Abstract
We used a heterogeneous stock of mice—UM-HET3, the first generation offspring of CByB6F1/J and C3D2F1/J parents—to test effects of six antiaging treatments on life span. In the first report of diet restriction in a structured, segregating heterogeneous population, we observed essentially the same increases in mean and maximum life span as found in CByB6F1/J hybrid positive controls. We also report results of treatment with N-acetyl-L-cysteine started at 7 months, and aspirin, nitroflurbiprofen, 4-hydroxy phenyl N-tert-butyl nitrone, and nordihydroguaiaretic acid, all started at 16–18 months. Only male UM-HET3 mice receiving N-acetyl-L-cysteine had significantly increased life span, and this may have been due to treatment-related inadvertent diet restriction. The other agents had no significant effects on life span. The use of UM-HET3 mice helps assure that these results are not the result of unresponsiveness of a single genotype but that they more broadly represent laboratory mice.
Keywords: Diet restriction, HET3, Life span, Antiaging interventions, Mice
A critical problem in mammalian aging research is overreliance on specific inbred strains of rats and mice (1). The popularity of inbred strains is well earned—availability, reliability, genetic homogeneity, and thorough characterization—but comes with a major risk: the possibility of generalizing strain-specific idiosyncrasies as traits of normal aging. This risk is a particular concern for life span studies because life span is especially susceptible to inbreeding depression, which has strain-specific manifestations and creates experimental confounds with senescence. Numerous approaches have been used to address this issue [reviewed in (2,3)]. Often the best choice is to use well-defined genetically segregating stocks, constructed directly from parental inbred strains chosen for their genetic diversity.
Crossing two diverse F1 hybrids in a four-strain cross is a convenient way to create such a structured segregating heterogeneous mouse population. Although each individual is genetically distinct, the population can be reproduced from the standard F1 hybrid parents at any time (4). The UM-HET3 (hereafter called HET3) cross, a specific four-strain cross that was developed by Dr. Richard A. Miller for the study of aging (5), is such a population. It comprises the first generation offspring of a cross between CByB6F1 females and C3D2F1 males. Exclusion of short-lived parental strains helps reduce the frequency of alleles that influence mortality independently of senescence. Also, the derivation of three of these four strains from completely independent sources (6) assures a high degree of genetic diversity. In fact, although the four strains of the HET3 cross represent only 7% of the 55 domesticated inbred strains of laboratory mice in the Sanger Institute database (http://www.sanger.ac.uk/Projects/M_musculus/), the genetic diversity is much broader: the 1,937,989 single nucleotide polymorphisms (SNPs) within the HET3 cross represent 35% of the total diversity (5,528,028 SNPs) among all 55 domesticated inbred strains, which includes inbred strains developed in Japan, Europe, and the United States. Finally, another advantage of the use of HET3 mice is that the stock has been characterized for many aging parameters (5,7–9).
The National Institute on Aging uses HET3 as the animal model for its Interventions Testing Program (ITP), which tests promising antiaging interventions (7). The genetic diversity designed into the HET3 stock ensures that causes of age-related mortality will be varied, and thus, that any significant increase in life span from an intervention will not result merely from a beneficial effect on a strain-specific disease. To date, the ITP has reported that lifelong treatment with aspirin or with nordihydroguaiaretic acid (NDGA) starting at 4 months of age significantly increases life span in male HET3 mice (10), and that treatment with rapamycin started at 20 months of age substantially increases life span in both male and female HET3 mice (11). Interventions being tested to date, and procedures to submit ideas for interventions to the ITP, are provided at http://www.nia.nih.gov/ResearchInformation/ScientificResources/InterventionsTestingProgram.htm
The current study further characterizes aging in HET3 mice using a classic treatment, diet restriction (DR). For the past 75 years, scientists have known that DR typically increases life span in healthy mouse and rat populations, thus setting the standard for interventions that increase mammalian life span (12,13). Recently, however, two DR studies using large collections of the inbred long sleep × inbred short sleep recombinant inbred strain set produced from the same two parental inbred strains (14,15) suggested that DR might not be as broadly effective across genotypes as previously assumed. In both of these studies, DR was as likely to decrease life span of a strain as it was to increase it. Although a few other studies have also reported failure of DR to increase life span in single inbred strains (see Discussion), the results of the recombinant inbred strain studies (14,15) were surprising in light of the conventional wisdom that DR generally increases life span. HET3 mice are ideal models for addressing this disparity because of their extremely broad range of genotypes and because of their segregating genetic configuration, which avoids inbreeding depression. In our study, DR increased life span in HET3 mice by 32%–47%, with no indication of a significant subgroup with shortened life span.
In addition, we report results of studies using HET3 mice to test the effectiveness of other potential antiaging treatments, including N-acetyl-L-cysteine (NAC), which may retard aging by several mechanisms. NAC is a synthetic acetylated derivative of cysteine, has a long history of clinical use, and appears to be well tolerated by humans. The major metabolites of NAC are L-cysteine and glutathione. NAC, like endogenous thiols, is an effective scavenger of free radicals, and is considered an antioxidant (16). Consequently, NAC has been tested as a potential auxiliary treatment in cardiovascular disease, pulmonary fibrosis, arthritic joint inflammation, HIV infection, renal failure, and cancer (17–22). Recently, Ackert-Bicknell et al. (23) have shown that chronic NAC treatment in mice can suppress levels of circulating insulin-like growth factor-1 by 20%–50%, depending on mouse strain. Therefore, NAC may retard senescence through both antioxidant and growth-retarding mechanisms.
We also report studies of aspirin, nitroflurbiprofen (NFP), 4-hydroxy phenyl N-tert-butyl nitrone (4-OH-PBN), and NDGA, previously tested in HET3 mice starting at 4 months of age (7,10). The current study started treatment when the HET3 females reached 16–18 months (equivalent to about 55 years in humans) to test the hypothesis that these interventions might be more effective if initiated at late middle age—specifically, to determine whether effects of aging or treatment duration might alter the response to treatment such that treatment initiated in older mice may be more effective (see Discussion). These treatments were chosen based on their antioxidant activity or anti-inflammatory activity or both [discussed in (7,10)].
