Abstract
The fluorescence of heme proteins is influenced by energy transfer from the excited tryptophan to the heme. Molecular dynamics simulations of the tryptophan and heme motions in sperm whale myoglobin were used to calculate the fluorescence intensity and anisotropy decays. The side chains underwent both small rapid orientational fluctuations and large infrequent transitions between conformations. The predicted motions of the tryptophans and the heme produce large fluctuations in the instantaneous rate of energy transfer, but no stable conformations in which energy transfer is suppressed were found. The calculated fluorescence anisotropies exhibited a large subpicosecond decay, corresponding to nondiffusive side-chain motions. The calculations adequately predict the observed fluorescence decay curve for myoglobin and the total anisotropy decay at 16-ps time resolution. The subnanosecond decays of anisotropy for tryptophan-14 in tuna myoglobin are not reproduced by the calculation.
Full text
PDF
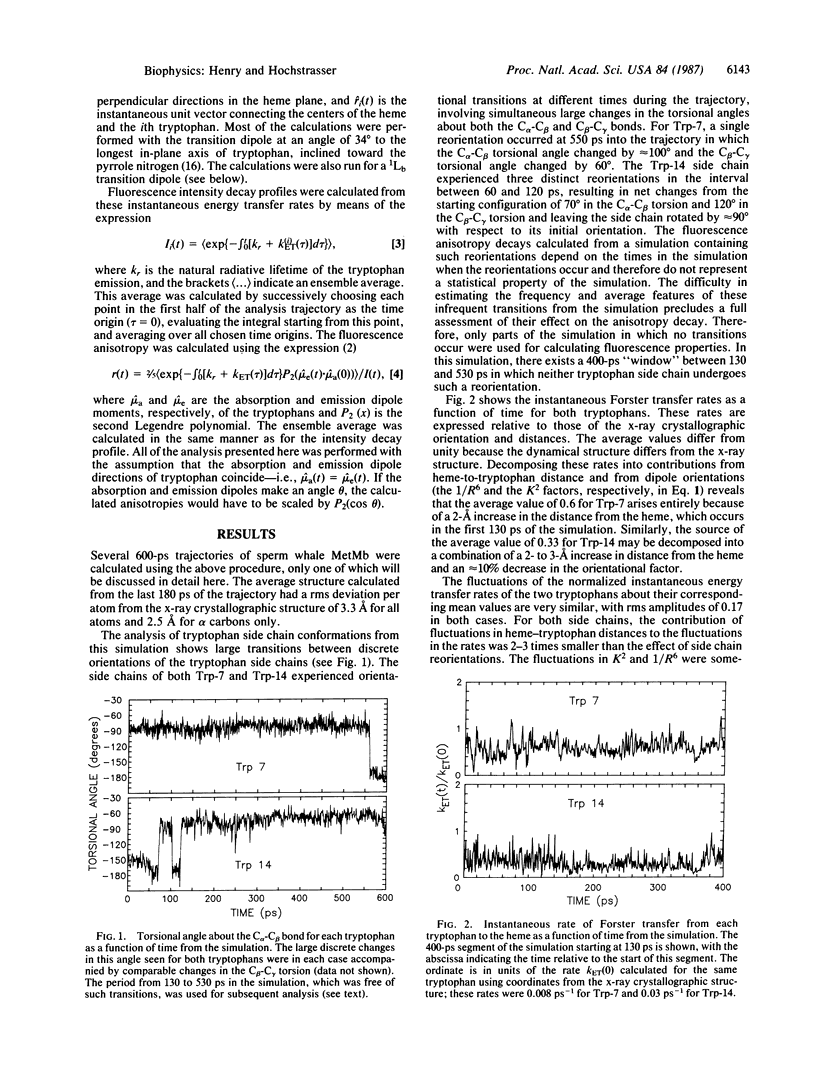
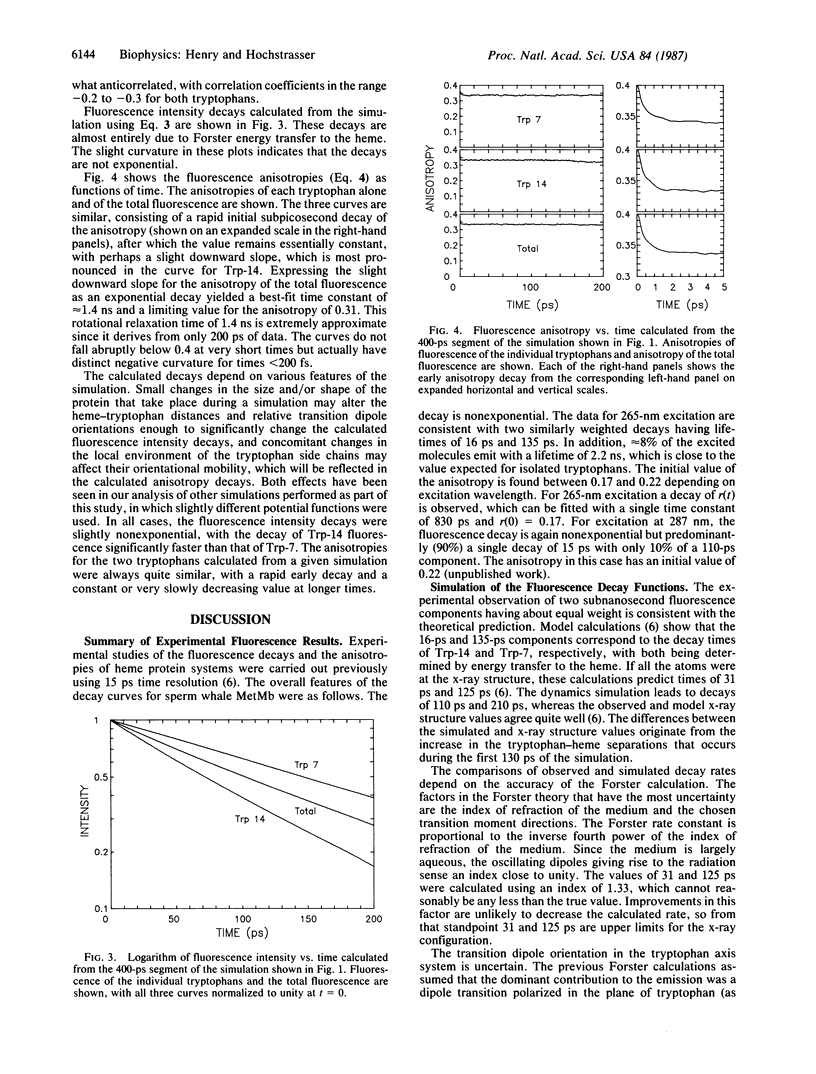


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Henry E. R., Levitt M., Eaton W. A. Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2034–2038. doi: 10.1073/pnas.82.7.