Abstract
Background.
Identification of gene variants that contribute to exceptional survival may provide critical biologic information that informs optimal health across the life span.
Methods.
As part of phenotype development efforts for the Long Life Family Study, endophenotypes that represent exceptional survival were identified and heritability estimates were calculated. Principal components (PCs) analysis was carried out using 28 physiologic measurements from five trait domains (cardiovascular, cognition, physical function, pulmonary, and metabolic).
Results.
The five most dominant PCs accounted for 50% of underlying trait variance. The first PC (PC1), which consisted primarily of poor pulmonary and physical function, represented 14.3% of the total variance and had an estimated heritability of 39%. PC2 consisted of measures of good metabolic and cardiovascular function with an estimated heritability of 27%. PC3 was made up of cognitive measures (h2 = 36%). PC4 and PC5 contained measures of blood pressure and cholesterol, respectively (h2 = 25% and 16%).
Conclusions.
These PCs analysis–derived endophenotypes may be used in genetic association studies to help identify underlying genetic mechanisms that drive exceptional survival in this and other populations.
Keywords: Heritability, Longevity, Endophenotypes
LONGEVITY and preservation of high levels of function with relative lack of disability are complex heritable traits and careful endophenotype development is necessary to maximize the ability to detect meaningful genetic signals (1,2). Endophenotypes comprising linear combinations of correlated physiologic measures may (a) better characterize exceptional survival than single measure traits and (b) improve detection of genes associated with high physical and cognitive function. Furthermore, such endophenotypes may be more useful measures of long life for other areas of clinical epidemiology aiming to identify nongenetic biomarkers or risk factors (3). The goal of this study is to develop heritable endophenotypes in the Long Life Family Study, a National Institute on Aging sponsored multicentered study of highly functional adults older than 90 years, their siblings, and their offspring.
METHODS
The Long Life Family Study is a family-based cohort study designed to characterize exceptional health well beyond what is expected in the general population. Families were recruited by four collection sites across the United States and Denmark. Family eligibility and ascertainment has been previously described (4,5). At the time of analysis, 480 families, consisting of 3,224 participants (1,228 siblings and 1,996 offspring) from all sites were collected. Spousal controls of the offspring generation were not used to estimate principal components (PCs) but were included in heritability estimates.
For endophenotype development, 28 measures were chosen based on availability across collection sites and on hypothesized physiologic significance to exceptional survival. Measures included (a) cognitive function: immediate memory, delayed memory, category fluency, and digit substitution forward and backward (6,7); (2) cardiovascular health: presence of hypertension, total cholesterol (milligrams per deciliter), high-density lipoprotein cholesterol (milligrams per deciliter), low-density lipoprotein cholesterol (milligrams per deciliter), triglycerides (milligrams per deciliter), systolic blood pressure (millimeter of mercury), diastolic blood pressure (millimeter of mercury), and pulse pressure (millimeter of mercury); (c) metabolic health: presence of diabetes, blood glucose (milligrams per deciliter), glycosylated hemoglobin, creatinine, body mass index (kg/m2), and waist circumference; (d) pulmonary health: presence of lung disease, forced expiratory volumes (FEV1 and FEV6, milliliters), and FEV1/FEV6 ratio; and (e) physical functioning: average and maximal grip strength (kilograms), walking speed (meter per second), and total physical activity (8). Variables were continuous and transformed to be normally distributed when necessary. Each variable was adjusted for family generation, which aimed to adjust for the large differences in means and standard deviations between generations, yet retain covariation among variables.
PCs analysis was used to develop endophenotypes of exceptional longevity (9). Simple random sampling of one person per family was repeated 1,000 times, and a matrix of the average correlations across iterations was used to perform factor analysis with principle component factor extraction and varimax rotation (10). The number of dominant PCs was assessed via (a) biologic relevance, (b) percent variance explained, and (c) scree plot evaluation. Biplots were plotted to assess orthogonality. Diagnostic regression analyses were used to assess if variable missingness accounted for any significant component loadings.
For subsequent heritability estimation, five component scores were calculated per individual to correspond to each observed dominant PC by standardizing each person’s generation-adjusted predictor values and multiplying them by the corresponding eigenvector for each PC.
Heritability scores were then estimated using the ASSOC program in the S.A.G.E. (Statistical Analysis in Genetic Epidemiology, version 5.4.1) statistical package, which calculates the amount of variance due to additive genetic effects divided by the total trait variance. Heritability analyses were adjusted for age, gender, and recruitment site (11).