Our results support the use of HET3 mice in studies of aging for the following reasons: HET3 mice are genetically well defined. They respond well to DR. And their use in intervention studies helps assure that both negative and positive findings are broadly applicable—that the results are dependent neither on inbreeding depression nor on an idiosyncratic response of a specific genotype.
METHODS
Mice
The HET3 mice used in the current study were produced by a cross between two different F1 parents from The Jackson Laboratory: (BALB/cByJ × C57BL/6J)F1 mothers (CByB6F1/J; JAX Mice stock #100009) and (C3H/HeJ × DBA/2J)F1 fathers (C3D2F1/J; JAX Mice stock #100004). Each HET3 mouse in the test population is genetically unique but shares one half of its (nuclear) genome, on average, with every other mouse in the population. CByB6F1 mice were diet restricted as a positive control in parallel with HET3 mice, to be certain that our DR methods gave the expected results with a standard F1 hybrid.
All mice in this study were produced and maintained at The Jackson Laboratory, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care, and all studies were conducted under a protocol approved by the Animal Care and Use Committee (ACUC) of The Jackson Laboratory. Mice were introduced into the aging colony at weaning (4–5 weeks of age). All mice were housed in double-sided plastic cages in isolated rooms under positive pressure with high efficiency particulate air-filtered air at room temperature (24 ± 1°C), lighted from 6 AM to 6 PM. Mice were housed four per side with others of the same sex and treatment. Results of quarterly screening for pathogens and other microorganisms at The Jackson Laboratory are provided online at http://jaxmice.jax.org/genetichealth/index.html. Mice in the current study were housed in either room C1 or D1; during the course of this study, neither room had any pathogens for which the The Jackson Laboratory routinely tests. We did not observe the common dermatitis (nor the associated prolapse) that is frequently seen in populations of B6 mice and related genotypes, which would typically require censoring of life span data. We did censor data if we observed persistent evidence of bite wounds or if mice were missing (see the Statistical Methods section, below).
Mice in our studies of life span were allowed to live until we were certain that they would have died shortly had we not euthanized them. Euthanasia was conducted according to an institutional procedure approved by The Jackson Laboratory’s Animal Care and Use Committee; the procedure is based on criteria that indicate death is imminent. These criteria include the following:
Unresponsiveness to touch.
Slow respiration.
Cold to the touch.
Hunched up with matted fur.
Sudden weight loss, failure to eat and drink, prominent-appearing ribs and spine, and sunken hips.
Among the criteria, severe nonresponsiveness by itself is critical enough to warrant euthanasia. Otherwise, all remaining criteria must be observed. Typically, however, if one symptom is present, all symptoms are present. If there is a question concerning the severity of morbidity, mice are not euthanized but are monitored until criteria are met.
Water and Feeding Protocols
Food and water.—
All mice received ad libitum chlorinated water, acidified to prevent growth of Pseudomonas. For the DR study, which was started upon weaning, both control and treated mice were fed autoclaved LabDiet 5K54 (PMI Nutrition International, Brentwood, MO). For all other treatment studies, both control and treated mice were fed LabDiet 5LG6 (PMI Nutrition International), an irradiated diet. Both diets have the following composition: 18% protein, not less than 5% fiber, not less than 4% fat, and not more than 8% ash. Gross energy content is 3.97 kcal/g, and metabolizable energy is 3.09 kcal/g. Specific amino acid, fat, carbohydrate, mineral, and vitamin contents are published by PMI Nutrition International.
Diet restriction.—
Starting at 4–5 weeks of age, mice treated with DR were fed a measured amount of food each day. Food quantity was 66%–70% of the average amount eaten by ad-lib-fed mice of the same sex and age. Mice were fed using an automated system of long troughs that rested across the tops of the mouse cages. Each trough was divided into equally spaced compartments, each of which held the appropriate quantity of food for a specific cage for 1 day. At 11:00 PM (23:00), a timing mechanism activated a small motor that slid the compartment dividers through the trough until a compartment aligned with an opening at the top of a cage and thus released the food into the cage. When numbers of mice in a cage were reduced, the amount of food was reduced proportionally.
NAC (Sigma #A7250).—
Starting at 7 months of age, mice were given drinking water containing either 5 g/L of NAC (lo-NAC; predicted at the start of the study to achieve about 600 mg/kg/d of NAC) or 10 g/L of NAC (hi-NAC; predicted at the start of the study to achieve about 1,200 mg/kg/d of NAC). Treated water was prepared fresh each week.
We based our dosage on the following: NAC supplementation at 250 mg/kg/d increased life span in a mouse model of lupus (24). In response to 1,200 mg/kg/d (our hi-NAC dose) in growing mice, serum insulin-like growth factor-1 levels were suppressed by 20%–50%, depending on strain, which was sufficient to diminish long bone growth and bone mineral density (23). Importantly, at this hi-NAC dose, food intake remained at normal levels (3 g per mouse per day). Therefore, we chose a “high” dose of NAC (1,200 mg/kg/d) and a lower dose (600 mg/kg/d) that is closer to the more commonly used “therapeutic” range.
In fact, in our study, NAC treatment reduced both food and water consumption. We measured water consumption during a 4- to 8-week period after NAC treatment was initiated to avoid effects of acclimation (see the Results section). Because NAC-treated mice reduced their water intake proportionally more than they reduced their body weight, their effective NAC dose was lower than originally estimated. We calculated the effective doses of NAC as follows: females, lo-NAC, 333 mg/kg/d; females hi-NAC, 536 mg/kg/d; males lo-NAC, 250 mg/kg/d; males hi-NAC, 421 mg/kg/d. Because the loss in body weight tended to stabilize (and body weight did not recover) after the 4-week acclimation period, we infer that food and water intake remained lower than normal throughout the life of the treated mice and that our effective dose of NAC remained lower than our initially calculated levels.
