Abstract
Chromosomal alterations are a feature of both aging and Alzheimer's disease (AD). This study examined if premature centromere division (PCD), a chromosomal instability indicator increased in AD, is correlated with aging or, instead, represents a de novo chromosomal alteration due to accelerating aging in AD. PCD in peripheral blood lymphocytes was determined in sporadic AD patients and gender and age-matched unaffected controls. Metaphase nuclei were analyzed for chromosomes showing PCD, X chromosomes with PCD (PCD,X), and acrocentric chromosomes showing PCD. AD patients, regardless of age, demonstrated increased PCD on any chromosome and PCD on acrocentric chromosomes in both genders, whereas an increase in frequency of PCD,X was expressed only in women. This cytogenetic analysis suggests that PCD is a feature of AD, rather than an epiphenomenon of chronological aging, and may be useful as a physiological biomarker that can be used for disease diagnosis.
Keywords: Age, Chromosome, Premature centromere division, Sporadic Alzheimer's disease
ALZHEIMER'S disease (AD), the most common cause of dementia in late life, is multifactorial with several genetic factors and environmental exposures playing a role in its etiology (1) and pathogenesis (2–4). AD occurs as a familial form in a small number of cases in which a clear pattern of inheritance exists (5). The sporadic form of AD (SAD), however, is far more common, representing 90%–95% of all cases. Although the specific etiology of SAD is not clearly understood, aging represents the major risk factor and is coincident with disease development (6). In addition, SAD, as with aging, is associated with alterations in chromosome number and/or structure (7).
Premature centromere division (PCD), a chromosomal alteration, is regarded as a phenomenon manifested as a loss of control of centromere separation and segregation and is characterized by distinctive separation of chromosome chromatids earlier than usual during interphase of mitosis (8–11). Cytogenetic analysis of peripheral blood lymphocytes shows increased aneuploidy and higher levels of PCD in patients with AD compared with controls (11–17). In fact, familial and SAD might be explained by the presence of the mosaic trisomy 21 in later life of normal individuals such that nondisjunction may underlie both AD and Down's syndrome (18). In this regard, compared with elderly controls, AD participants have a higher frequency of aneuploidy of chromosome 21 as well as chromosome 13 in peripheral blood lymphocytes (15). Aside from chromosome 21, based on the known unequal gender distribution, the most intriguing chromosomes in AD patients are the sex chromosomes, which also show aneuploidy in the course of aging (19–21).
Given the overlap in abnormalities between normal aging and AD, the goal of this study was to examine the incidence of PCD of metaphase chromosomes in peripheral blood lymphocytes and not only greatly expand upon previous studies, thereby allowing gender and age comparisons of SAD patients with healthy controls, but also, for the first time, directly and simultaneously compare PCD in three separate chromosomes. Specifically, we assessed (a) the number of metaphases with at least one chromosome showing PCD (PCD,C), (b) the number of metaphases with at least one X chromosome showing PCD (PCD,X), and (c) the number of metaphases with at least one acrocentric chromosome showing PCD phenomenon (PCD,A). Our data clearly indicate that the PCD phenomenon in SAD patients is not simply a consequence of chronological aging but rather represents a more specific process linked to the etiology and/or pathogenesis of the disease process.
MATERIALS AND METHODS
Participants
Blood samples were collected by venous puncture from SAD patients and age-matched control participants. Probable AD was diagnosed clinically and met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke—Alzheimer's Disease Association (22). Because of familial history, all patients were classified as sporadic cases. The AD patients comprised 13 females (ages 53–80 years; mean ± SEM = 69.3 ± 2.6 years) and 11 males (ages 60–81 years; mean ± SEM = 68.2 ± 1.7 years) and control patients comprised 13 females (ages 55–83 years; mean ± SEM = 68.9 ± 2.8 years) and 11 male volunteers (ages 58–80 years; mean ± SEM = 68.8 ± 2.0 years) and were in general good health and without a history of neurological and/or psychiatric disorders. The ethical committee of the Medical School at the University of Belgrade approved the study, and written informed consent was obtained from all participants or from their families.