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser R. M., Negus D. K. Picosecond fluorescence decay of tryptophans in myoglobin. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4399–4403. doi: 10.1073/pnas.81.14.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiye T., Karplus M. Fluorescence depolarization of tryptophan residues in proteins: a molecular dynamics study. Biochemistry. 1983 Jun 7;22(12):2884–2893. doi: 10.1021/bi00281a017. [DOI] [PubMed] [Google Scholar]
- Janes S. M., Holtom G., Ascenzi P., Brunori M., Hochstrasser R. M. Fluorescence and energy transfer of tryptophans in Aplysia myoglobin. Biophys J. 1987 Apr;51(4):653–660. doi: 10.1016/S0006-3495(87)83390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Dynamics of proteins: elements and function. Annu Rev Biochem. 1983;52:263–300. doi: 10.1146/annurev.bi.52.070183.001403. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Levitt M., Meirovitch H. Integrating the equations of motion. J Mol Biol. 1983 Aug 15;168(3):617–620. doi: 10.1016/s0022-2836(83)80305-2. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Wolynes P. G., Karplus M. Picosecond dynamics of tyrosine side chains in proteins. Biochemistry. 1979 Mar 20;18(6):927–942. doi: 10.1021/bi00573a001. [DOI] [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem Photobiol. 1977 May;25(5):441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]