RESULTS
Eigenvalues and eigenvectors for the five most dominant components are shown in Table 1. Combinations of measures across physiologic domains characterized the most significant PCs. However, as the percent variance decreased, PCs were more likely to be characterized by a single physiologic domain. The most dominant PC consisted of measures of pulmonary, physical function, and metabolic health accounting for 14.3% of the variability in the data set. The second component included metabolic and cardiovascular measures and accounted for 11.9% of variability. The third component was defined solely by cognitive measures and accounted for 8.9% of variability. The fourth component included cardiovascular, specifically blood pressure–related measures, accounting for 8.3% of the variability. Lastly, the fifth component included cardiovascular (lipid-related) measures and accounted for 6.2% of the variance. Missingness for any particular predictor did not seem to significantly affect loadings.
Table 1.
PCA Results for Five Most Dominant Components
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| Eigenvalue (% variance explained) | |||||
| Domain | 4.01 (14.3) | 3.33 (11.9) | 2.50 (8.9) | 2.31 (8.3) | 1.73 (6.2) |
| Cognition | |||||
| Animal recall | 0.17 | −0.07 | 0.56 | −0.09 | 0.08 |
| Vegetable recall | −0.14 | −0.12 | 0.60 | −0.09 | 0.07 |
| Digit forward | 0.06 | 0.04 | 0.46 | −0.04 | −0.20 |
| Digit backward | 0.04 | 0.03 | 0.56 | −0.01 | −0.14 |
| Immediate memory | 0.00 | 0.01 | 0.78 | 0.06 | 0.05 |
| Delayed memory | 0.01 | −0.01 | 0.78 | 0.03 | 0.08 |
| Cardiovascular | |||||
| Presence of hypertension | −0.09 | 0.11 | −0.07 | 0.79 | −0.06 |
| Systolic BP | −0.06 | 0.05 | −0.06 | 0.86 | 0.08 |
| Diastolic BP | 0.14 | −0.01 | 0.00 | 0.81 | 0.13 |
| Pulse pressure | −0.17 | 0.08 | 0.03 | 0.17 | 0.20 |
| Total cholesterol | −0.09 | −0.14 | −0.04 | 0.12 | 0.93 |
| HDL cholesterol | −0.29 | −0.56 | 0.10 | 0.06 | 0.15 |
| LDL cholesterol | 0.02 | −0.07 | −0.07 | 0.10 | 0.91 |
| Triglycerides | 0.05 | 0.52 | −0.08 | 0.06 | 0.41 |
| Metabolic | |||||
| Presence of diabetes | −0.17 | 0.59 | 0.02 | −0.04 | −0.04 |
| Estimated BMI | 0.20 | 0.66 | 0.00 | 0.20 | −0.04 |
| Creatinine | 0.35 | 0.21 | −0.16 | −0.04 | −0.05 |
| Glucose | −0.07 | 0.67 | −0.01 | 0.02 | 0.05 |
| Glycosylated hemoglobin | −0.19 | 0.68 | 0.03 | −0.06 | −0.02 |
| Waist circumference | 0.17 | 0.68 | −0.08 | 0.14 | −0.02 |
| Physical activity | |||||
| Average grip strength | 0.88 | 0.14 | −0.02 | 0.09 | −0.09 |
| Maximum grip strength | 0.88 | 0.14 | −0.02 | 0.09 | −0.09 |
| Gait speed | 0.42 | −0.20 | 0.31 | −0.03 | 0.10 |
| Total physical activity | 0.42 | −0.15 | 0.31 | 0.01 | 0.14 |
| Pulmonary | |||||
| Presence of lung disease | −0.15 | 0.10 | −0.02 | 0.07 | −0.11 |
| FEV1 | 0.85 | 0.00 | 0.08 | −0.09 | 0.05 |
| FEV6 | 0.86 | −0.02 | 0.07 | −0.06 | 0.00 |
| FEV1/FEV6 ratio | 0.10 | 0.07 | 0.05 | −0.14 | 0.19 |
Note: Loading variables per PC are presented for each variable. Values in bold indicate the strongest correlations to that particular PC. BMI = body mass index; BP = blood pressure; FEV = forced expiratory volume; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PCs = principal components.