Blood Culture
Peripheral blood lymphocyte culture stimulations, cell harvests, and slide preparations were performed as previously described (23). Briefly, heparinized whole blood samples (0.8 mL) were added to vials with 9.2 mL RPMI 1640 medium (Gibco, Grand Island, NY), supplemented with 10% fetal calf serum (Gibco, Eggenstein, Germany), 5 μg/mL phytohemagglutinin (Inep-Zemun, Serbia), and a 1% cocktail of antibiotics penicillin/streptomycin (Bio Whitaher, Barcelona, Spain). Cultures were incubated at 37°C for 72 hours, and 2 hours before cultures were harvested, 0.05 μg/mL colcemid (Ciba, Basel, Switzerland) was added to the media. Cells were treated with hypotonic solution (20 minutes), fixed in 3:1 methanol/acetic acid (3 × 20 minute), then placed on clean grease-free chilled glass slides, and air dried over a flame. The slides were aged for the next 5–7 days and then stained using the G-banding technique (24) to identify and verify PCD of chromosomes.
PCD Counting
Slides were examined using Olympus BX 50 microscope (Olympus Optical Co., GmbH, Hamburg, Germany) under oil immersion. For each sample, 100 cells at metaphase were analyzed. The following parameters were recorded: the number of metaphases with at least one PCD chromosome (PCD,C), the number of metaphases with at least one X chromosome showing PCD (PCD,X), and metaphases containing at least one acrocentric chromosome with PCD (PCD,A)
Data Analysis and Statistics
The data are presented as group means ± SEM. The impact of two factors (disease and gender) and their interaction on the frequency of PCD were compared by the multivariate general linear model. When the F value was significant, the comparisons between groups were done by the nonparametric Mann–Whitney test because the Levene's test for equality of variances revealed that some groups of data did not come from populations with the same variance. The correlation between PCD frequency and age was also tested in both AD and control patients by the Spearman's nonparametric correlation. p values of <.05 were considered significant. Statistical software SPSS for Windows (Version 7.5) was used.
RESULTS
Statistical analysis revealed that the diagnosis of AD significantly affected the frequency of PCD,C; PCD,X; and PCD,A. Gender, however, influenced the frequency of PCD,C and PCD,X but not PCD,A. On the other hand, interaction of gender and disease was only expressed in PCD,X frequency (Table 1).
Table 1.
Influence of AD and Gender on the Frequency of PCD,C; PCD,X; and PCD,A in the Peripheral Blood Lymphocytes of Female and Male AD Patients and Control Groups as Assessed by the Multivariate General Linear Model statistics
| Factor | Dependent Variable | df | F | Significance |
| AD | PCD,C | 1 | 47.25 | 0.000 |
| PCD,X | 1 | 13.12 | 0.001 | |
| PCD,A | 1 | 12.04 | 0.001 | |
| Gender | PCD,C | 1 | 16.48 | 0.000 |
| PCD,X | 1 | 34.02 | 0.000 | |
| PCD,A | 1 | 2.08 | 0.156 | |
| AD × Gender | PCD,C | 1 | 1.96 | 0.168 |
| PCD,X | 1 | 6.41 | 0.015 | |
| PCD,A | 1 | 1.37 | 0.248 |
Note: AD = Alzheimer's disease; df = degrees of freedom; PCD = premature centromere division.
