receptors may be predominantly regulated by the ER itself, with only a smaller participation of mitochondrial Ca2+ buffering. Upon stimulation with caffeine, an increased sensitivity of ryanodine receptors to cytosolic Ca2+ may overcome ER luminal inhibition and initiate regenerative Ca2+ waves from multiple sites within ICC-LCs.
The Ca2+ release from ER through ryanodine receptors is regulated by both cytosolic and luminal Ca2+ concentration (Györke and Györke, 1998). In the present study, CPA inhibition of SERCA prevented Ca2+ wave generation, which was associated with a rise in basal Ca2+ level, and the subsequent caffeine caused a single rudimentary Ca2+ release from ER. Thus, ER function appears to require continuous Ca2+ uptake by SERCA, and reduced SERCA activity instantly results in a substantial fall of ER Ca2+ contents. In HeLa cells, thapsigargin caused a rapid, substantial fall in ER content (Arnaudeau et al., 2001). When ER Ca2+ content falls, caffeine-induced increases in the sensitivity of ryanodine receptors to cytosolic Ca2+ may not be able to cause any further Ca2+ release, presumably via their inhibition by low luminal Ca2+ in the ER store. Ryanodine also prevented the generation of spontaneous Ca2+ waves, which was associated with a rise in basal Ca2+. Subsequent cell exposure to caffeine evoked only a single, small Ca2+ transient that was equivalent to those observed upon CPA treatment, suggesting ryanodine may effectively diminish ER Ca2+ content by forming a long-lasting, subconductance state of the Ca2+-releasing channels (Rousseau et al., 1987).
Inhibition of mitochondrial Ca2+ release by CGP37157 suppressed the initial gradual rise in basal Ca2+ and reduced the frequency of Ca2+ waves, suggesting that mitochondrial Ca2+ efflux through the Na+ : Ca2+ exchanger may contribute to the gradual rise in basal Ca2+. Such gradual rises in basal Ca2+ that precede each Ca2+ transient are observed in ICC-LCs of the rabbit portal vein in which spontaneous Ca2+ waves also originate from the perinuclear regions (Harhun et al., 2006). Transfer of Ca2+ from mitochondria to the ER as the mitochondrial Na+ : Ca2+ exchanger releases Ca2+ close to SERCA, and its importance in the ER Ca2+ refilling has been reported in HeLa cells (Arnaudeau et al., 2001; Ishii et al., 2006) and HUVECs (Malli et al., 2005). In HeLa cells, ER regions close to mitochondria release sites maintain a higher Ca2+ content than regions lacking mitochondria due to this substantial mobilization of Ca2+ from mitochondria to ER (Arnaudeau et al., 2001). Consistent with our observations, CGRP37157 has been shown to take some 20 min to reduce the frequency of Ca2+ waves in the gall bladder (Balemba et al., 2008). Because mitochondrial Ca2+ efflux may also depend on Na+-independent Ca2+ efflux, the effects of CGRP37157, and therefore the relative contribution of mitochondrial Na+ : Ca2+ exchanger to mitochondrial Ca2+ efflux, may vary between cells. In three ICC-LCs, CGP37157 eventually suppressed spontaneous Ca2+ waves in a manner that was associated with a rise in the basal Ca2+ level, suggesting that an excessive rise in mitochondrial Ca2+ content subsequent to Na+ : Ca2+ exchanger inhibition may result in the suppression of mitochondrial Ca2+ uptake (Chalmers and McCarron, 2009) as it relies on both ΔΨm and the Ca2+ gradient across the inner membrane.
Although spontaneous Ca2+ transients in the urethral ICC-LCs primarily rely on Ca2+ mobilization through the ER in association with mitochondrial Ca2+ buffering, nominally Ca2+-free solution readily prevented the generation of Ca2+ transients associated with a reduction in the basal Ca2+. This is consistent with the previous demonstration that the frequency of Ca2+ transients in ICC-LCs critically depends on the extracellular Ca2+ concentrations (Johnston et al., 2005). Previous studies also indicate that the Na+ : Ca2+ exchanger on the plasma membrane operating in reverse mode, rather than store-operated Ca2+ entry, plays an important role in maintaining cytosolic Ca2+ concentrations at levels which allow the refilling of the ER (Bradley et al., 2005; 2006;). Because low subplasmalemmal Ca2+ concentrations are necessary before Ca2+ influx can take place through the plasmalemmal Na+ : Ca2+ exchanger, mitochondria may also play a role in buffering Ca2+ microdomains around these exchangers to regulate the rate of ER refilling.