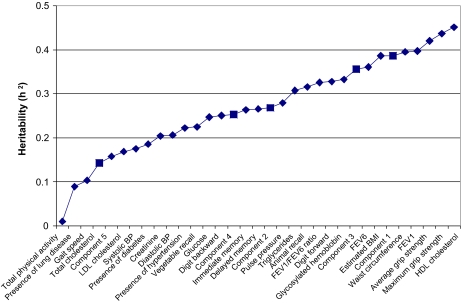
Heritability estimates for 28 individual predictor variables and for the five dominant PC-derived endophenotypes exhibited wide variability (Figure 1). High-density lipoprotein cholesterol, grip strength, FEV1, and waist circumference exhibited the highest heritabilities (h2 ≥ 0.40). Overall, individual variables that loaded into any particular PC demonstrated wide ranges of heritabilities; despite these ranges, four of the five derived PCs showed modestly high heritability compared with its components. For example, the seven variables that loaded into PC1 had heritabilities ranging from 0.01 to 0.44. Construction of an endophenotype (PC1) to characterize these variables resulted in a heritability estimate of 0.39. Likewise, PC2 was estimated to have a heritability of 0.27, yet was characterized by variables with heritabilities ranging from 0.19 to 0.45. Heritability estimates for PC3 and PC4 were higher than any single loading variable, possibly due to the fact that loading variables for these PCs had more narrow ranges of heritability ( = 0.36 with single variable ranging 0.22–0.33 and with single variable ranging 0.17–0.22, respectively). Although loadings were strong for PC5, this component demonstrated low heritability .
Figure 1.
Heritability estimates for individual variables and for five principal components analysis–derived endophenotypes. Diamonds represent heritability estimates for individual trait variables, whereas squares represent heritability estimates for the five most dominant endophenotypes. BMI = body mass index; BP = blood pressure; FEV = forced expiratory volume; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
DISCUSSION
The most dominant PC, made up of pulmonary physical function measures, accounted for 14.3% of the variance and was moderately heritable (h2 = 0.39). Physical function and pulmonary health are highly associated at older ages, possibly due to a significant decline in skeletal muscle strength (12). Physically active older adults have reportedly better overall and cardiovascular-related survival compared with sedentary counterparts (13). Respiratory muscle strength has been associated with reduced walking capacity, whereas active older adults have been shown to have greater diaphragm thickness and greater maximal inspiratory and expiratory pressures, suggesting that genes that help maintain muscle function later in life may influence both traits (14–16). Physical function and pulmonary health may also be associated via shared pathways of chronic inflammation (17–19).
The second most dominant PC, characterized by metabolic and cholesterol-related traits, accounted for 11.9% of the variance and had modest heritability (h2 = 0.25). Heritable metabolic phenotypes such as low insulin resistance, absence of obesity, and hyperglycemia have previously been associated with longevity, suggesting that they play an important role in the reduction of overall disease burden (20–22).
PC3 was related to global cognition, accounting for 8.9% of the underlying variance with heritability of 0.36. Reports have estimated that 50%–60% of the variance in cognitive function can be accounted for by genetic differences (23,24). Apolipoprotein E and cholesterol ester transfer protein have been proposed as candidate genes consistently associated with both longevity and memory function (25,26).
Lastly, PC4 was mainly characterized by blood pressure measures, accounting for 8.3% of the variance with an estimated heritability of 0.25. A study of cardiovascular-related risk factors estimated a derived “blood pressure” factors to have heritabilities ranging 0.15–0.27, similar to our estimates (27). Gene variants related to blood pressure regulation, including some in the angiotensin-converting enzyme gene, have emerged as potentially valuable candidates that may influence aging processes (28,29). Genome-wide association studies of blood pressure have identified additional candidate genes that may influence hypertension.
The ability to maintain health and functional status well into older ages is most likely related to interconnected biologic mechanisms that preserve functionality across domains. Findings from these analyses have broader implications. Endophenotypes developed in this study show that combined measures of pulmonary and physical function and of metabolic and Cardiovascular function are important for functional longevity. This preliminary finding may be an indication of pleiotropic effects of combined genotypes among disparate biologic pathways. Validation of these PC-derived endophenotypes to other populations would help further clarify their utility for genetic association analysis of long and healthy life.
FUNDING
This project was funded by National Institute on Aging (NIA), Claude D. Pepper Older Americans Independence Center, grant P30AG021334 and NIA-grant T32AG000120. The Long Life Family Study is funded by U01AG023749, U01AG023744 and U01AG023712.