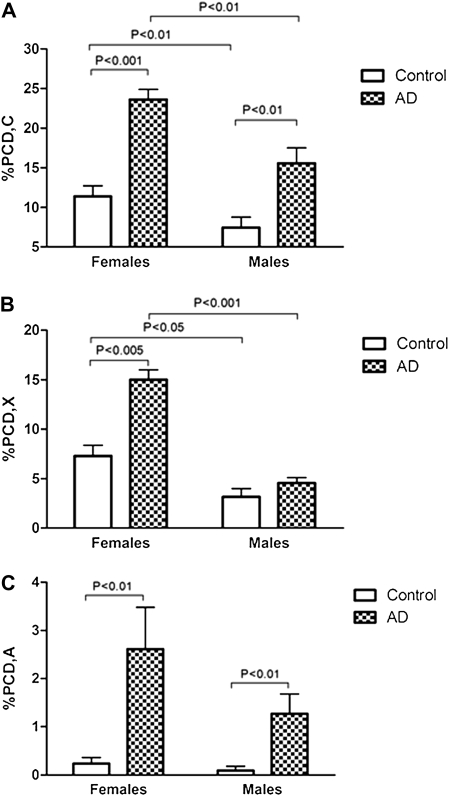
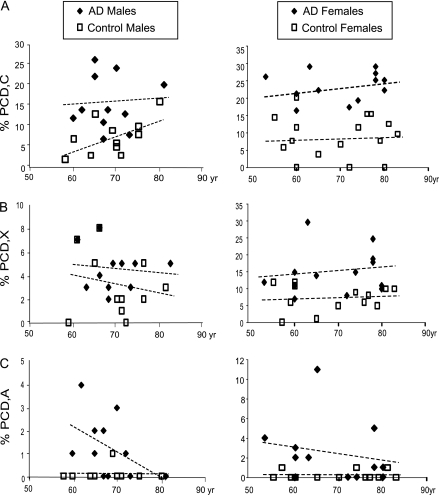
Post hoc comparisons between corresponding groups showed that in women, those with a diagnosis of AD had a markedly and significantly increased frequency of PCD,C (Figure 1A), PCD,X (Figure 1B), and PCD,A (Figure 1C) in comparison with control group. In male patients with AD, the frequencies of PCD,C (Figure 1A) and PCD,A (Figure 1C) were significantly greater than in corresponding controls. No difference was found in the percentage of PCD,X in males patients (Figure 1B). Importantly, in the sampled population of female individuals aged 53–80 years old, neither the AD cases nor the control cases showed any statistically significant correlation between the frequency of PCD and age (Figure 2). Similarly, in the sampled population of male individuals aged 58–81 years old, the frequency of PCD,C; PCD,X; and PCD,A did not significantly correlate with age in either the AD or the control cohorts (Figure 2).
Figure 1.
Alzheimer's disease (AD) cases of both genders significantly demonstrate increased PCD,C and PCD,A in peripheral blood lymphocytes compared with controls (A and C). Gender-related differences were found in the percentage of PCD,X, which only was affected in women (B). Female participants, both control and AD, had higher frequency of PCD,C and PCD,X than their corresponding male groups (A and B), which was not the case with PCD,A (C). The data are expressed as mean ± SEM (n = 11–13), and statistically significant differences are labeled. For each participant, 100 metaphase nuclei were analyzed.
Figure 2.
The frequency of premature centromere division (PCD) does not correlate with age in either the Alzheimer's disease (AD) or the control cohorts for PCD,C (A), PCD,X (B), and PCD,A (C) in males or females.
Figure 3.
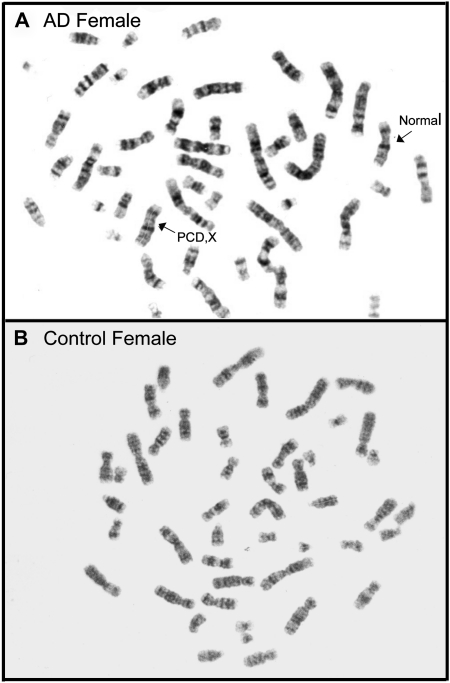
Representative chromosomes from peripheral blood lymphocytes, G banded, from a control female (B) and an Alzheimer's disease (AD) female showing a normal X chromosome and an X chromosome with PCD,X (A).