Aspirin, NFP, 4-OH-PBN, and NDGA.—
These compounds were tested only in female mice, starting at 16–18 months of age. Compounds were individually mixed in the powdered irradiated diet (LabDiet 5LG6) at Purina TestDiet, Inc. (Richmond, IN), which then prepared the diets as pellets. Doses were chosen based on recommendations by those nominating the intervention to the ITP (7) and were checked against published literature to be certain that they produced no toxicity. The rationale for testing these interventions and results of testing starting at 4 months has been published (7,10). Concentrations of these compounds, based on weight ratios, are reported in the following (assuming mice weighed 30 g and ate 5 g of food per day):
Aspirin was added to chow at 21 mg/kg food to produce a daily dose of about 3.5 mg/kg body weight. This dose is equivalent to the higher end of the dose range recommended for daily treatment of humans at risk for heart attack. We were not aware of reports of effects of aspirin treatment in this dose range on life span in rodents at the time we initiated this study. To induce an ulcerogenic activity of aspirin in rodents, a dose 1,000-fold greater than 3.5 mg/kg is typically required (25).
NFP was added at 200 mg/kg food to produce a daily dose of about 33 mg/kg body weight. In studies of rats and mice, this dose, given orally, has been shown to provide protection in a variety of disease models. For example, it diminished onset and severity of disease in a mouse model of experimental autoimmune encephalomyelitis (26).
4-OH-PBN was added to chow at 315 mg/kg food to produce a daily dose of about 52 mg/kg body weight. A preliminary study in mice given 4-OH-PBN (10 mg/kg/d) orally, beginning at 23–24 months of age, indicated that the treatment increased median survival (maximum survival was not determined) (27); treatment of rats with 37.5 mg/kg/d (by drinking water) started at 24.5 months, significantly increased mean and maximum life span (by 4%–5%) (28). When 4-OH-PBN was given to rats intraperitoneally at a dose of 32 mg/kg/d beginning at 24 months, median survivorship, compared with controls, increased by about 16% (29), suggesting the possibility that effective oral doses higher than 37.5 mg/kg/d may increase life span even more.
NDGA was added to chow at 2,500 mg/kg (0.25%) food to produce a daily dose of about 417 mg/kg body weight. NDGA is tolerated by mice at 1% of total dietary intake for periods of 6 months to 1 year (30). In his proposal of NDGA for treatment studies by the ITP, K. L. Hensley reported preliminary results showing that mice tolerate 0.25% NDGA for at least 1 year with no signs of frank or tissue-level pathology, and in fact demonstrate better motor performance than untreated controls (unpublished data, 2003).
Statistical Methods
For the DR study, we analyzed life span data using the likelihood ratio chi-square tests of main effects (sex, DR, stock [HET3 or CByB6F1]) and their interactions in a proportional hazards survival model (JMP 7.0; SAS Institute Inc. 2007). Date of death was the date the mouse was euthanized as moribund or the date the carcass was found. When neither of these conditions was met, the date of death could not be determined; therefore, we censored the life span data for those mice. Censoring treats the survival data as known until the date of censoring and as missing after the date of censoring. For a mouse whose carcass was never found (eg, it may have been eaten by cage mates, or the mouse may have escaped without being detected during cage changing), we assigned the censoring date as the date the mouse was noted as missing from the cage. When there was no death date or missing date recorded, we used the date of the last body weight as the censoring date.
We first tested main effects and their interactions in the full proportional hazards model (including all two-way interactions and the three-way interaction). Nonsignificant interactions were then removed hierarchically (interactions with the largest p values were removed one at a time) to produce a minimal model; in the DR study, none of the interactions were significant in the full model or in any reduced model.
For the NAC study, we first tested effects of treatment and sex and their interaction, as in the DR study. Because the interaction was significant, we then ran a separate analysis for each sex.
We also considered statistics for maximum life span because in populations that experience senescence, progressively more information about the impact of aging rates on mortality risk is contained in the survival data as the data are drawn from progressively older survivorship subsets (2). One of the best strategies for analysis of maximum life span uses Fisher’s Exact Test to evaluate treatment effects on the proportion of treated versus control mice that survive to the longest-lived 10% of the study population (31). However, this approach requires large sample sizes to obtain reasonable statistical power. Therefore, we lowered the maximum survival criterion to the longest-lived 40%, which can provide, for studies of laboratory mice, even greater power than analysis of total population life span (32), and which still focuses on a subset of survival data that contains the greatest information about aging rates in the populations. Because we wanted to analyze multiple independent variables in a single model, and because Fisher’s Exact Test is used when only a single independent variable is analyzed, we used the likelihood ratio chi-square test of effects in a nominal logistic model that we generated using JMP 7.0 (SAS Institute Inc. 2007). This model permits analysis of multiple independent variables and their interactions when the dependent variable is categorical (nominal). The null hypothesis in this test is that the ratio of treated to untreated mice (or male to female, or HET3 to CByB6F1 mice) in the longest-lived 40% of the population is equal to the ratio in the shorter-lived 60%.
The nominal logistic model does not take into account censored data. We included censored data in our analysis of maximum life span if the age of the mouse at censoring was in the longest-lived 40% because we could unequivocally assign the life span to the longest-lived group; we deleted censored data if the age of the mouse at censoring was in the shorter-lived 60% because we could not know which survivorship that mouse would have belonged to.
We analyzed effects of DR or NAC treatment on body weights using analysis of variance.
RESULTS
Diet Restriction
DR significantly increased life span in both the HET3 and the CByB6F1 population (Table 1 and Figure 1). The relative response to DR was the same for both stocks, and it did not differ between the sexes. This conclusion is based on the absence of two-way interactions and the three-way interaction among sex, genotype, and diet treatment, as indicated by likelihood ratio chi-square tests. Therefore, we combined the two genotypes and sexes for tests of main effects in the proportional hazards model (Table 1 and Figure 1).