Acknowledgments
Author contributions: All authors contributed to project concept and design, project implementation, and preparation of manuscript.
Sponsor’s role: None.
References
- 1.Perls TT, Wilmoth J, Levenson R, et al. Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci U S A. 2002;99(12):8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen K, McGue M, Yashin A, et al. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol Med Sci. 2000;55(8):M446–M452. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 5.Sebastiani P, Hadley EC, Province M, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170(12):1555–1562. doi: 10.1093/aje/kwp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K, Kessler J. WMS-R Manual. Bern, Switzerland: Huber Verlag; 2000. [Google Scholar]
- 7.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’ Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 9.Jolliffe IT. 2nd ed. New York: Springer-Verlag Inc; 2002. Principal Component Analysis; p. 502. Springer Series in Statistics, ed. Springer-Verlag. [Google Scholar]
- 10.SAS, I. Cary, NC: SAS Institute; 1987. SAS Languages and Procedures: Introduction, Version 9.1. [Google Scholar]
- 11.S.A.G.E. Statistical Analysis for Genetic Epidemiology. Release 6.0.1. http://darwin.cwru.edu/. 2009. Accessed August 25, 2010. [Google Scholar]
- 12.Burchfiel CM, Enright PL, Sharp DS, et al. Factors associated with variations in pulmonary function among elderly Japanese-American men. Chest. 1997;112(1):87–97. doi: 10.1378/chest.112.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Rengo G, Galasso G, Vitale DF. An active lifestyle prior to coronary surgery is associated with improved survival in elderly patients. J Gerontol A Biol Sci Med Sci. 2010;65(7):758–763. doi: 10.1093/gerona/glp216. [DOI] [PubMed] [Google Scholar]
- 14.Summerhill EM, Angov N, Garber C, McCool FD. Respiratory muscle strength in the physically active elderly. Lung. 2007;185(6):315–320. doi: 10.1007/s00408-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 15.Watsford ML, Murphy AJ, Pine MJ. The effects of ageing on respiratory muscle function and performance in older adults. J Sci Med Sport. 2007;10(1):36–44. doi: 10.1016/j.jsams.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Buchman AS, Boyle PA, Wilson RS, et al. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31(3):174–180. doi: 10.1159/000154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 18.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci. 2010;65(5):532–537. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haren MT, Malmstrom TK, Miller DK, et al. Higher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African Americans. J Gerontol A Biol Sci Med Sci. 2010;65(3):274–281. doi: 10.1093/gerona/glp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296(19):2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 21.Yates LB, Djousse L, Kurth T, Buring JE, Gaziano JM. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168(3):284–290. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 22.Austin MA, Edwards KL, McNeely MJ, et al. Heritability of multivariate factors of the metabolic syndrome in nondiabetic Japanese Americans. Diabetes. 2004;53(4):1166–1169. doi: 10.2337/diabetes.53.4.1166. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen NL, McClearn GE, Plomin R, et al. The Swedish Adoption Twin Study of Aging: an update. Acta Genet Med Gemellol. 1991;40(1):7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- 24.McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Exp Aging Res. 2002;28(4):435–451. doi: 10.1080/03610730290080416. [DOI] [PubMed] [Google Scholar]
- 25.Mayeux R, Small SA, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiol Aging. 2001;22(4):683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 26.Barzilai N, Atzmon G, Derby CA, Bauman JM, Lipton RB. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology. 2006;67(12):2170–2175. doi: 10.1212/01.wnl.0000249116.50854.65. [DOI] [PubMed] [Google Scholar]
- 27.Huang P, Kraja AT, Tang W, et al. Factor relationships of metabolic syndrome and echocardiographic phenotypes in the HyperGEN study. J Hypertens. 2008;26(7):1360–1366. doi: 10.1097/HJH.0b013e3282ffdc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schachter F, Faure-Delanef L, Guenot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6(1):29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 29.Galinsky D, Tysoe C, Brayne CE, et al. Analysis of the apo E/apo C-I, angiotensin converting enzyme and methylenetetrahydrofolate reductase genes as candidates affecting human longevity. Atherosclerosis. 1997;129(2):177–183. doi: 10.1016/s0021-9150(96)06027-3. [DOI] [PubMed] [Google Scholar]