Furthermore, our results demonstrated that in control patients, PCD,C and PCD,X were significantly more frequent in women than in men (Figure 1A and B). These gender differences were expressed in both AD and control patients. However, the frequency of acrocentrics was similar both in control and AD groups of different genders (Figure 1C). Representative chromosomes analyzed are shown in Figure 3.
DISCUSSION
In this study, the frequency of PCD in peripheral blood lymphocytes was determined in male and female clinical patients diagnosed with SAD and compared with age-matched control patients. Replication, separation, and segregation of human chromosomes are highly controlled processes through the cell cycle. PCD, as a cause of improper chromosome separation, is found to be increased in patients with AD. In fact, loss of control of the chromosome separation may be regarded as a manifestation of chromosome instability (25).
In this work, using cases carefully chosen spanning 53–83 years old, PCD was not found to correlate with age after the sixth decade of life. A classic study evaluated PCD in both young and aged normal women and compared the percentage of metaphase cells exhibiting PCD. In women aged 22–39 years, PCD occurred less than 1% of the time, women aged 40–59 years old showed an average of 2.5 % PCD, and aged women of 60–89 years had an average PCD occurrence of 4% (26). The data in the present work found similar values for the advanced age control individuals and importantly found that after the age of 53 years, PCD no longer increases significantly with advancing age within any group of male or female aged or normal people. Yet, our results do demonstrate that AD patients, regardless of age, have significantly increased incidence of PCD in peripheral blood lymphocytes both in male and in female participants. Therefore, PCD likely represents an important contributor, in its own right, to the etiology and pathogenesis of SAD.
Our analysis of three separate PCD events (PCD,C; PCD,X; and PCD,A) showed that the principal gender-related difference was in the frequency of PCD,X, which was only increased in female AD patients. Because AD affects twice as many women as men (27), this finding may have important pathological implications. Indeed, in AD, the X chromosome frequently shows aneuploidy and PCD phenomenon (19–21), and gender clearly plays an important role in the pathogenesis of AD, influencing the risk of developing AD (28–30). One possible explanation for this gender-related difference in PCD,X is the finding that partially inactive X chromosomes in lymphocytes from elderly women are more susceptible to PCD (19,31,32). Consistent with this, X chromosome analysis by fluorescence in situ hybridization of peripheral blood lymphocytes revealed a higher percentage of PCD in female AD patients than in corresponding controls (11,13). Additionally, PCD,X occurs much earlier than the metaphase of mitosis, that is, in interphase of the cell cycle, immediately after DNA replication (11). PCD,X has also been detected in the interphase nuclei of neurons in the frontal cerebral cortex in SAD females (13).
In acrocentrics (chromosomes 21,13,14,15, and 22), PCD is markedly increased both in female and in male AD patients compared with corresponding controls. Such a result is not surprising because aneuploidy of chromosomes 21 and 13 is well established in AD patients (15,33). In fact, both familial and SAD can be explained by the presence of the mosaic trisomy of chromosome 21, and given this, it is likely not coincidental that patients with Down syndrome (i.e., trisomy 21) invariably develop the pathological and clinical characteristics of AD (16,18,34,35). In AD patients, aneuploidy of chromosome 21 is more frequent than that of chromosome 13 (15). Although the molecular mechanisms of chromosome instability are still not fully understood, in relation to AD, it is known that mutations in the presenilin 1 gene lead to abnormal presenilin function giving rise to defect in the cell cycle, increased number of abnormal mitotic spindles, and improper chromosome segregation (33).
It is well known that there is a sequential segregation of replicated genetic material in normal mammalian cells (36–40). Between the end of replication and the moment when segregation of replicated genetic material begins in the mitotic anaphase, two sister chromatids are tightly held together in the centromere region (41). In humans, the centromeres of chromosomes 2, 8, 17, and 18 separate earliest, whereas chromosomes 13, 14, 15, 21, and 22 are among the last to split into two subunits (42,43). In addition, chromosomes from D and G group participate in satellite association. PCD of acrocentric chromosomes are connected with aneuploidy, such as that occurs on chromosome 21 in Roberts syndrome (44), in chronic myelogenous leukemia (42), and in AD (15). In fact, increased aneuploidy and PCD may account for increased hybridization (fluorescence in situ hybridization) spots in neurons in AD compared with controls (21), although a defective ectopic cell cycle reentry cannot be discounted (45,46).