Table 1.
Effects of Diet Restriction on Life Spans and Weights of UM-HET3 (HET3) and CByB6F1/J Mice
| Weight (g), Mean ± SD | ||||||||
| Stock | Sex | Diet | Life Span, Mean ± SD | n | Oldest 2 Mice (d) | 90 d | 240 d | 480 d |
| HET3 | F | Ad lib | 836 ± 171 | 16 | 994; 1,032 | 24 ± 2 | 35 ± 7 | 41 ± 9 |
| DR | 1,169 ± 195 | 16 | 1,382; 1,383 | 19 ± 2 | 22 ± 2 | 21 ± 2 | ||
| M | Ad lib | 831 ± 237 | 14* | 1,115; 1,320 | 34 ± 5 | 45 ± 6 | 47 ± 4 | |
| DR | 1,101 ± 256 | 16 | 1,412; 1,426 | 26 ± 2 | 29 ± 2 | 25 ± 2 | ||
| CByB6F1 | F | Ad lib | 859 ± 205 | 16 | 1,164; 1,178 | 25 ± 3 | — | — |
| DR | 1,283 ± 137 | 16 | 1,421; 1,653 | 17 ± 1 | 20 ± 1 | — | ||
| M | Ad lib | 839 ± 227 | 16 | 1,108; 1,108 | 34 ± 2 | 48 ± 4 | 51 ± 6 | |
| DR | 1,154 ± 185 | 16 | 1,370; 1,370 | 20 ± 1 | 24 ± 2 | 28 ± 3 | ||
Notes: DR = diet restriction. HET3 mice are the first-generation four-way cross mice of CByB6F1/J females crossed with C3D2F1/J males. Results of statistical tests for life span are given in Figure 1.
Two mice were not included in the determination of mean life span because they were recorded as missing before they were 1 year old, presumably due to reasons not reflecting aging; the remaining censored mice (see Figure 1) were included in the mean life span calculation with the age at censoring as their life span.
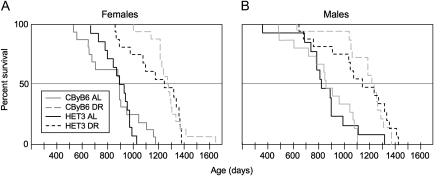
Figure 1.
Kaplan–Meier plots of survival curves comparing mice of two stocks—CByB6F1/J and UM-HET3 (HET3), a first-generation four-way cross—treated with diet restriction (DR) or ad lib (AL) fed. At the start of the study, each group consisted of 32 mice, 16 of each sex. We initiated DR at 4–5 weeks of age. Treatment was continued until mice died or became moribund. DR increased life span of both stocks and both sexes (p < .0001, likelihood ratio chi-square tests in a proportional hazards model). The relative response to DR was the same for both stocks, and it did not differ between the sexes (no two-way interactions or three-way interaction among stock, sex, and diet in the proportional hazards model). Data were censored for missing mice (see the Methods section) at the following ages (days): two female HET3 AL mice, both at 478 days; two female HET3 DR mice at 987 and 1,292 days; one male CByB6F1 AL mouse at 460 days; two male CByB6F1 DR mice, both at 1,000 days; two male HET3 AL mice at 92 and 477 days; and one male HET3 DR mouse at 932 days. We censored the life span data for one HET3 AL mouse at 266 days due to bite wounds. No data were censored for female CByB6F1 mice. Overall, we censored data for 6 of the 63 AL mice and 5 of the 64 diet-restricted mice.
Although the combined group sizes for tests of the main effects were 32 mice per group (including censored data), subtle effects of sex or genotype could have been missed in our analysis. For example, the effect of DR on maximum life span (proportion of diet-restricted mice among the longest-lived 40%) was sex dependent (interaction of sex and diet in the nominal logistic model, p = .04). Therefore, we separated further analysis of maximum life span by sex. Among both males and females, DR comparably increased maximum life span (Figure 2, p < .0001, likelihood ratio chi-square test for the nominal logistic model) in both HET3 and CByB6F1 mice (no significant interaction of stock with diet for either sex).
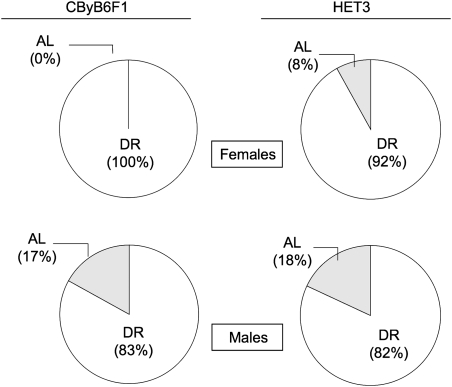
Figure 2.
Maximum survivorship in response to diet restriction (DR) is comparable in CByB6F1/J and UM-HET3 (HET3) mice. The figure shows the effect of DR on the proportion of diet-restricted mice in the longest-lived 40% of females and 40% of males for each stock. Statistical analysis employed a nominal logistic model to compare the proportion of diet-restricted mice in the longer-lived 40% survivorship with the proportion of DR mice in the shorter-lived 60% survivorship. Because the effect of DR was sex dependent (interaction of sex and diet, p = .04, likelihood ratio chi-square test), further analysis was separated by sex. Among both males and females, DR increased maximum life span (p < .0001, likelihood ratio chi-square test) comparably in both HET3 and CByB6F1 mice (no significant interaction of stock with diet for either sex). Mice are the same as in Figure 1, except that we dropped all censored mice but one (the “longest-lived” censored mouse) because they could not be unequivocally assigned to a survivorship group. Group sizes were as follows: CByB6F1 females, 32 mice; HET3 females, 29 mice; CByB6F1 males, 29 mice; and HET3 males, 28 mice. Numbers of mice in the lower 60% survivorship were as follows—CByB6F1 females: 16 AL, 3 DR; HET3 females: 13 AL, 4 DR; CByB6F1 males: 13 AL, 4 DR; and HET3 males: 11 AL, 6 DR. AL = ad lib.