Because SERCA activity is vital in maintaining ER function as a primary step of Ca2+ wave generation, continuous and sufficient ATP must be supplied to SERCA by intracellular energy production systems. In atypical smooth muscle cells of the mouse renal pelvis, inhibition by oligomycin of mitochondrial ATP synthesis reduced the frequency of spontaneous Ca2+ transients arising from Ca2+ movement between the ER and mitochondria (Hashitani et al., 2009). However, oligomycin did not prevent the generation of Ca2+ waves in urethral ICC-LCs (Johnston et al., 2005), suggesting that mitochondrial ATP production may not be fundamental in maintaining SERCA function in the ER membrane. In HeLa cells in which ATP is mainly generated by glycolysis, mitochondrial ATP generation also does not contribute significantly to SERCA function (Jouaville et al., 1999; Arnaudeau et al., 2001). In the present experiments and consistent with this notion, caffeine was capable of generating oscillatory Ca2+ transients after the cessation of spontaneous Ca2+ waves with CCCP. Although inhibition of glycolysis, with 2DG and glucose omission, abolished Ca2+ waves, the subsequent application of caffeine still evoked oscillating Ca2+ transients, suggesting that inhibition of glycolysis did not critically reduce ATP supply to SERCA, and that the effects of 2DG resulted from a reduced mitochondrial Ca2+ uptake subsequent to the reduced availability of mitochondrial substrates. Because the supply of mitochondrial substrates, particularly glucose, is fundamental in maintaining mitochondrial respiration and thus the inner ΔΨm, inhibition of glycolysis would be expected to subsequently reduce Ca2+ uptake that relies on this voltage gradient. ICC-LCs may store substantial amounts of creatine phosphate by consuming ATP produced by their numerous mitochondria, and thus may be able to maintain SERCA activity for short periods during a reduction in the supply of glucose and oxygen (i.e. during ischaemia). Thus, perinuclear mitochondria in ICC-LCs may sense cellular metabolic demands, as well as local metabolic supply, by altering their Ca2+-buffering capacity, and play a key role in determining spatio-temporal Ca2+ dynamics that critically link a number of diverse cellular functions.
Acknowledgments
This project was supported by a Grant-in-Aid for Scientific Research (B) from JSPS to H.H. (no. 19390418) and by the NHMRC (Australia) to R.J.L.
Glossary
Abbreviations
- ΔΨm
mitochondrial inner membrane potential
- 2DG
2-deoxy-glucose
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CPA
cyclopiazonic acid
- DS
dispersal solution
- ER
endoplasmic reticulum
- ICC-LC
interstitial cells of Cajal-like cell
- SERCA
sarco-endoplasmic reticulum Ca2+ ATPase
- STD
spontaneous transient depolarization
- STIC
spontaneous inward current
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional supporting information may be found in the online version of this article.
Video Clip S1 An ICC-LC was loaded with fluo-4 (1 μM), and then the mitochondria were stained with MitoTracker Red (10 nM). Fluorescence images of the ICC-LC showing the intracellular distribution of mitochondria were superimposed over the fluo-4 Ca2+ fluorescence movie. Note that a nonpropagating Ca2+ transient appears in the cell periphery on the left side, while the propagating Ca2+ wave originated from the perinuclear mitochondria cluster on the right side of the nucleus.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;29:G467–G476. doi: 10.1152/ajpgi.00415.2007. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca2+ signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, McHale NG, Thornbury KD, Sergeant GP. Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am J Physiol Cell Physiol. 2005;289:C625–C632. doi: 10.1152/ajpcell.00090.2005. [DOI] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, et al. Contribution of reverse Na+–Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol. 2006;574:651–661. doi: 10.1113/jphysiol.2006.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater M, MacNeil HF, Brading AF. Regulation of urethral tone in pig urethral smooth muscle. J Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Bruce JL, Giovannucci DR, Blinder G, Shuttleworth TJ, Yule DI. Modulation of [Ca2+]i signaling dynamics and metabolism by and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J Biol Chem. 2004;279:12909–12917. doi: 10.1074/jbc.M309070200. [DOI] [PubMed] [Google Scholar]
- Chalmers S, McCarron JG. Inhibition of mitochondrial calcium uptake rather than efflux impedes calcium release by inositol-1,4,5-trisphosphate-sensitive receptors. Cell Calcium. 2009;46:107–113. doi: 10.1016/j.ceca.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Cole L, Davies D, Hyde GJ, Ashford AE. ER-Tracker dye and BODIPY-brefeldin A differentiate the endoplasmic reticulum and Golgi bodies from the tubularvacuole system in living hyphae of Pisolithus tinctorius. J Microscopy. 2000;197:239–248. doi: 10.1046/j.1365-2818.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhun M, Gordienko D, Kryshtal D, Pucovský V, Bolton T. Role of intracellular stores in the regulation of rhythmical [Ca2+]i changes in interstitial cells of Cajal from rabbit portal vein. Cell Calcium. 2006;40:287–298. doi: 10.1016/j.ceca.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J Physiol. 2007;583:505–519. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Lang RJ, Mitsui R, Mabuchi Y, Suzuki H. Distinct effects of CGRP on typical and atypical smooth muscle cells involved in generating spontaneous contractions in the mouse renal pelvis. Br J Pharmacol. 2009;158:2030–2045. doi: 10.1111/j.1476-5381.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AD, Gardiner TA, McCloskey KD. Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int. 2007;99:687–694. doi: 10.1111/j.1464-410X.2006.06617.x. [DOI] [PubMed] [Google Scholar]
- Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnóczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Bradley E, Thornbury KD, McHale NG, Hollywood MA. Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol. 2008;586:4631–4642. doi: 10.1113/jphysiol.2008.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Csordás G, Hantash BM, Thomas AP, Hajnóczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- Ward SM, Ordög T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