Advanced age is the major contributing factor for increased risk of developing AD (47,48). Beyond the age of 65 years, every 5 years represents a doubling of the risk for the development of AD such that more than 30% of individuals aged 80 years and older are affected (49–51). Although PCD does increase between the ages of 20 and 60 years (26), the present work found no effect of age on the PCD frequency in the lymphocytes of either AD patients or control patients after the age of 53 years. Other factors such as oxidative stress that also are increased early in the development of AD (52) and are found to be increased, decades before disease onset, undoubtedly contribute to the disease (53). Chromosome stability alterations, therefore, likely occur a number of years prior to clinical diagnosis of AD, a supposition consistent with the hypothesis that both genetic and SAD may be predicted by certain chromosome alterations in apparently normal individuals (34). Additionally, PCD might also prove useful as cytogenetic biomarkers for the diagnosis of AD or to monitor progression of the disease. As a noninvasive method using blood samples, this assay could be adapted as a screening tool for predicting disease. Current thoughts on the use of biomarkers for identifying patients at risk for developing AD stress the fact that any single biomarker is not reliable. However, the combination of biomarkers with genetic, cognitive, and imaging analyses may be valid (54). PCD assays would fill another niche in the broad range of measurements necessary for adequate biomarker development and future clinical use.
Although aging per se is associated with chromosome abnormalities (19,20,55), a noticeable difference between lymphocyte PCD frequency in AD patients and their age-matched controls certainly questions the notion that cytogenetic alterations are inherent to the cellular aging process but are in fact specifically related to AD (55,56). In fact, we would suggest that our data show that PCD and chromosome centromere instability in AD are distinct from an epiphenomenon of the aging process and in fact likely are etiological and pathological contributors to the disease phenotype (3,4).
FUNDING
The work was supported by the Serbian Ministry of Science (grant #143018) and by the National Institutes of Health (AG028679 and AG031364).
References
- 1.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X, Castellani RJ, Takeda A, et al. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 5.Schellenberg GD. Early Alzheimer's disease genetics. J Alzheimers Dis. 2006;9:367–372. doi: 10.3233/jad-2006-9s341. [DOI] [PubMed] [Google Scholar]
- 6.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojda A, Zietkiewicz E, Mossakowska M, Pawlowski W, Skrzypczak K, Witt M. Correlation between the level of cytogenetic aberrations in cultured human lymphocytes and the age and gender of donors. J Gerontol A Biol Sci Med Sci. 2006;61:763–772. doi: 10.1093/gerona/61.8.763. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald PH, Archer SA, Morris CM. Evidence for the repeated primary non-disjunction of chromosome 21 as a result of premature centromere division (PCD) Hum Genet. 1986;72:58–62. doi: 10.1007/BF00278818. [DOI] [PubMed] [Google Scholar]
- 9.Mehes K, Buhler EM. Premature centromere division: a possible manifestation of chromosome instability. Am J Med Genet. 1995;56:76–79. doi: 10.1002/ajmg.1320560117. [DOI] [PubMed] [Google Scholar]
- 10.Spremo-Potparevic; B, Verbic; V, Stevanovic; M. Experimental model for studying premature centromere division (PCD) in all phases of the cell cycle. Balkan J Med Genet. 2000;3:29–34. [Google Scholar]