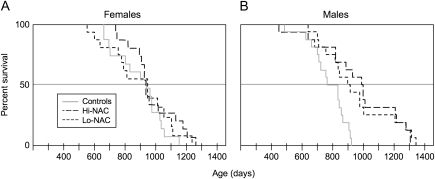
Figure 3.
Kaplan–Meier plots of survival curves comparing a control group with N-acetyl-L-cysteine (NAC)–treated groups. Each group consisted of 32 UM-HET3 (HET3) mice, 16 mice of each sex. We treated mice with either 10 g (hi-NAC) or 5 g (lo-NAC) NAC per liter of drinking water starting at 7 months of age. Treatment was continued until mice died or became moribund. The control group was a separate group of HET3 mice from the control group in the diet restriction study. The effect of NAC treatment on life span differed by sex (interaction of sex and treatment in the proportional hazards model, p = .02, likelihood ratio chi-square test); therefore, we analyzed females separately from males. (A) NAC treatment did not affect life span in females. (B) NAC treatment increased life span in males (p = .004, likelihood ratio chi-square test). Each NAC dose had a similar effect (low dose p = .008; high dose p = .0007, likelihood ratio chi-square tests in separate proportional hazards models for each dose). Data were censored for missing mice at the following ages (days): female control, 629 days; female low dose, 791 and 868 days; and female high dose, 292 days. No data were censored for male mice.
The individual group sizes were too small (14–16 mice of each sex and stock for control and for diet-restricted mice) to evaluate effects on the longest-lived 10%—a criterion for analysis of maximum life span recommended by Wang et al. (31). Therefore, to illustrate the effects of DR on maximum life span as conventionally defined, we reported the life spans of the longest-lived two mice in each group (Table 1). DR comparably increased life span for the longest-lived HET3 and CByB6F1 females by about 1 year, and by somewhat less for the longest-lived HET3 and CByB6F1 males.
Diet-restricted HET3 mice also weighed less than controls. Based on body weight data, this protocol appeared to greatly diminish postmaturational growth. For example, male weights at 3, 8, and 16 months were as follows: HET3 ad-lib-fed controls averaged 34, 45, and 47 g; diet-restricted HET3 males averaged 26, 29, and 25 g. Again, results in the HET3 population were very similar to those in the CByB6F1 hybrids (Table 1).
N-Acetyl-L-Cysteine
The effect of NAC treatment on total life span differed by sex (interaction of sex and treatment in the proportional hazards model, p = .02, likelihood ratio chi-square test); therefore, we analyzed females and males separately. In females, NAC treatment did not significantly affect total life span. In males, both the hi- and the lo-NAC doses increased total life span, although neither was as effective as DR (Figure 3).
As with total life span, there was a sex difference in effects of NAC on maximum life span. For females, neither high nor low NAC treatment affected the proportion that survived to the longest-lived 40%. For males, however, those treated with the high dose of NAC showed a significant increase compared with controls: 11 of 16 treated males versus 2 of 16 control males (p = .0008). Those treated with the low dose of NAC showed a marginal increase compared with controls: 9 of 16 treated males were in the longest-lived 40% survivorship, versus 4 of 16 of the control males (p = .07, likelihood ratio chi-square test in a nominal logistic model).
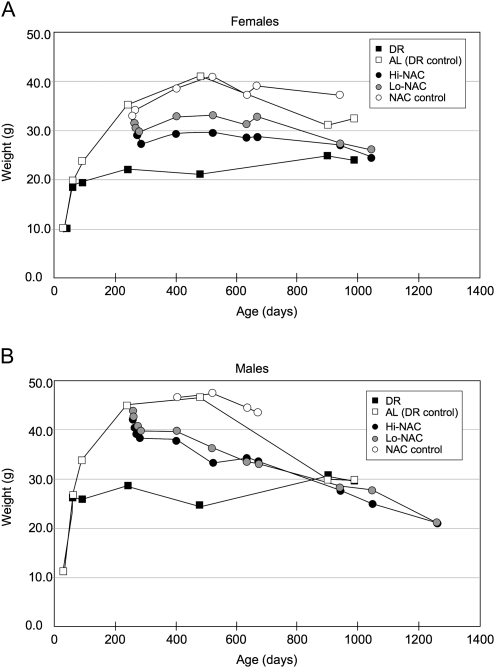
Figure 4 compares body weights. We weighed NAC mice of both sexes frequently because weights dropped suddenly—about 5 g for lo-NAC and 7 g for hi-NAC—during the first 2 weeks after NAC treatment was started. The rate of weight loss then became much slower, especially in females, but the slow loss continued until about 900 days of age, when weights of the NAC-treated groups were similar to those of diet-restricted mice (Figure 4). From weeks 4 to 8 of NAC treatment, we recorded amounts of water consumed per four-mouse cage (four cages per sex and treatment). NAC treatment caused water consumption to decline: For female controls, consumption averaged 4.0 mL per day per mouse, whereas lo-NAC and hi-NAC mice averaged 2.0 and 1.5 mL, respectively; for male controls, consumption averaged 4.2 mL per day per mouse, whereas lo-NAC and hi-NAC mice averaged 2.0 and 1.6 mL, respectively.
Figure 4.
Body weights of UM-HET3 mice on diet restriction (DR) or N-acetyl-L-cysteine (NAC) treatment. Mice were diet restricted from 4 weeks of age or treated with NAC from 7 months of age. Standard errors generally were 1–2 g. AL = ad lib.