- 11.Spremo-Potparevic B, Zivkovic L, Djelic N, Bajic V. Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through the cell cycle. Exp Gerontol. 2004;39:849–854. doi: 10.1016/j.exger.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Nordenson I, Adolfsson R, Beckman G, Bucht G, Winblad B. Chromosomal abnormality in dementia of Alzheimer type. Lancet. 1980;1:481–482. doi: 10.1016/s0140-6736(80)91020-x. [DOI] [PubMed] [Google Scholar]
- 13.Spremo-Potparevic B, Zivkovic L, Djelic N, Plecas-Solarovic B, Smith MA, Bajic V. Premature centromere division of the X chromosome in neurons in Alzheimer disease. J Neurochem. 2008;106:2218–2223. doi: 10.1111/j.1471-4159.2008.05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward BE, Cook RH, Robinson A, Austin JH. Increased aneuploidy in Alzheimer disease. Am J Med Genet. 1979;3:137–144. doi: 10.1002/ajmg.1320030204. [DOI] [PubMed] [Google Scholar]
- 15.Migliore L, Botto N, Scarpato R, Petrozzi L, Cipriani G, Bonuccelli U. Preferential occurrence of chromosome 21 malsegregation in peripheral blood lymphocytes of Alzheimer disease patients. Cytogenet Cell Genet. 1999;87:41–46. doi: 10.1159/000015389. [DOI] [PubMed] [Google Scholar]
- 16.Migliore L, Testa A, Scarpato R, Pavese N, Petrozzi L, Bonuccelli U. Spontaneous and induced aneuploidy in peripheral blood lymphocytes of patients with Alzheimer's disease. Hum Genet. 1997;101:299–305. doi: 10.1007/s004390050632. [DOI] [PubMed] [Google Scholar]
- 17.Zivkovic L, Spremo-Potparevic B, Djelic N, Bajic V. Analysis of premature centromere division (PCD) of the chromosome 18 in peripheral blood lymphocytes in Alzheimer disease patients. Mech Ageing Dev. 2006;127:892–896. doi: 10.1016/j.mad.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Potter H. Review and hypothesis: Alzheimer disease and Down syndrome—chromosome 21 nondisjunction may underlie both disorders. Am J Hum Genet. 1991;48:1192–1200. [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald PH, McEwan CM. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet. 1977;39:329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- 20.Bajnoczky K, Mehes K. Parental centromere separation sequence and aneuploidy in the offspring. Hum Genet. 1988;78:286–288. doi: 10.1007/BF00291679. [DOI] [PubMed] [Google Scholar]
- 21.Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- 24.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 25.Mehes K. Centromere separation and malignancy. Orv Hetil. 2000;141:2479–2481. [PubMed] [Google Scholar]
- 26.Fitzgerald PH. A mechanism of x chromosome aneuploidy in lymphocytes of aging women. Humangenetik. 1975;28:153–158. doi: 10.1007/BF00735748. [DOI] [PubMed] [Google Scholar]
- 27.Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 28.Blass JP. Metabolic alterations common to neural and non-neural cells in Alzheimer's disease. Hippocampus. 1993;3:45–53. Spec No. [PubMed] [Google Scholar]
- 29.Payami H, Zareparsi S, Montee KR, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 30.Webber KM, Casadesus G, Marlatt MW, et al. Estrogen bows to a new master: the role of gonadotropins in Alzheimer pathogenesis. Ann N Y Acad Sci. 2005;1052:201–209. doi: 10.1196/annals.1347.020. [DOI] [PubMed] [Google Scholar]
- 31.Galloway SM, Buckton KE. Aneuploidy and ageing: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet. 1978;20:78–95. doi: 10.1159/000130842. [DOI] [PubMed] [Google Scholar]
- 32.Abruzzo MA, Mayer M, Jacobs PA. Aging and aneuploidy: evidence for the preferential involvement of the inactive X chromosome. Cytogenet Cell Genet. 1985;39:275–278. doi: 10.1159/000132157. [DOI] [PubMed] [Google Scholar]
- 33.Boeras DI, Granic A, Padmanabhan J, Crespo NC, Rojiani AM, Potter H. Alzheimer's presenilin 1 causes chromosome missegregation and aneuploidy. Neurobiol Aging. 2008;29:319–328. doi: 10.1016/j.neurobiolaging.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geller LN, Potter H. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer's disease. Neurobiol Dis. 1999;6:167–179. doi: 10.1006/nbdi.1999.0236. [DOI] [PubMed] [Google Scholar]