Aspirin, NFP, 4-OH-PBN, and NDGA
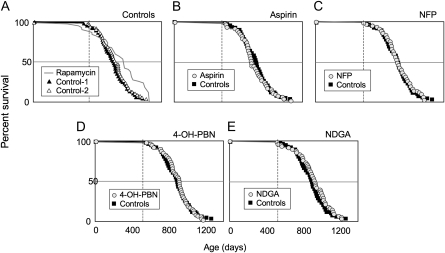
We tested four additional interventions starting at 16–20 months of age in HET3 females. Aspirin, NFP, 4-OH-PBN, and NDGA had been tested previously starting at 4 months of age. In that study, none of the treatments increased life span in females. Aspirin and NDGA increased life span slightly, but significantly, only in males (10). We started these interventions later in life (16–18 months of age) and saw no significant effect on survival (Figure 5). We observed no initial effect of any of the four treatments on body weight; therefore, we continued to monitor body weight only about every 3 months. Mice in all groups, including the control group, continued to gain weight until 20–23 months of age. At 37 months of age, when we stopped taking body weights, mice were 28% lighter than their initial weight at 14 months (all groups combined; no significant difference among the groups). Aspirin treatment may have delayed the onset of this age-related weight loss: at 27 months of age, aspirin-treated mice were still 13% heavier than they were at 14 months, whereas control mice were already 8% lighter (p = .03, interaction of age and aspirin treatment, analysis of variance). Although aspirin treatment may retard an aging process that causes late age-related weight loss, we consider this interpretation provisional because by 31 months, and afterward, aspirin-treated mice weighed the same as controls and the other treated mice.
Figure 5.
Survival curves for control and treated UM-HET3 (HET3) mice. Starting at 16–18 months of age, groups of about 60 females were given diets containing the following: aspirin, 21 ppm; nitroflurbiprofen (NFP), 200 ppm; 4-hydroxy phenyl N-tert-butyl nitrone (4-OH-PBN), 315 ppm; and nordihydroguaiaretic acid (NDGA), 2,500 ppm. Here, 1 ppm is 1 mg/kg of diet. None of these four treatments affected overall life span or maximum life span (proportion of mice in the longest-lived 40% survivorship). Note: The results from rapamycin-treated mice at The Jackson Laboratory are redrawn from Harrison et al. (11); they are provided here as an illustration of the potential for increased life span of HET3 mice when treatment is started late in life.
DISCUSSION
The Genetic Configuration of Mouse Models in Gerontological Research
Because gerontologists must study their animal models at ages when senescence and age-related disease interact, the influence of the genetic configuration itself on disease becomes an important consideration. Homozygous genotypes, such as inbred mouse strains, possess two inherent drawbacks: diminished “physiological buffering” that can limit stress response (33) and the late expression of deleterious recessive alleles. Both characteristics can contribute to susceptibility to age-related disease, which can shorten life span for reasons unrelated to senescence. In fact, Chai (34) directly demonstrated that life span of various types of crosses between the same two strains (eg, F1, F2, N2) is inversely correlated with the degree of homozygosity.
F1 hybrid mice made from unrelated strains minimize homozygosity, and their robustness permits normal aging to proceed and to be measured, while minimizing the complication of disease-associated impairments. A single F1 stock, however, still represents only a single genotype. Thus, as with an inbred strain, generalization may be limited. A useful alternative is a four-way cross model, typically comprising the first-generation offspring of two different F1 hybrids. When parental strains are unrelated—as with the strains of the HET3 cross—a four-way cross produces a genetically highly diverse population that minimizes homozygosity and presents its alleles in a virtually infinite assortment because every unlinked polymorphic locus segregates. Reliability is ensured because the genetic makeup of a four-way cross population can be reproduced at any time directly from the inbred parental strains. The increased genetic variance of a four-way cross may require a greater sample size to maintain statistical power at a desired level. Yet, this additional expense is often justifiable, especially for preclinical studies, because the enhanced reliability and broad applicability of results can help assure judicious decisions when designing costly clinical trials.
In this group of studies, we used the HET3 four-way cross mice to test effects of six antiaging treatments on life span, with the goal of generating results that would have broad applicability for the field of gerontology.
Diet Restriction
Although HET3 mice had been well characterized for aging, no published studies had documented their response to DR. DR markedly increased both overall mean and maximum life span among the HET3 mice. Although we cannot eliminate the possibility that a few of the individual genotypes in our HET3 population may have responded to DR with a shortened life span, all the diet-restricted HET3 mice in our study survived past the age when the ad-lib-fed control HET3 mice began to die; in fact, the first age-related death among diet-restricted HET3 males did not occur until half the ad-lib-fed HET3 males had already died. These results are important for two specific reasons. First, we showed that DR increases life span in mice across the broadest range of genotypes tested to date. Second, we did not see the early deaths sometimes observed for individual diet-restricted mice within an inbred strain.
Differences in experimental design and husbandry from lab to lab could, by themselves, account for different results [eg, we initiated DR at 4–5 weeks age, whereas Liao et al. (14) initiated DR at 2–5 months]. We suggest that DR-related shortened life spans, such as those reported for the inbred recombinant inbred lines studied by Liao et al. (14) and Rikke et al. (15), are a consequence of the amplification of the stress of DR as it interacts with particulars of husbandry and experimental design when inbred strains are used. A review of the studies that report a failure of DR to increase life span in rodents is instructive.
Barrows and Roeder (35) observed no increased life span for diet-restricted Sprague-Dawley and Wistar rats. However, mean life spans of ad-lib-fed rats in this study were very short (22 and 20 months), indicating that conditions in their conventional colony may have interfered with potentially beneficial effects of DR. Results such as these underscore the importance of maintaining supportive husbandry, including specific pathogen-free conditions, when studying life span in rodents (2,36).
We reported that DR, using a 96WA diet, shortened life span of B6 male mice (37). In the same study, DR increased the life span of B6CBAF1 males. In light of the numerous reports of DR-induced increases in life span of B6 mice [eg, Goodrick et al. (38), Turturro et al. (39), Forster et al. (40)], we interpret the isolated failure of DR to increase life span in B6 males as the result of a particular requirement of inbred B6 male mice for some nutrient that was deficient in the specific diet.
Fernandez et al. (41) and Forster et al. (40) report that DR shortened life span in DBA/2 (D2) strains of mice. This may be a consequence of the interaction of some specific genetic element in D2 mice with the inbred genetic configuration, which affects the response to DR; in both studies, the same DR treatment increased life span in F1 mice, including, in the study of Forster et al. (40), B6D2 F1 mice. Similarly, in the large cohorts of mice studied in the National Center for Toxicological Research collaboration with the National Institute on Aging (39), DBA/2JNia mice showed an increase, in response to DR, only in maximum life span, not in median life span. Yet, the F1 cross of these mice with C57BL/6NNia mice showed robust increases in both median and maximum life span in response to DR, as did B6C3F1 mice. In fact, our survey of the literature found that F1 hybrid, and other noninbred, mouse and rat lines tested under specific pathogen free conditions responded to DR with increased life span.
The study of Harper et al. (42) using a genetically mixed colony of grandoffspring of wild-trapped mice may be particularly instructive. Mean life span of mice in this colony was not increased by DR; DR diminished the Gompertz slope for these wild-derived mice due to a significantly increased early mortality, compared with ad-lib-fed controls. This result serves as a reminder that DR is a stress and that, at some level, DR produces starvation. The particular degree of restriction that produces starvation is presumably influenced by genetic makeup; Harper et al. (42) suggest that domestication of mice may have co-selected for genotypes that are particularly suited to survive starvation. Thus, crosses of domesticated mice may be well suited to permit the expression of the underlying antiaging effects of DR.
N-Acetyl-L-Cysteine
NAC has attracted interest among gerontologists as a potential “antiaging” treatment because of its antitumor, antioxidant, and other antiaging characteristics. In HET3 females, we detected no benefits of NAC treatment at either a low or a high treatment dose. In HET3 males, we observed increases in overall life span at both low and high doses, and an increase in maximum life span at the higher dose. In both males and females, however, both doses of NAC caused a sudden drop in body weight, followed by a further slow decline (Figure 4). At 900 days of age, body weights in NAC-treated mice were the same in males and only 2 g heavier in females than weights of age-matched mice treated with DR. Thus, beneficial effects of NAC on life span may have resulted simply from self-imposed DR. It would be interesting to perform a complementation test, in which diet-restricted mice are given NAC, to determine whether effects on life span are additive or complementary, and to determine if any antiaging benefits correlate with antitumor effects or can be predicted by increased glutathione or decreased insulin-like growth factor-1 levels.
Late-Life Interventions
The possibility of a successful antiaging treatment started in late middle age to old age is very appealing for translational application. It is unlikely that most people will consider antiaging treatments when they are young. Our recent demonstration that dietary rapamycin initiated late in life significantly increased survival so effectively (11) suggests that other treatments to retard aging also might be successful when initiated later in life. This strategy might work because physiological changes with age, such as diminished clearance, might render mice more responsive to treatments started later in life. Furthermore, late-onset treatment could diminish tolerance that might develop when treatments are initiated in young adulthood.
For aspirin, NFP, 4-OH PBN, or NDGA, we found no effect of treatment started in late middle age in HET3 females at the doses we used. All four interventions had been tested previously, starting in younger mice (10); aspirin and NDGA increased life spans slightly in males but not in females when started at 4 months. These results indicate that the failure of these treatments, when started at 4 months, to increase life span in females, was not a consequence of iatrogenic effects from very long-term treatment.
Life Span in HET3 Mice
Given the high degree of heterozygosity in HET3 mice, greater life spans than we observed here might be predicted. Those we report, however, are comparable with life spans reported in more extensive studies conducted for the ITP (at The Jackson Laboratory, University of Michigan, and University of Texas, San Antonio). Thus, we believe that the life span data we report here are reliable. It is possible that the beneficial effect of heterosis on life span in HET3 mice is partially offset by genetic determinants from the two parental strains (C3H/HeJ and DBA/2J) with modest life spans. For example, although the HET3 mice include genetic elements from B6 mice, which is the longest lived among the 32 strains in the Mouse Phenome Database life span study (www.jax.org/phenome), the other three HET3 parental strains have intermediate life spans. If there was no effect of heterozygosity, we might expect the life span of HET3 mice to reflect an average life span of the parental strains. Instead, both male and female HET3 mice have a mean life span that is about 10% longer than the mean life span of the four parental inbred strains (based on the Mouse Phenome Database data). Of course, determination of the actual degree of heterosis would require direct comparison under identical conditions; however, the HET3 mouse model provides a reasonably long-lived model that includes genetic elements that represent strains with intermediate life spans and a strain with a long life span.
CONCLUSIONS
In choosing animal models for studies of mammalian aging interventions, no single genetic configuration is ideal. Researchers must consider the relative merits of each genetic configuration for their model in the context of the goals of their research. For the broadest application of results, one needs to sample multiple genotypes. One of the most effective ways to do this is to use a multistrain, structured segregating cross such as the HET3 stock. Our use of the HET3 mice in a DR study clarifies a current controversy concerning the breadth of effect of DR: We confirmed that DR consistently increased life span across a broad range of genotypes. Our use of HET3 mice in our other studies to test interventions with potential clinical application assures that the failure of a treatment is not the result of the unresponsiveness of a single genotype. Our results support the use of a genetically mixed model, with reproducible allelic composition, for studies in aging research.
FUNDING
This work was supported by National Institute on Aging grants to D.E.H.: AG022308, AG026074, and AG032333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health.
Acknowledgments
We thank Elizabeth Adler, Patricia J. Harrison, Bee Stork, and Pamala J. Krason for reliable technical assistance; Paul T. Smith Jr. for calculations of the genetic diversity of HET3 mice; and Joanne Currer for editorial assistance.