- 35.Potter H. Down's syndrome and Alzheimer's disease: two sides of the same coin. Future Neurol. 2008;3:29–37. [Google Scholar]
- 36.Vig BK. Centromere separation: existence of sequences. Experientia. 1981;37:566–567. doi: 10.1007/BF01990051. [DOI] [PubMed] [Google Scholar]
- 37.Vig BK, Broccoli D. Sequence of centromere separation: differential replication of pericentric heterochromatin in multicentric chromosomes. Chromosoma. 1988;96:311–317. doi: 10.1007/BF00286919. [DOI] [PubMed] [Google Scholar]
- 38.Vig BK, Wodnicki J. Separation of sister centromeres in some chromosomes from cultured human leukocytes. A preliminary survey. J Hered. 1974;65:149–152. doi: 10.1093/oxfordjournals.jhered.a108486. [DOI] [PubMed] [Google Scholar]
- 39.Mehes K. Non-random centromere division: a mechanism of non-disjunction causing aneuploidy? Hum Hered. 1978;28:255–260. doi: 10.1159/000152965. [DOI] [PubMed] [Google Scholar]
- 40.Mehes K. Letter: nonrandom anaphase segregation of mitotic chromosomes. Acta Genet Med Gemellol (Roma) 1975;24:175. [PubMed] [Google Scholar]
- 41.Broccoli D, Paweletz N, Vig BK. Sequence of centromere separation: characterization of multicentric chromosomes in a rat cell line. Chromosoma. 1989;98:13–22. doi: 10.1007/BF00293330. [DOI] [PubMed] [Google Scholar]
- 42.Vig BK. Out-of-phase separation of a G-group chromosome in a woman with chronic myelogenous leukemia. Cancer Genet Cytogenet. 1984;12:167–169. doi: 10.1016/0165-4608(84)90129-8. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Orad A, Vig BK, Aucoin D. Separation vs. replication of inactive and active centromeres in neoplastic cells. Cancer Genet Cytogenet. 2000;120:18–24. doi: 10.1016/s0165-4608(99)00242-3. [DOI] [PubMed] [Google Scholar]
- 44.Van Den Berg DJ, Francke U. Roberts syndrome: a review of 100 cases and a new rating system for severity. Am J Med Genet. 1993;47:1104–1123. doi: 10.1002/ajmg.1320470735. [DOI] [PubMed] [Google Scholar]
- 45.McShea A, Wahl AF, Smith MA. Re-entry into the cell cycle: a mechanism for neurodegeneration in Alzheimer disease. Med Hypotheses. 1999;52:525–527. doi: 10.1054/mehy.1997.0680. [DOI] [PubMed] [Google Scholar]
- 46.Bowser R, Smith MA. Cell cycle proteins in Alzheimer's disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–254. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- 47.Thomas P, Fenech M. A review of genome mutation and Alzheimer's disease. Mutagenesis. 2007;22:15–33. doi: 10.1093/mutage/gel055. [DOI] [PubMed] [Google Scholar]
- 48.Joseph J, Shukitt-Hale B, Denisova NA, Martin A, Perry G, Smith MA. Copernicus revisited: amyloid beta in Alzheimer's disease. Neurobiol Aging. 2001;22:131–146. doi: 10.1016/s0197-4580(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 49.Burns A, Byrne EJ, Maurer K. Alzheimer's disease. Lancet. 2002;360:163–165. doi: 10.1016/S0140-6736(02)09420-5. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- 51.Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 52.Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 53.Smith MA. Oxidative stress and iron imbalance in Alzheimer disease: how rust became the fuss! J Alzheimers Dis. 2006;9:305–308. doi: 10.3233/jad-2006-9s334. [DOI] [PubMed] [Google Scholar]
- 54.Gustaw-Rothenberg K, Lerner A, Bonda DJ, et al. Biomarkers in Alzheimer's disease: past, present and future. Biomark Med. 2010;4:15–26. doi: 10.2217/bmm.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kormann-Bortolotto MH, de Arruda Cardoso Smith M, Toniolo Neto J. Alzheimer's disease and ageing: a chromosomal approach. Gerontology. 1993;39:1–6. doi: 10.1159/000213508. [DOI] [PubMed] [Google Scholar]
- 56.Chamla Y. C-anaphases in lymphocyte cultures versus premature centromere division syndromes. Hum Genet. 1988;78:111–114. doi: 10.1007/BF00278177. [DOI] [PubMed] [Google Scholar]