References
- 1.Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–B88. doi: 10.1093/geronj/46.3.b87. [DOI] [PubMed] [Google Scholar]
- 2.Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research, Vol III. 2nd ed. Burlington, MA: Elsevier Academic Press; 2007. pp. 637–672. [Google Scholar]
- 3.Austad SN. Issues in the choice of genetic configuration for animal aging models. Exp Gerontol. 1997;32:55–63. doi: 10.1016/s0531-5565(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 4.Roderick TH. Selection for radiation resistance in mice. Genetics. 1963;48:205–216. doi: 10.1093/genetics/48.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RA, Burke D, Nadon N. Announcement: four-way cross mouse stocks: a new, genetically heterogeneous resource for aging research. J Gerontol A Biol Sci Med Sci. 1999;54A:B358–B360. doi: 10.1093/gerona/54.8.b358. [DOI] [PubMed] [Google Scholar]
- 6.Flurkey K, Currer JM. Chapter 2: Some basic genetics of the mouse. In: Flurkey KF, Currer JM, Leiter E, Witham B, editors. The Jackson Laboratory Handbook on Genetically Standardized Mice. Bar Harbor, Maine: The Jackson Laboratory; 2009. pp. 9–23. [Google Scholar]
- 7.Miller RA, Harrison DE, Astle CM, et al. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller RA, Chrisp C. T cell subset patterns that predict resistance to spontaneous lymphoma, mammary adenocarcinoma, and fibrosarcoma in mice. J Immunol. 2002;169:1619–1625. doi: 10.4049/jimmunol.169.3.1619. [DOI] [PubMed] [Google Scholar]
- 9.Harper JM, Galecki AT, Burke DT, Miller RA. Body weight, hormones and T cell subsets as predictors of life span in genetically heterogeneous mice. Mech Ageing Dev. 2004;125:381–390. doi: 10.1016/j.mad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 13.Masaro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. 2010;45:691–701. doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafarulla M, Li WQ, Sylvester J, Ahman M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu MH, Coimbra TM, de Araujo M, Menezes LF, Seguro AC. N-acetylcysteine attenuates the progression of chronic renal failure. Kidney Int. 2005;68(5):2208–2217. doi: 10.1111/j.1523-1755.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti G, Lodola E, Licciardello L, Colombo A. Use of N-acetylcysteine in the management of coronary artery diseases. Cardiologia. 1999;44(7):633–637. [PubMed] [Google Scholar]
- 20.Roederer M, Staal FJ, Ela SW, Harzenberg LA, Harzenberg LA. N-acetylcysteine: potential for AIDS therapy. Pharmacology. 1993;46(3):121–129. doi: 10.1159/000139037. [DOI] [PubMed] [Google Scholar]
- 21.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3(2):114–127. [PubMed] [Google Scholar]
- 22.Delneste Y, Jeannin P, Potier L, Romero P, Bonnefoy JY. N-acetyl-L-cysteine exhibits antitumoral activity by increasing tumor necrosis factor alpha-dependent T-cell cytotoxicity. Blood. 1997;90(3):1124–1132. [PubMed] [Google Scholar]
- 23.Ackert-Bicknell C, Beamer WG, Rosen CJ. N-Acetylcysteine supplementation of growing mice: effects on skeletal size, bone mineral density and serum IGF-1. In: Burckhardt P, Dawson-Hughes B, Heaney RP, editors. Nutritional Aspects of Osteoporosis. 2nd ed. Burlington, MA: Elsevier Academic Press; 2004. pp. 369–377. [Google Scholar]
- 24.Suwannaroj S, Lagoo A, Keisler D, McMurray R. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus. Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 25.Zollei I, Szabo A, Kaszaki J, Tiszlavicz L, Ghyczy M, Boros M. Betaine-palmitate reduces acetylsalicylic acid-induced gastric damage in rats. Scand J Gastroenterol. 2001;36:811–816. doi: 10.1080/003655201750313324. [DOI] [PubMed] [Google Scholar]
- 26.Furlan R, Kurne A, Bergami A, et al. A nitric oxide releasing derivative of flurbiprofen inhibits experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;150:10–19. doi: 10.1016/j.jneuroim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Forster MJ, Wang Y, Nguyen L, Kelleher-Anderson J. American Aging Association (AGE) 28th Annual Meeting. New York: Springer: 1999. Anti-aging actions of novel nitrones. Abstract 60. p. 36. [Google Scholar]
- 28.Sack CA, Socci DJ, Crandall BM, Arendash GW. Antioxidant treatment with phenyl-a-tert-butyl nitrone (PBN) improves the cognitive performance and survival of aging rats. Neurosci Lett. 1996;205:181–184. doi: 10.1016/0304-3940(96)12417-4. [DOI] [PubMed] [Google Scholar]
- 29.Saito K, Yoshioka H, Cutler RG. A spin trap, N-tert-butyl-a-phenylnitrone extends the life span of mice. Biosci Biotechnol Biochem. 1998;62:792–794. doi: 10.1271/bbb.62.792. [DOI] [PubMed] [Google Scholar]
- 30.Cranston EM, Jensen MJ, Moren A, Brey T, Bell ET, Bieter RN. The acute and chronic toxicity of nordihydroguaiaretic acid. Fed Proc. 1947;6:318–319. [PubMed] [Google Scholar]
- 31.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan.”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Klebanov S, Harrison DE. Optimizing detection of QTLs retarding aging: choice of statistical model and animal requirements. Mech Ageing Dev. 2002;123:131–144. doi: 10.1016/s0047-6374(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 33.Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49:B1–11. doi: 10.1093/geronj/49.1.b1. [DOI] [PubMed] [Google Scholar]
- 34.Chai CK. Life span in inbred and hybrid mice. J Heredity. 1959;50:203–208. [Google Scholar]
- 35.Barrows CH, Jr, Roeder LM. The effect of reduced dietary intake on enzymatic activities and life span of rats. J Gerontol. 1965;20:69–71. doi: 10.1093/geronj/20.1.69. [DOI] [PubMed] [Google Scholar]
- 36.Russell ES. Lifespan and aging patterns. In: Green EL, editor. Biology of the Laboratory Mouse. 2nd ed. New York: McGraw-Hill; 1966. pp. 511–529. [Google Scholar]
- 37.Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- 38.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Div. 1990;1:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 39.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 40.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci USA. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harper JM, Leathers CS, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]